Virus Evolution Mechanisms of viral evolution Evolution the



- Slides: 20

Virus Evolution

Mechanisms of viral evolution Evolution: the constant change of a viral population in the face of selective pressures. 1. 2. 3. 4. Mutation Recombination Reassortment Selection Positive and negative pressure select for particular preexisting mutants.

Basic point: Virus evolution is fast • Fast generation time. • Produce large numbers of progeny. • High rates of mutation.

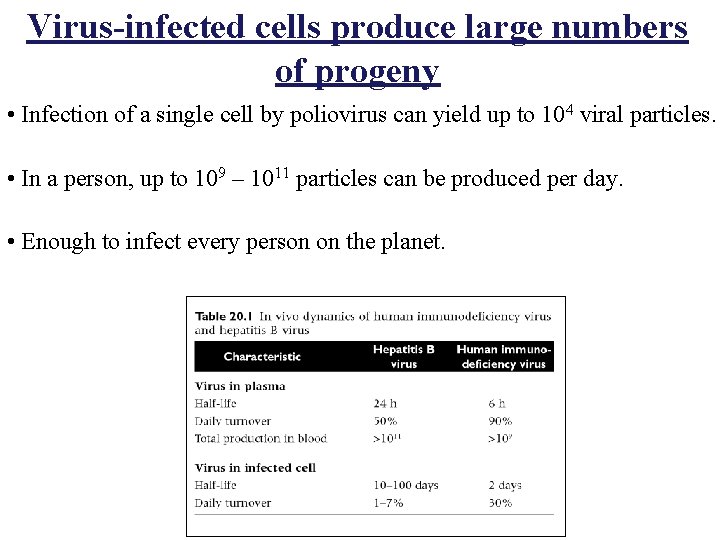
Virus-infected cells produce large numbers of progeny • Infection of a single cell by poliovirus can yield up to 104 viral particles. • In a person, up to 109 – 1011 particles can be produced per day. • Enough to infect every person on the planet.

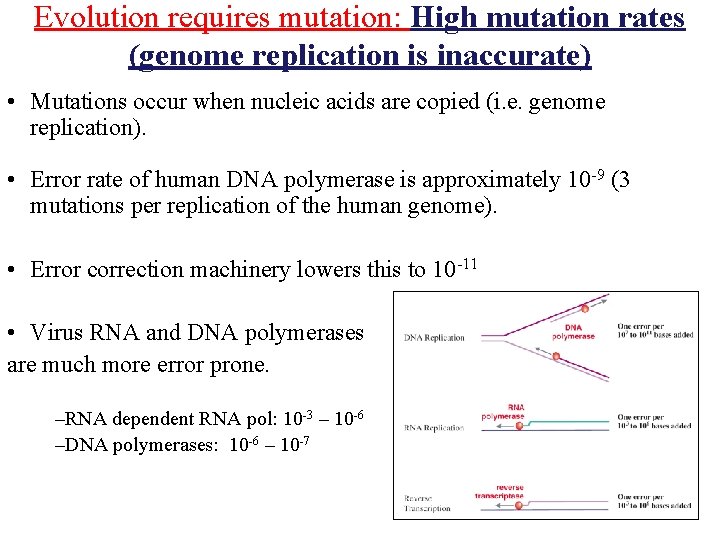
Evolution requires mutation: High mutation rates (genome replication is inaccurate) • Mutations occur when nucleic acids are copied (i. e. genome replication). • Error rate of human DNA polymerase is approximately 10 -9 (3 mutations per replication of the human genome). • Error correction machinery lowers this to 10 -11 • Virus RNA and DNA polymerases are much more error prone. –RNA dependent RNA pol: 10 -3 – 10 -6 –DNA polymerases: 10 -6 – 10 -7

