Viral diseases of shrimps Viral diseases of shrimps













- Slides: 32

Viral diseases of shrimps

Viral diseases of shrimps Currently, at least 14 virus diseases of cultured shrimp are recognised. The major virus families present in the crustaceans include Parvoviridae, Baculoviridae, Picornaviridae, Reoviridae, Togaviridae, Cornaviridae.

The parvo-like viruses are characterised by their small size (20 – 30 mm), single stranded DNA genome and unique cytopathology. 1 a) Hepatopancreatic parvo–like virus (HPV) • HPV has been widely distributed in indo-pacific. • In cases of dual infections, mortality range from 50 -100% • HPV infections - characterized by atrophied and white hepatopancreas, reduced growth rate, anorexia, poor preening activity, and opacity of abdominal muscle, surface and gill fouling and secondary infections by opportunistic pathogens like vibrio spp.

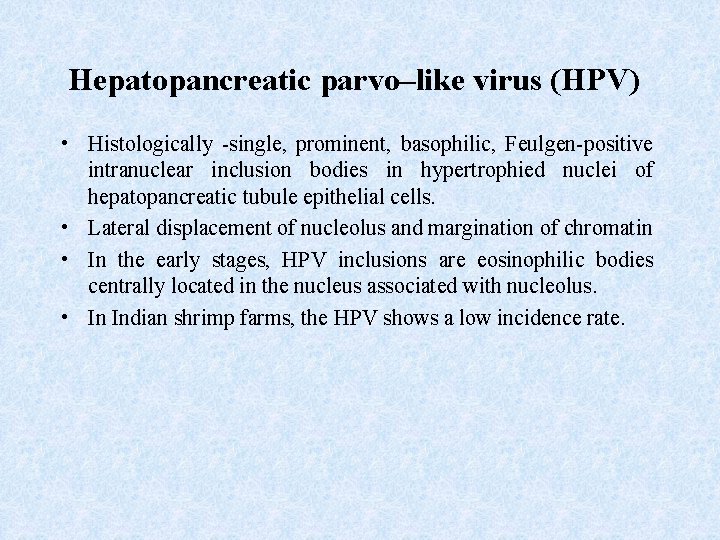
Hepatopancreatic parvo–like virus (HPV) • Histologically -single, prominent, basophilic, Feulgen-positive intranuclear inclusion bodies in hypertrophied nuclei of hepatopancreatic tubule epithelial cells. • Lateral displacement of nucleolus and margination of chromatin • In the early stages, HPV inclusions are eosinophilic bodies centrally located in the nucleus associated with nucleolus. • In Indian shrimp farms, the HPV shows a low incidence rate.

