Dengue and other Viral hemorrhagic fevers Dr SELVI
















- Slides: 43

Dengue and other Viral hemorrhagic fevers Dr. SELVI Medical Microbiology Block: Hematology Session 2011/2012

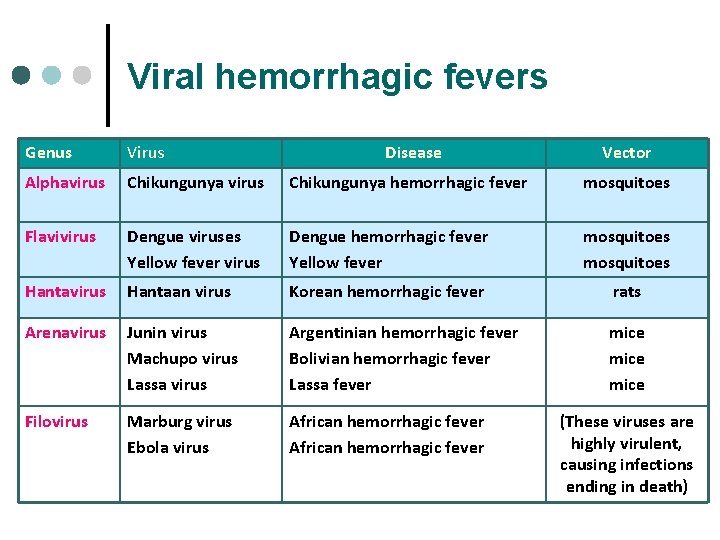
Viral hemorrhagic fevers Genus Virus Disease Vector Alphavirus Chikungunya hemorrhagic fever mosquitoes Flavivirus Dengue viruses Yellow fever virus Dengue hemorrhagic fever Yellow fever mosquitoes Hantavirus Hantaan virus Korean hemorrhagic fever rats Arenavirus Junin virus Machupo virus Lassa virus Argentinian hemorrhagic fever Bolivian hemorrhagic fever Lassa fever mice Filovirus Marburg virus Ebola virus African hemorrhagic fever (These viruses are highly virulent, causing infections ending in death)

Dengue is a mosquito-borne infection caused by a flavivirus. ¤ is also called “breakbone” fever. ¤ occurs in South-East Asia, Pacific areas, India, South and Central America. ¤ is characterized by fever, muscle and joint pain, lymphadenopathy and rash. ¤

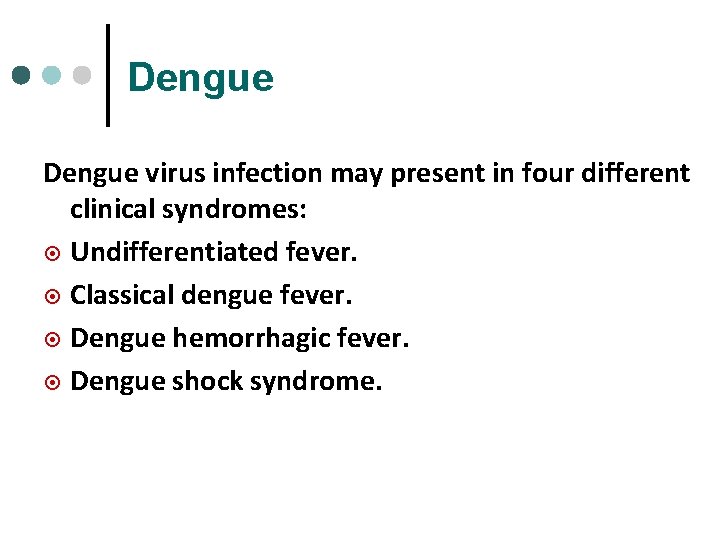
Dengue virus infection may present in four different clinical syndromes: ¤ Undifferentiated fever. ¤ Classical dengue fever. ¤ Dengue hemorrhagic fever. ¤ Dengue shock syndrome.