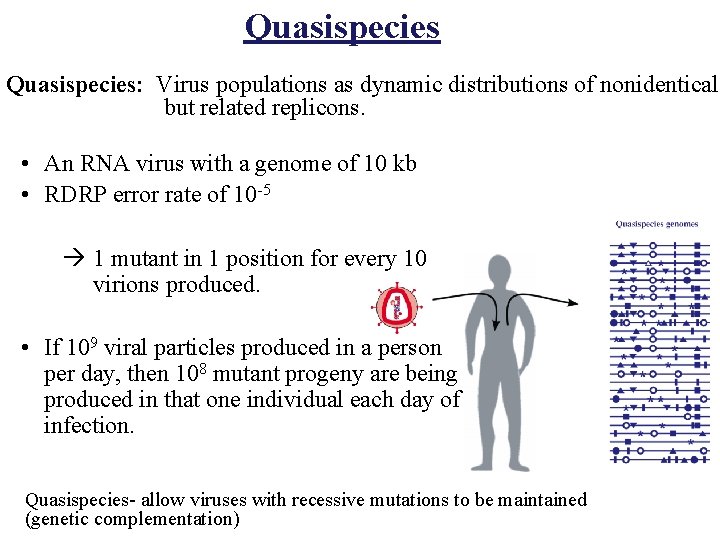
Quasispecies: Virus populations as dynamic distributions of nonidentical but related replicons. • An RNA virus with a genome of 10 kb • RDRP error rate of 10 -5 à 1 mutant in 1 position for every 10 virions produced. • If 109 viral particles produced in a person per day, then 108 mutant progeny are being produced in that one individual each day of infection. Quasispecies- allow viruses with recessive mutations to be maintained (genetic complementation)

Error Threshold The error threshold: Error threshold is a mathematical parameter that measures the complexity of the information that must be maintained to ensure survival of the population. The point at which accumulated mutations reduce fitness • Too much mutation can be lead to loss of vital information • Too little mutation can lead to host defenses overcoming the virus. Fitness: the replicative adaptability of an organism to its environment. The greatest fitness is when mutation rates approach the error threshold.

Error Threshold • Factors limiting mutations/evolution 1. mutations in cis acting factor required for replication, m. RNA synthesis, packaging of genomes will decrease fitness. 2. Constrained by interactions with the host. 3. Genome size. • Antimutators: mutations in polymerase that reduce frequency of incorporation errors. Increased fidelity is not advantageous in nature and not selected. • Error Catastrophe: extinction of an virus as the result of excessive RNA mutations. example: the antiviral drug ribavirin

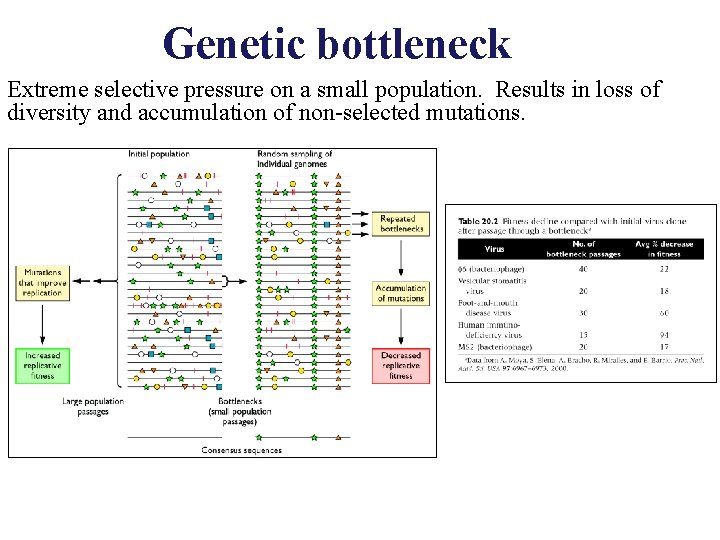
Genetic bottleneck Extreme selective pressure on a small population. Results in loss of diversity and accumulation of non-selected mutations.