HPV

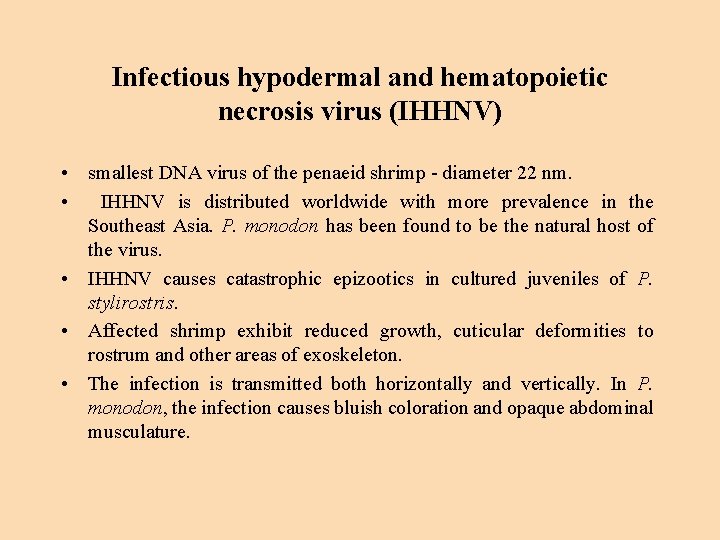
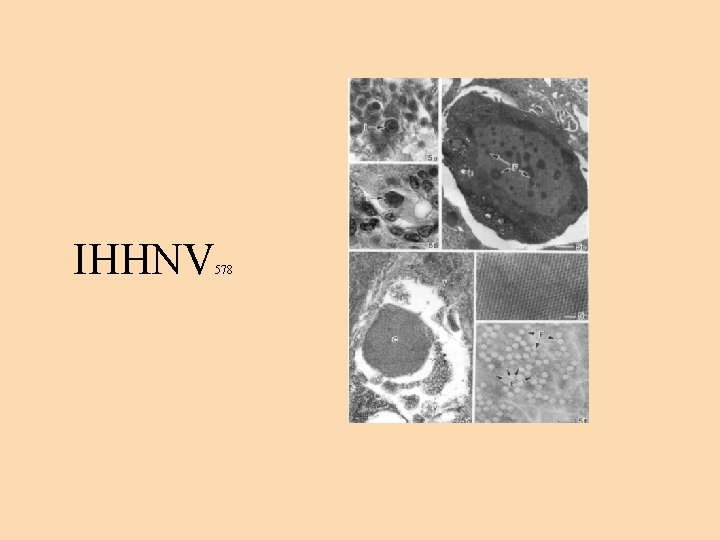
Infectious hypodermal and hematopoietic necrosis virus (IHHNV) • smallest DNA virus of the penaeid shrimp - diameter 22 nm. • IHHNV is distributed worldwide with more prevalence in the Southeast Asia. P. monodon has been found to be the natural host of the virus. • IHHNV causes catastrophic epizootics in cultured juveniles of P. stylirostris. • Affected shrimp exhibit reduced growth, cuticular deformities to rostrum and other areas of exoskeleton. • The infection is transmitted both horizontally and vertically. In P. monodon, the infection causes bluish coloration and opaque abdominal musculature.

IHHNV 578

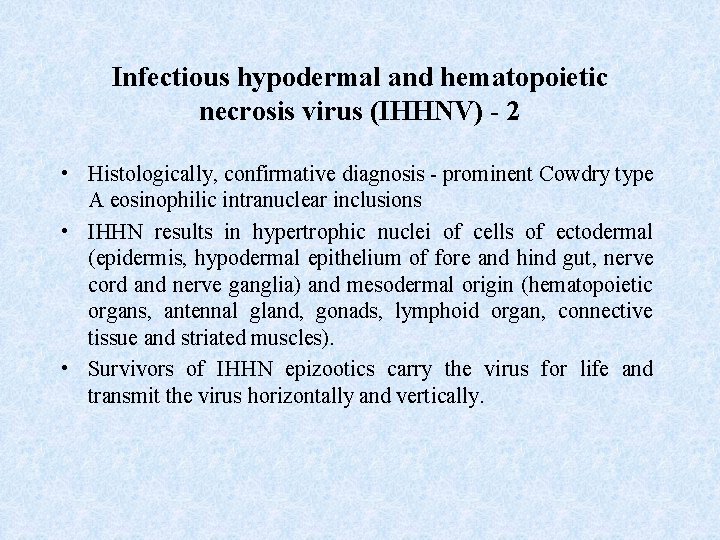
Infectious hypodermal and hematopoietic necrosis virus (IHHNV) - 2 • Histologically, confirmative diagnosis - prominent Cowdry type A eosinophilic intranuclear inclusions • IHHN results in hypertrophic nuclei of cells of ectodermal (epidermis, hypodermal epithelium of fore and hind gut, nerve cord and nerve ganglia) and mesodermal origin (hematopoietic organs, antennal gland, gonads, lymphoid organ, connective tissue and striated muscles). • Survivors of IHHN epizootics carry the virus for life and transmit the virus horizontally and vertically.