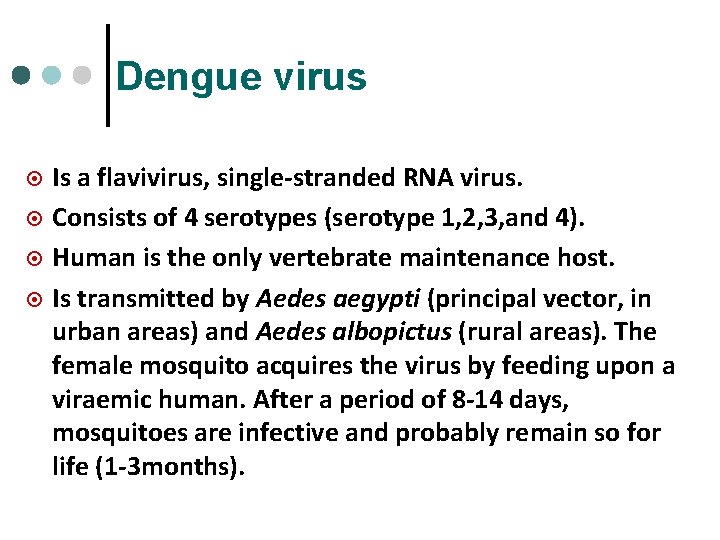
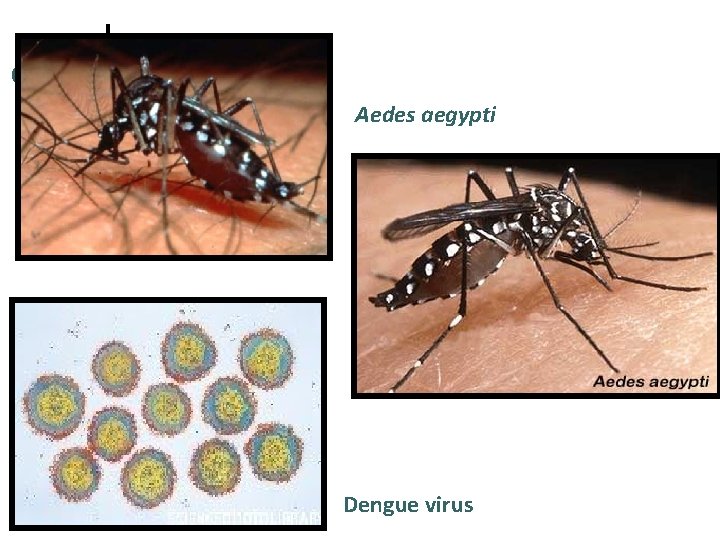
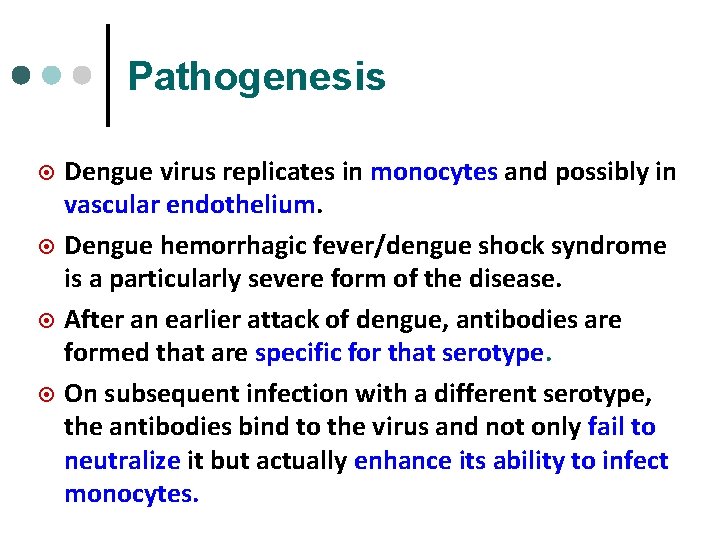
Dengue virus ¤ ¤ Is a flavivirus, single-stranded RNA virus. Consists of 4 serotypes (serotype 1, 2, 3, and 4). Human is the only vertebrate maintenance host. Is transmitted by Aedes aegypti (principal vector, in urban areas) and Aedes albopictus (rural areas). The female mosquito acquires the virus by feeding upon a viraemic human. After a period of 8 -14 days, mosquitoes are infective and probably remain so for life (1 -3 months).