Genetic information exchange Genetic information is exchanged by recombination of genome segments. Infection of a cell by two different viruses can result in exchange of genetic information, resulting in production of mixed progeny. 1. Recombination of genome segments. 2. Reassortment. 3. Acquisition of cellular genes

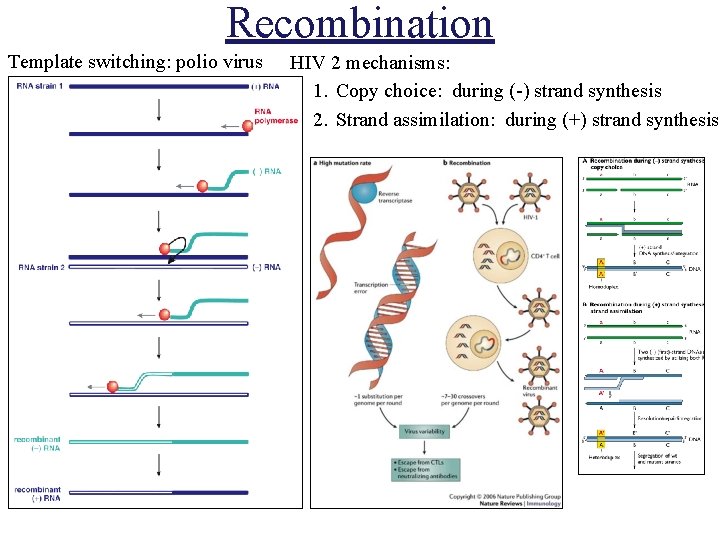
Recombination Template switching: polio virus HIV 2 mechanisms: 1. Copy choice: during (-) strand synthesis 2. Strand assimilation: during (+) strand synthesis

Reassortment

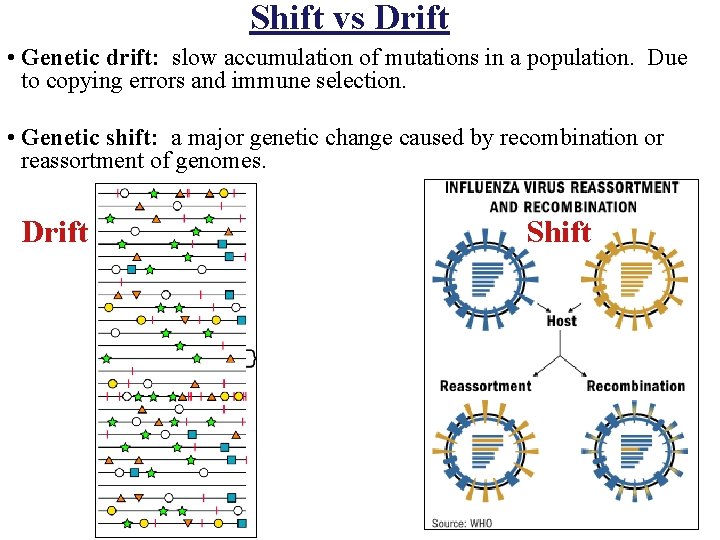
Shift vs Drift • Genetic drift: slow accumulation of mutations in a population. Due to copying errors and immune selection. • Genetic shift: a major genetic change caused by recombination or reassortment of genomes. Drift Shift