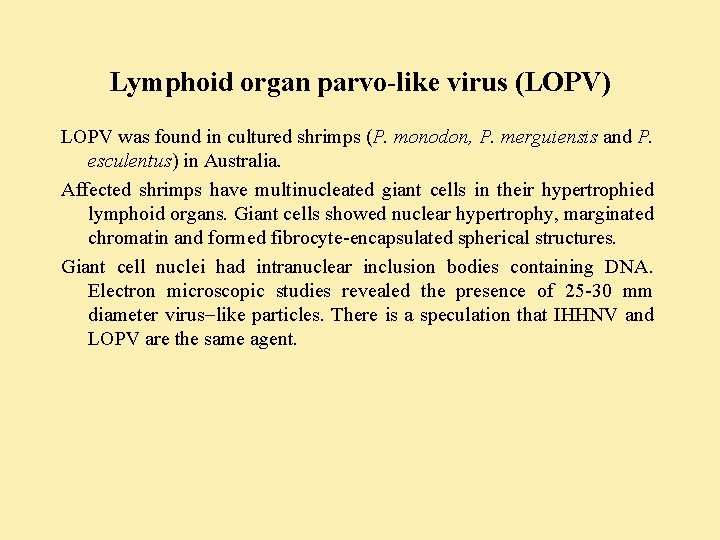
Lymphoid organ parvo-like virus (LOPV) LOPV was found in cultured shrimps (P. monodon, P. merguiensis and P. esculentus) in Australia. Affected shrimps have multinucleated giant cells in their hypertrophied lymphoid organs. Giant cells showed nuclear hypertrophy, marginated chromatin and formed fibrocyte-encapsulated spherical structures. Giant cell nuclei had intranuclear inclusion bodies containing DNA. Electron microscopic studies revealed the presence of 25 -30 mm diameter virus like particles. There is a speculation that IHHNV and LOPV are the same agent.

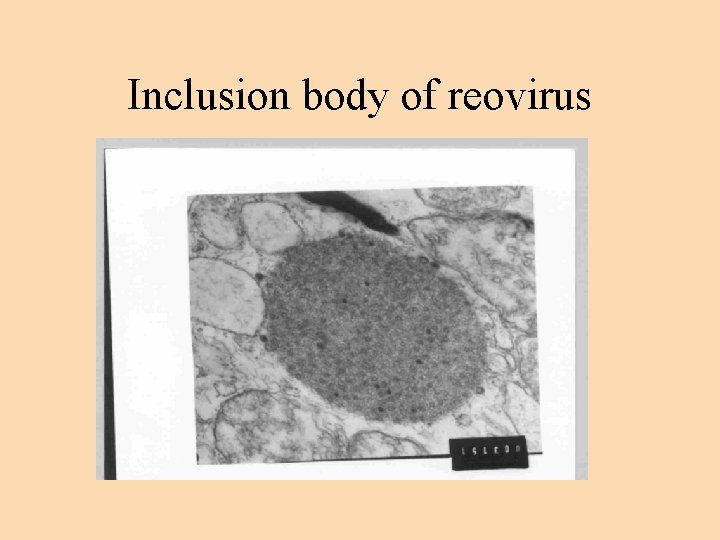
Reo like viruses Reo-like viruses are reported from P. japonicus, P. monodon, P. chinensis and P. vannamei. Hepatopancreas has been suggested as the principal target for both the viral strains. Presumptive diagnosis of infections can be made with routine histology and staining. Feulgen negative cytoplasmic inclusion bodies are demonstrated in I & R type cells of atrophied hepatopancreas. Transmission electron microscopic (TEM) pictures show crystalline array of virus particles of 50 -60 mm diameter. Reovirus infections were always reported in mixed infections. Hence the role of reovirus as pathogens is not completely clear.

REOVIRUS 580

Inclusion body of reovirus

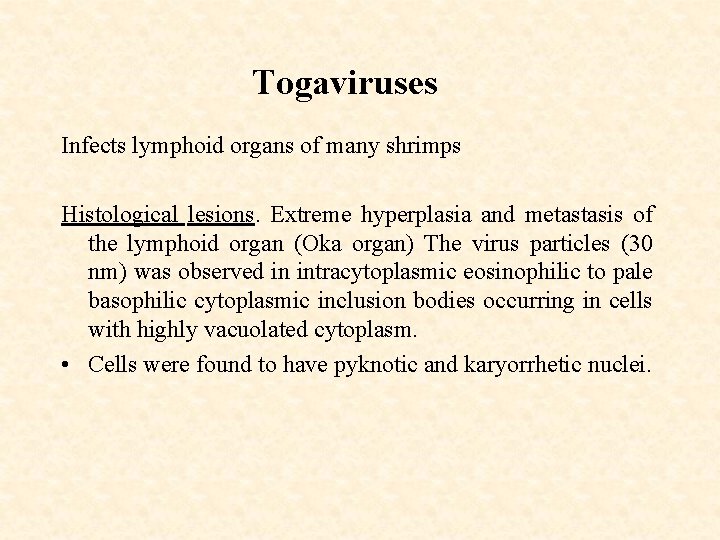
Togaviruses Infects lymphoid organs of many shrimps Histological lesions. Extreme hyperplasia and metastasis of the lymphoid organ (Oka organ) The virus particles (30 nm) was observed in intracytoplasmic eosinophilic to pale basophilic cytoplasmic inclusion bodies occurring in cells with highly vacuolated cytoplasm. • Cells were found to have pyknotic and karyorrhetic nuclei.