Aedes aegypti Dengue virus

Pathogenesis ¤ ¤ Dengue virus replicates in monocytes and possibly in vascular endothelium. Dengue hemorrhagic fever/dengue shock syndrome is a particularly severe form of the disease. After an earlier attack of dengue, antibodies are formed that are specific for that serotype. On subsequent infection with a different serotype, the antibodies bind to the virus and not only fail to neutralize it but actually enhance its ability to infect monocytes.

Pathogenesis ¤ ¤ ¤ The Fc portion of the virus-bound immunoglobulin molecule attaches to Fc receptors on monocytes, and entry into the cell by this route increases the efficiency of infection. Infection of increased numbers of monocytes results in an increased release of cytokines into the circulation. This leads to vascular damage, shock and hemorrhage, especially into the gastrointestinal tract and the skin.

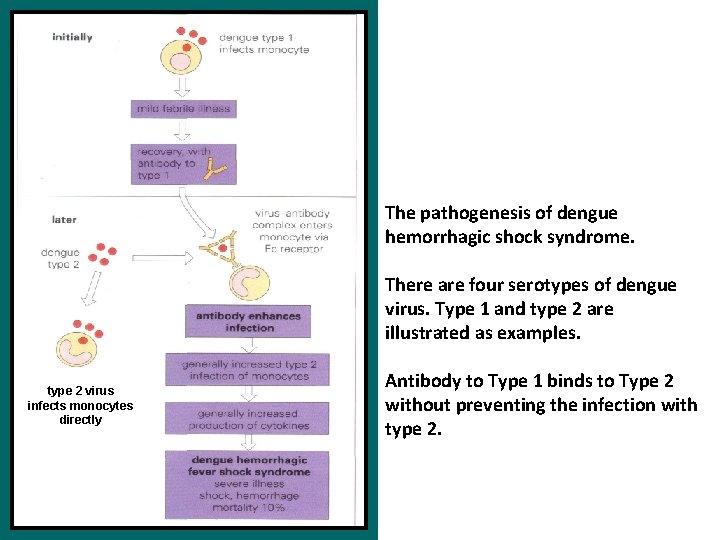
The pathogenesis of dengue hemorrhagic shock syndrome. There are four serotypes of dengue virus. Type 1 and type 2 are illustrated as examples. type 2 virus infects monocytes directly Antibody to Type 1 binds to Type 2 without preventing the infection with type 2.

Clinical features which point to diagnosis of dengue fever: ¤ ¤ ¤ ¤ High continuous fever of 3 days or more. Headache, backache and retro-orbital pain. Abdominal pain, vomiting, loose stools. Petechial hemorrhage and/or spontaneous bleeding. Rash – generalized flushing/maculopapular. Hepatomegaly. Fall in platelet count that precedes or occurs simultaneously with a rise in the hematocrit. volume percentage (%) of red blood cells in blood. ¤ ¤ ¤ Normal WBC or leukopenia with relative lymphocytosis. Normal ESR (<20 mm first hour). Shock.

Classical dengue fever: Clinical features ¤ ¤ ¤ ¤ Fever - The temperature returns to normal after 5 -6 days and rise again about 5 -8 days after onset (“saddleback” form). Headache Retro-orbital pain. Myalgia and deep bone pain (breakbone fever) Arthralgia (joint pain) Rash (maculopapular or morbilliform) may appear on the 3 rd day or 4 th day and last for 1 -5 days. Hemorrhagic manifestations: Positive Hess test /tourniquet test. Leukopenia with relative lymphocytosis is common.

Dengue hemorrhagic fever (DHF) ¤ The critical stage is reached at the end of the febrile phase of illness, accompanying or shortly after a rapid drop in temperature, varying degrees of circulatory disturbance occurs. This phase rarely lasts longer than 48 hours. The following features must be present: ¤ Fever or history of acute fever, lasting 2 -7 days.

Dengue hemorrhagic fever ¤ Hemorrhagic tendencies evidenced by at least one of the following - Positive tourniquet test. - Petechiae and ecchymosis. - Bleeding from the mucosa, gastrointestinal tract, injection sites or other locations. ¤ Thrombocytopenia (100, 000/mm 3 or less).