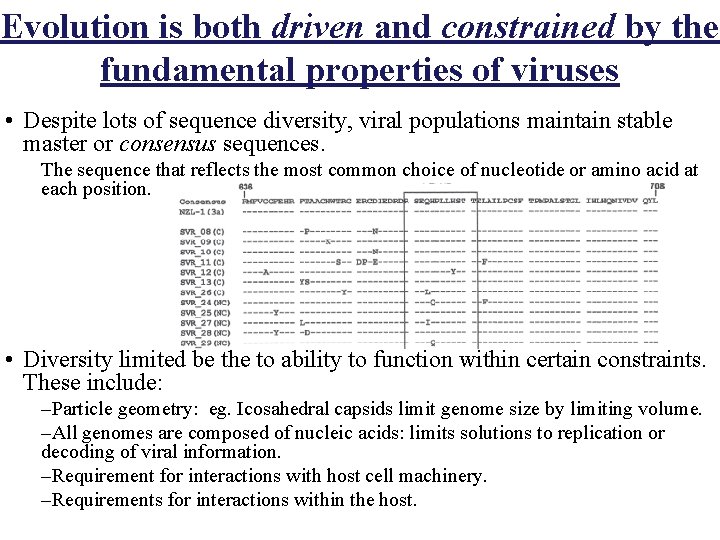
Evolution is both driven and constrained by the fundamental properties of viruses • Despite lots of sequence diversity, viral populations maintain stable master or consensus sequences. The sequence that reflects the most common choice of nucleotide or amino acid at each position. • Diversity limited be the to ability to function within certain constraints. These include: –Particle geometry: eg. Icosahedral capsids limit genome size by limiting volume. –All genomes are composed of nucleic acids: limits solutions to replication or decoding of viral information. –Requirement for interactions with host cell machinery. –Requirements for interactions within the host.

Two general pathways for virus evolution is inescapable • Co-evolution with host Advantage: prosperous host = prosperous virus Disadvantage: virus shares same fate as host. Typically used by DNA viruses. • Infection of multiple host species. Advantage: if one host species is compromised the virus can replicate in another Disadvantage: cannot optimize for any one situation. ( a mutation that enhances replication in one host may decrease replication in another) Typically used by RNA viruses

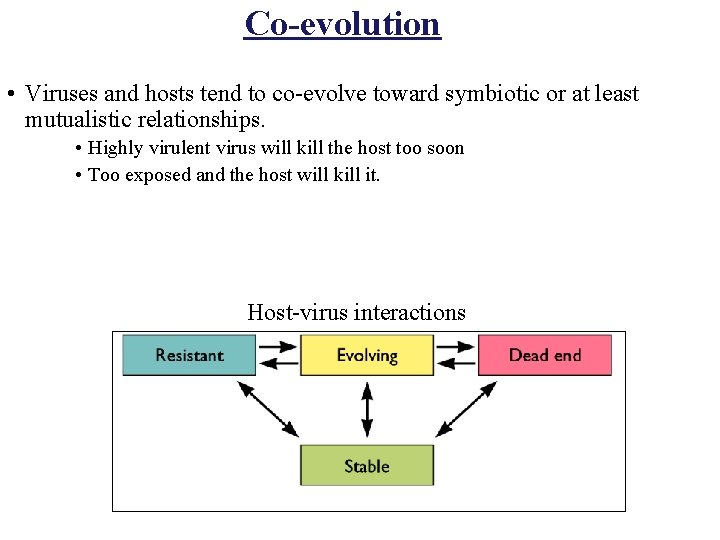
Co-evolution • Viruses and hosts tend to co-evolve toward symbiotic or at least mutualistic relationships. • Highly virulent virus will kill the host too soon • Too exposed and the host will kill it. Host-virus interactions

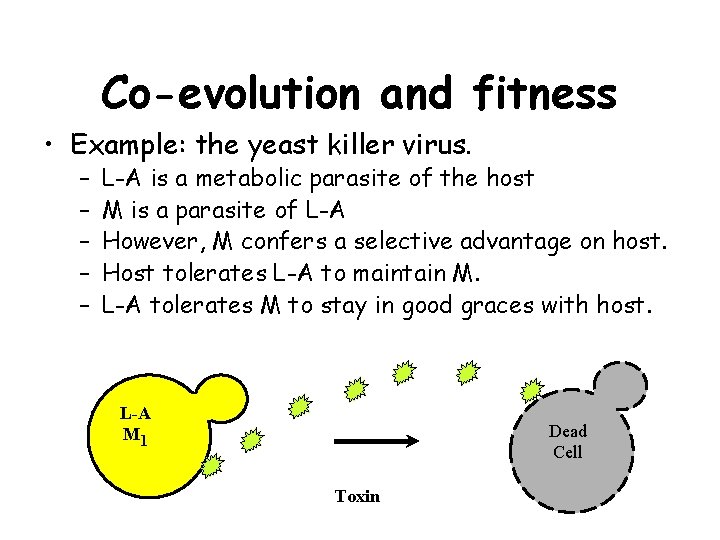
Co-evolution and fitness • Example: the yeast killer virus. – – – L-A is a metabolic parasite of the host M is a parasite of L-A However, M confers a selective advantage on host. Host tolerates L-A to maintain M. L-A tolerates M to stay in good graces with host. L-A M 1 Dead Cell Toxin