Baculoviruses • (1) Type A occlusion body forming viruses BP and MBV and • (2) Type C non occluded baculoviruses BMN, TCBV, Owen’s hemocyte–infecting baculovirus and WSDV. • Transmission: horizontal, some transmit vertically • In hatcheries, BP and BMN often cause serious epizootics in larvae and postlarvae (PL) • MBV produce serious infections and mortalities in the late PL and juvenile stages of hosts.

BP Type baculoviruses • BP (Baculovirus penaei): diagnosis: demonstration of prominent tetrahedral occlusion bodies in unstained squash preparations of hepatopancreas, midgut or faeces and also in histological sections. • In histological sections, occlusion bodies are found in single or multiple, eosinophilic, usually triangular within hypertrophied nuclei of hepatopancreatic tubule epithelial cells or in midgut epithelial cells. • BP has not been reported outside of the Americas and Hawaii

BP 573

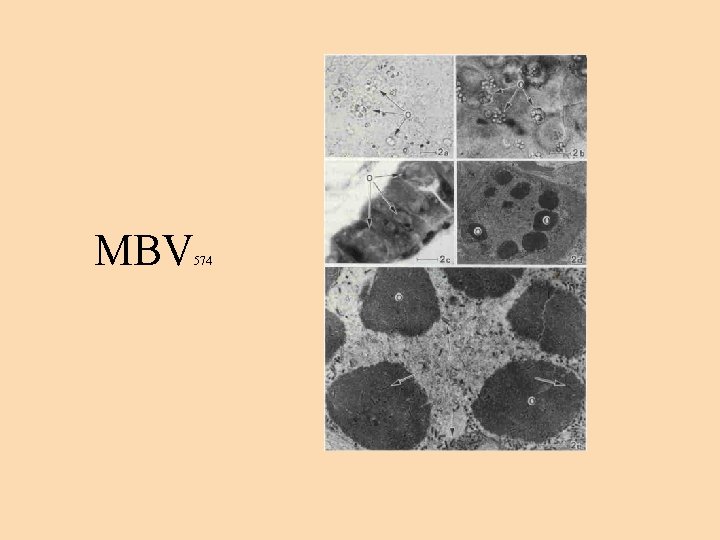
Monodon type baculoviruses • MBV enjoy a world-wide distribution. These are type A baculoviruses measuring 75 x 324 mm. • Diagnosis: presence of single or multiple, generally spherical intranuclear occlusion bodies in hepatopancreas and midgut epithelial cells. Squash preparation (0. 05% aqueous malachite green), epifluorescence microscopy and acridine orange staining visualizes MBV occlusions

MBV 574

• MBV was first discovered in a quarantined population of P. monodon that had originated from Taiwan. • Despite the world distribution, MBV is not a highly virulent pathogen of P. monodon. MBV is found in healthy prawns and in disease epizootics, P. monodon has been found to frequently have mixed infections by MBV and other viral, bacterial or protozoan pathogens.