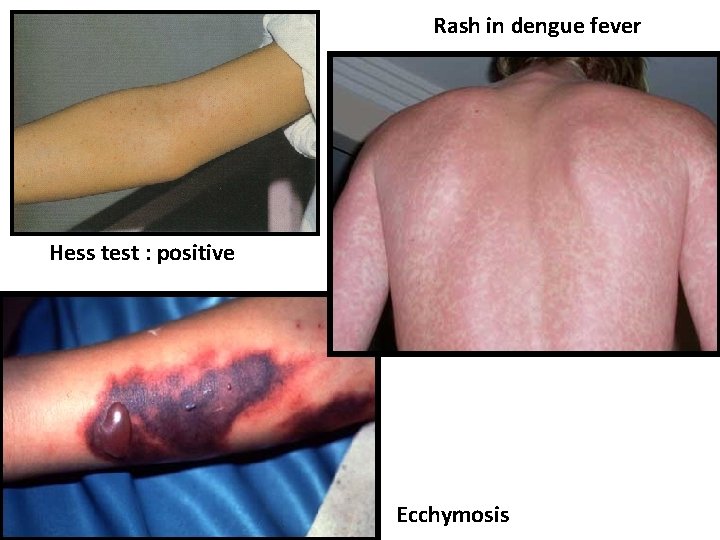
Rash in dengue fever Hess test : positive Ecchymosis

Hess Test: To perform the test, pressure is applied to the forearm with a blood pressure cuff. After removing the cuff, the number of petechiae in a 5 cm diameter circle of the area under pressure is counted. Normally less than 15 petechiae are seen. 15 or more petechiae indicate capillary fragility, which occurs due to poor platelet function, thrombocytopenia, and can be seen in cases of Dengue fever. Ecchymosis: Nonraised skin discoloration caused by the escape of blood into the tissues from ruptured blood vessels.

Dengue hemorrhagic fever ¤ Evidence of plasma leakage due to increased vascular permeability manifested by at least one of the following: - Hemoconcentration (decrease of the fluid content of the blood, with increased concentration of formed elements). ¤ Drop in hematocrit (percentage (%) of red blood cells in blood). Signs of plasma leakage evidenced by pleural effusion. Other clinical manifestations suggestive of DHF are - Hepatomegaly which may be tender. - Circulatory disturbance.

Dengue shock syndrome (DSS) All the 4 criteria for DHF must be present, plus evidence of circulatory failure manifested by: ¤ Rapid and weak pulse ¤ Narrow pulse pressure of less than 20 mm. Hg (the difference between systolic and diastolic pressure) ¤ Hypotension ¤ Cold clammy skin and restlessness.

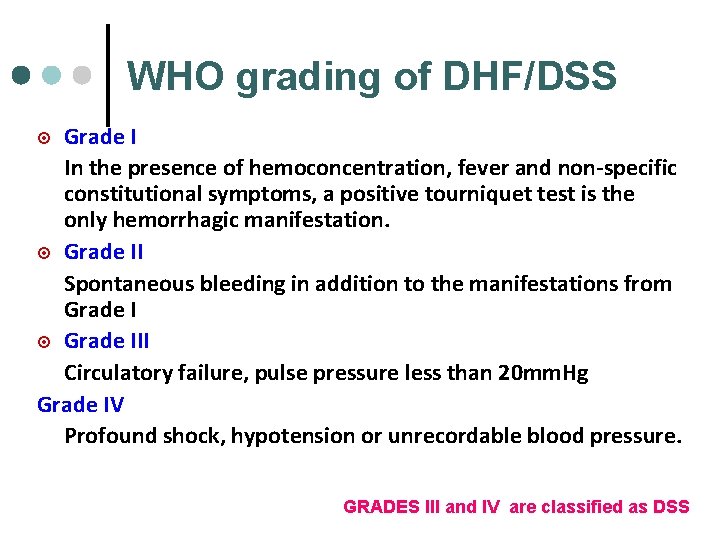
WHO grading of DHF/DSS Grade I In the presence of hemoconcentration, fever and non-specific constitutional symptoms, a positive tourniquet test is the only hemorrhagic manifestation. ¤ Grade II Spontaneous bleeding in addition to the manifestations from Grade I ¤ Grade III Circulatory failure, pulse pressure less than 20 mm. Hg Grade IV Profound shock, hypotension or unrecordable blood pressure. ¤ GRADES III and IV are classified as DSS