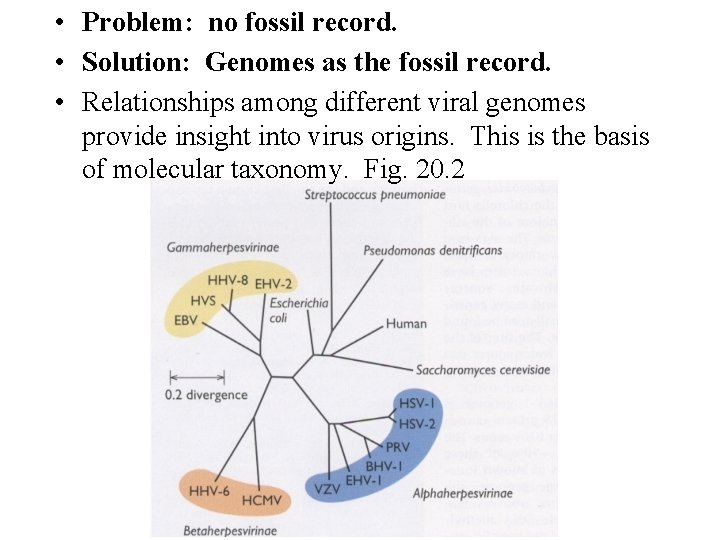
• Problem: no fossil record. • Solution: Genomes as the fossil record. • Relationships among different viral genomes provide insight into virus origins. This is the basis of molecular taxonomy. Fig. 20. 2

Co-evolution with host populations. • Association of a given viral genome sequence with a particular host group. – e. g. different papillomaviruses subtypes are more prevalent in different human populations. • Can use viruses to trace human origins

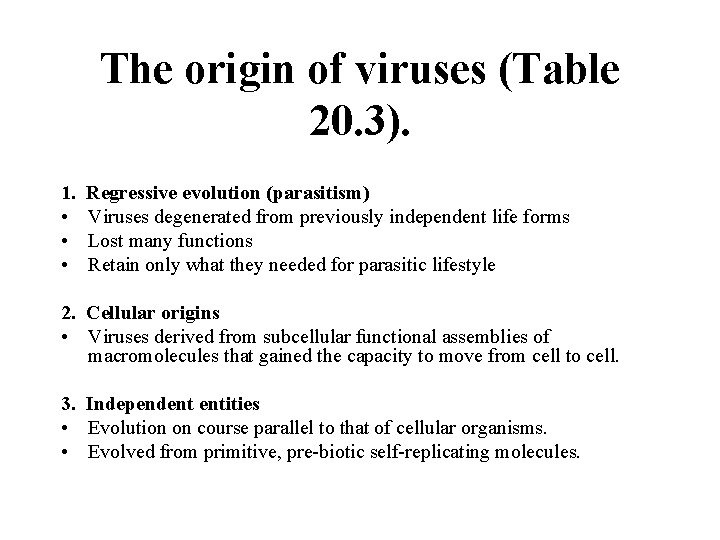
The origin of viruses (Table 20. 3). 1. • • • Regressive evolution (parasitism) Viruses degenerated from previously independent life forms Lost many functions Retain only what they needed for parasitic lifestyle 2. Cellular origins • Viruses derived from subcellular functional assemblies of macromolecules that gained the capacity to move from cell to cell. 3. Independent entities • Evolution on course parallel to that of cellular organisms. • Evolved from primitive, pre-biotic self-replicating molecules.