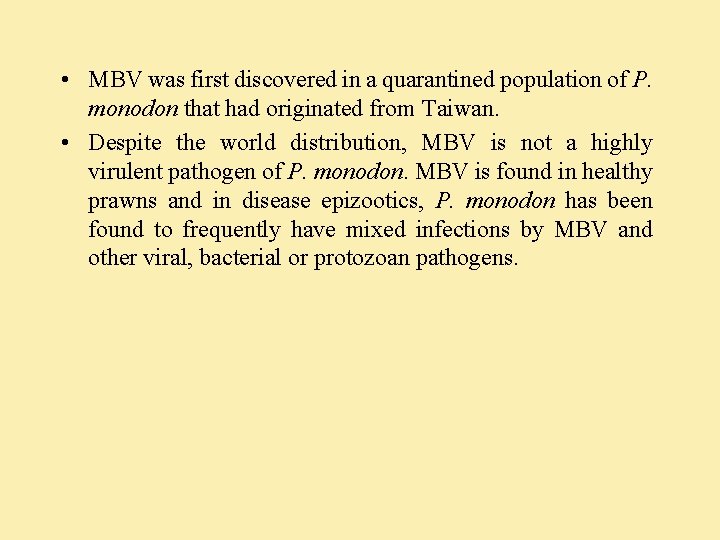
BMN (Baculoviral midgut gland necrosis virus) and other type C baculoviruses • Does not produce occlusion body in the nuclei of infected cells. • Diagnosis: necrotic hepatopancreatic (midgut gland) tubule, epithelial cells that have hypertrophied nuclei with marginated chromatin, diminished nuclear chromatin, nucleolar dissociation and no occlusion bodies. • Type C baculoviruses are enveloped viruses of 72 -310 nm and may occur in dense aggregates especially near to nuclear membrane and surrounding virogenic areas.

BMN 576

BMN (Baculoviral midgut gland necrosis virus) and other type C baculoviruses. . 2 • BMN causes serious epizootics in hatchery reared P. japonicus larvae in Southern Japan. Causes sudden onset and high mortality rate. The disease subsides by PL 20. • Diagnosis: The infected larvae float inactively on the surface and have a white turbid midgut line through the abdomen. • Histological conformation: presence of necrotic hepatopancreatic tubule epithelial cells with hypertrophied nuclei having marginated and diminished chromatin, nucleolar dissociation and the absence occlusion bodies.

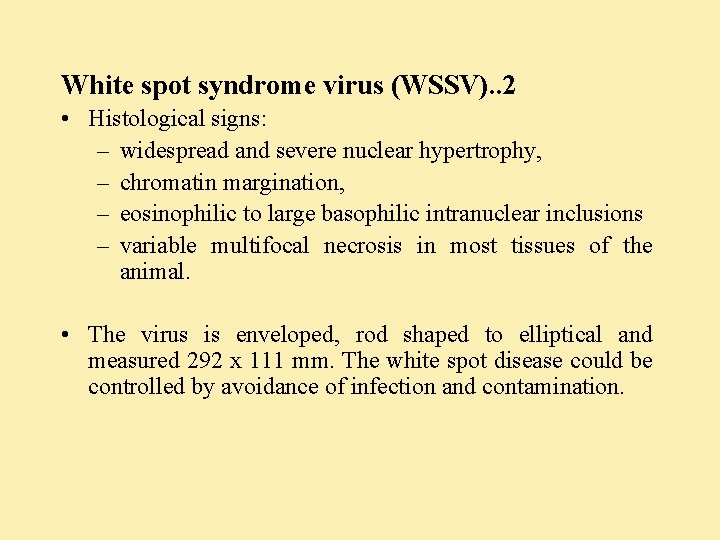
White spot syndrome virus (WSSV) • WSSV, formerly known as SEMBV is a non occluded baculovirus – like agent • Epizootic of white spot disease cause mortalities ranging up to 80 100% in 2 7 days susceptible species: On growing juvenile shrimp of many species of all ages but mostly from 1 - 3 months old in the grow-out ponds. • WSSV outbreak occurs in all types of farming systems irrespective of stocking density, water quality and salinity. Diagnosis: Infected shrimp swim to the surface and gather at the pond dykes. • Broken antennae, white spots of 1 mm size in the cuticle and / or reddish discoloration, empty guts and cuticular epibiont fouling and lymphoid organ swelling.

White spot syndrome virus (WSSV). . 2 • Histological signs: – widespread and severe nuclear hypertrophy, – chromatin margination, – eosinophilic to large basophilic intranuclear inclusions – variable multifocal necrosis in most tissues of the animal. • The virus is enveloped, rod shaped to elliptical and measured 292 x 111 mm. The white spot disease could be controlled by avoidance of infection and contamination.