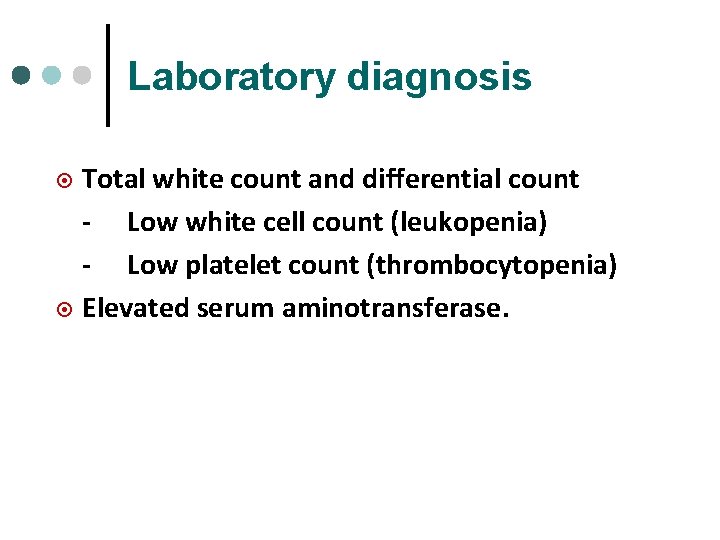
Laboratory diagnosis Total white count and differential count - Low white cell count (leukopenia) - Low platelet count (thrombocytopenia) ¤ Elevated serum aminotransferase. ¤

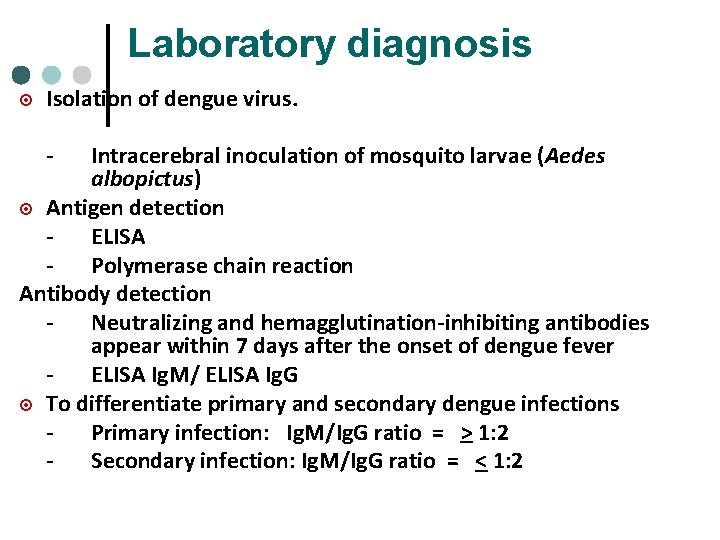
Laboratory diagnosis ¤ Isolation of dengue virus. - Intracerebral inoculation of mosquito larvae (Aedes albopictus) ¤ Antigen detection ELISA Polymerase chain reaction Antibody detection Neutralizing and hemagglutination-inhibiting antibodies appear within 7 days after the onset of dengue fever ELISA Ig. M/ ELISA Ig. G ¤ To differentiate primary and secondary dengue infections Primary infection: Ig. M/Ig. G ratio = > 1: 2 Secondary infection: Ig. M/Ig. G ratio = < 1: 2

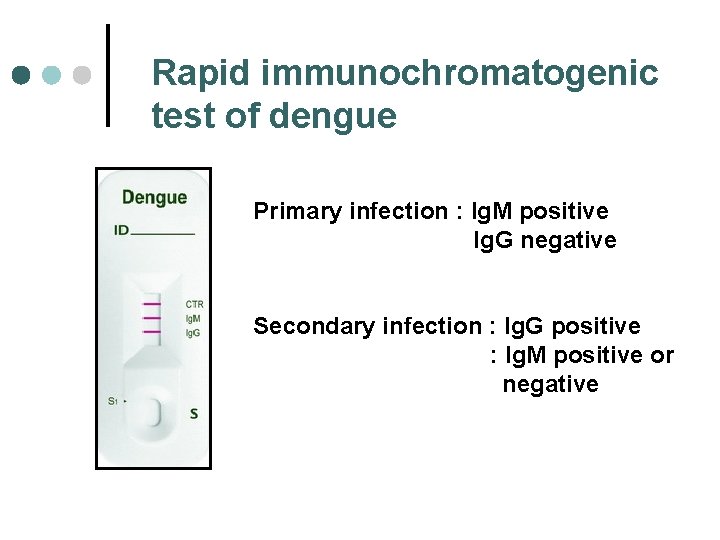
Rapid immunochromatogenic test of dengue Primary infection : Ig. M positive Ig. G negative Secondary infection : Ig. G positive : Ig. M positive or negative

Immunity ¤ ¤ Four serotypes of the virus can be distinguished by neutralization tests/ PCR. Infection confers life-long protection against that serotype, but cross-protection between serotypes is of short duration. Reinfection with a virus of a different serotype after the primary attack is more likely to result in severe disease (dengue hemorrhagic fever). Dengue and yellow fever viruses are antigenically related, but this does not result in significant crossimmunity.

Management of classical dengue fever ¤ Treatment of classical dengue fever is the same as for any acute uncomplicated viral infection - Plenty of oral fluids - Paracetamol for relief of fever and body ache. - Avoid aspirin & NSAIDS (anti-platelet effect) - Seek further medical advice if there is deterioration.

Management of dengue shock syndrome ¤ ¤ ¤ HDU/ICU* care indicated whenever available. Rapid volume replacement (colloids or plasma expander). Continuous monitoring of vital signs. Hematocrit monitoring every 4 to 6 hours as clinically indicated. Correct electrolyte imbalance and acidosis. Blood transfusion (packed cells) if there is - Obvious bleeding or - Persistent shock with falling hematocrit and hemoglobin following volume replacement.

Prevention and control Control depends upon anti-mosquito measures eg. elimination of breeding places and the use of insecticides. ¤ Vaccine development is difficult because a vaccine must provide protection against all four serotypes of virus, otherwise, recipients may be at increased risk of dengue hemorrhagic fever. ¤

Yellow fever virus is transmitted by mosquitoes and is restricted to Africa, Central and South America and the Caribbean. ¤ Yellow fever virus is a flavivirus and there is only one antigenic type. ¤

Transmission ¤ ¤ ¤ Yellow fever virus is transmitted by two different cycles: - From human to human by the mosquito Aedes aegypti, known as “urban yellow fever”. - From infected monkeys to humans by mosquitoes such as Haemagogus. This is “jungle yellow fever” and is seen in Africa and south America. Yellow fever is not transmitted directly from human to human by day-to-day contact. Transmission from ill patients to healthcare workers has been reported, notably after needle stick injury.

Clinical features ¤ ¤ ¤ Infects vascular endothelium and liver. Sudden onset of fever, headache and muscular aches. Prostration and shock are not uncommon, severe liver damage may result in death. Coagulation defects (largely due to prothrombin deficiency) cause hemorrhage into the gastrointestinal tract (manifest as hematemesis and melena). Renal damage is also seen with proteinuria and occasionally tubular necrosis. The case mortality rate is in the range 5 -50% (hepato-renal failure).

Diagnosis ¤ ¤ Diagnosis is usually clinical. Virus detection and isolation - Virus antigen or nucleic acid can be identified in tissue specimens using immunohistochemistry, ELISA antigen capture and polymerase chain reaction. - The virus can be recovered from the blood the first 4 days after onset or from post-mortem tissues by intracerebral inoculation of mice or by use of cell lines. Post-mortem diagnosis: severe mid-zonal changes and acidophilic inclusion bodies seen in the liver. Serology: Virus-specific Ig. M antibodies are detectable after a week.