WHITE SPOT DISEASE

Yellow Head Virus (YHV) • YHV is an RNA virus reported only from P. monodon in Thailand. • All ages of juveniles could be infected and mass mortalities up to 100% are observed within 3 - 5 days • Diagnosis: Pale body colour with yellowish gills and hepatopancreas. It affects many tissues such as gills, lymphoid organ, hemocytes and connective tissue. • Histology: Degenerative changes in nuclei and presence of cytoplasmic basophilic inclusion bodies.

Management of shrimp disease 1. Early and effective detection of pathogens by improved diagnostic methodologies 2. Use of biosecure, closed or semi closed recycle system with reduced water exchange 3. Maintain good water quality and treat water before use especially for hatcheries. 4. Construct reservoirs for storing water without directly taking from the sea and treat reservoir water

5. Bio-security approach for preventing pathogen introduction by screening entry of infected hosts, carriers like crabs, fish or birds, stray dogs, avoiding contaminated inanimate objects 6. In case of a disease outbreak, disinfect contaminated water before discharge. 7. Maintain good pond preparation by sun drying pond bottom, desilting and ploughing. Application of 100 ppm Ca. O (burnt lime). 8. Avoid over stocking to reduce the stress on the animals and chances of water quality deterioration

9. Production of specific pathogen free (SPF) shrimps and the • • • development of specific pathogen resistant (SPR) strains SPF animals are produced by selecting animals free of known and detectable pathogens and raising them under controlled and strict sanitary conditions. The SPR animals are developed through selective breeding of animals known to be less susceptible to specific pathogens. This is useful for production of high health (HH) post-larvae, free of, or resistant to, known pathogens. Reports indicate that Penaeus merguiensis has a higher resistance to WSSV than P. monodon. However, in certain cases SPF stocks may perform poorly when exposed to pathogens in the field.

10. Feed nutritionally balanced diet avoiding excess feed. 11. Use of immunostimulants and vaccines 12. Development of co-operative disease control strategies. Cooperative program involving farmers, health care specialists, scientists and government agencies to track the movement of a potential pathogen, for notification of neighbouring farms of an infection and proper disinfection of the infected stock and water before discharge 13. Locate hatchery away from the shrimp farm to prevent cross contamination of hatchery source-water and air borne contamination of pathogens from the infected farm. 14. Use only virus-free broodstock to prevent vertical transmission of the viral diseases in particular.

15. Avoid importing of broodstock and larvae. 16. Maintain broodstocks of different sources in separate holding tanks and rear the larval population in separate batches to prevent cross contamination. 17. Do not use trash fish and shellfish to feed broodstock which can act as carriers of viral pathogens. In case of feeding with trash fish, care has to be taken for properly cooking the feed before use.

Thank you
 Viral shedding
Viral shedding An acute highly contagious viral disease
An acute highly contagious viral disease Viral dna
Viral dna Hemolyzed serum sample
Hemolyzed serum sample Inklüzyon cisimcikleri
Inklüzyon cisimcikleri Vacinas subcutânea
Vacinas subcutânea Hepatitis viral
Hepatitis viral Viral infection
Viral infection Viral receptors
Viral receptors Spasmodic croup vs viral croup
Spasmodic croup vs viral croup Viral induced wheeze vs asthma
Viral induced wheeze vs asthma Eline's viral
Eline's viral Viral life cycle
Viral life cycle Rotarix live attenuated
Rotarix live attenuated Que causa la meningitis
Que causa la meningitis Viral
Viral Method of cultivation of virus
Method of cultivation of virus Csf analysis
Csf analysis Streptococcus
Streptococcus Variola varicela
Variola varicela Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Viral communications
Viral communications Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Viral entry
Viral entry Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Viral recombination
Viral recombination Viral arthritis
Viral arthritis Mobilivirus
Mobilivirus Equine synonym
Equine synonym Decapsidação
Decapsidação Section 24-1 viral structure and replication
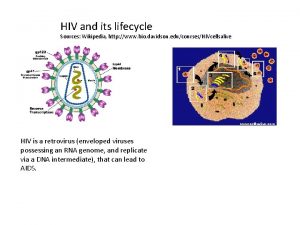
Section 24-1 viral structure and replication Hiv wikipedia
Hiv wikipedia The dynamics of viral marketing
The dynamics of viral marketing