Immunity ¤ Neutralizing antibodies develop about a week into the illness and are responsible for viral clearance. ¤ Neutralizing antibodies endure for life and provide complete protection from disease. ¤ Demonstration of neutralizing antibodies is the only useful test for immunity to yellow fever.

Prevention and control ¤ ¤ Control of vectors (insecticides, attention to breeding sites) and reduced exposure (insect repellents, mosquito nets) are also important. Vaccination Live attenuated 17 D yellow fever vaccine to be given to - Those who may be exposed to the disease. - Travelers from endemic areas to other countries. - To be given subcutaneously 10 days before travel to endemic areas. - Protection rate : 95% for 10 years.

Hemorrhagic fever with renal syndrome (HFRS) ¤ ¤ First reported during the Korean war in 1950 s but the causative agent, hantaan virus was only isolated in 1977 from a rodent, Apodermus agrarius. Hantaan virus is single-stranded RNA virus. Urban rats are known to be persistently infected with hantaan virus and it had been suggested that rats on the trading ships may have dispersed hantaan virus worldwide. In Malaysia, anti-hantaan virus antibody was detected in rats, Rattus.

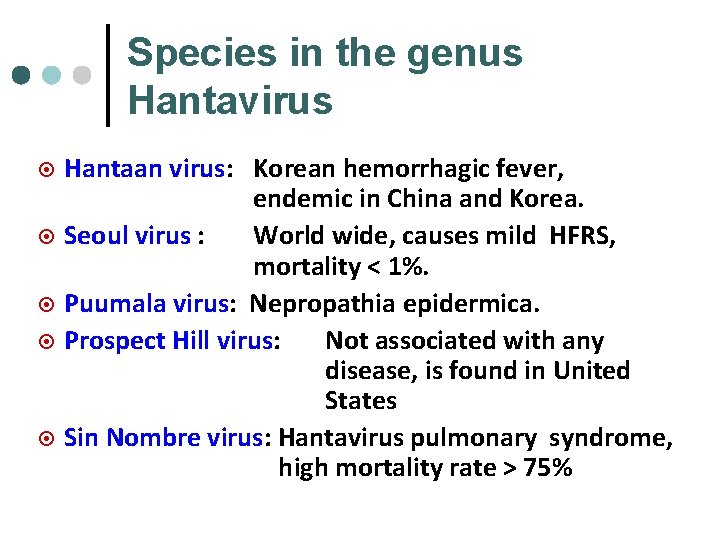
Species in the genus Hantavirus ¤ ¤ ¤ Hantaan virus: Korean hemorrhagic fever, endemic in China and Korea. Seoul virus : World wide, causes mild HFRS, mortality < 1%. Puumala virus: Nepropathia epidermica. Prospect Hill virus: Not associated with any disease, is found in United States Sin Nombre virus: Hantavirus pulmonary syndrome, high mortality rate > 75%

Hemorrhagic fever with renal syndrome (Hantaan virus) ¤ High grade fever for 3 -7 days, associated with malaise, myalgia, anorexia and headache. ¤ Severe form: generalized hemorrhage with acute renal insufficiency and renal failure.

Laboratory diagnosis ¤ Detection of viral nucleic acid by PCR (polymerase chain reaction) ¤ Serology: - Immunofluorescent test. - Hemagglutination-inhibition test. - EIA (enzyme immunoassay) Ig. M test.

Treatment and Prevention ¤ Symptomatic treatment. ¤ The antiviral drug ribavirin is of some benefit. ¤ Preventive measures: - Rodent control and avoidance of contact with rodents and rodents dropping.

Hantavirus Pulmonary syndrome (HPS) In 1993, an outbreak of severe respiratory illness in the United States, now designated as hantavirus pulmonary syndrome. ¤ It was found to be caused by a hantavirus (Sin Nombre virus). ¤ Subclinical infections appear to be unusual. HPS is generally severe, with reported mortality rate of >30%. ¤

Clinical features The disease is characterized by prodromal fever, myalgia, and other symptoms including cough, headache , nausea and vomiting followed by rapidly progressive pulmonary edema. ¤ There is no signs of hemorrhage. ¤ The pathogenesis of the pulmonary findings is not known but may reflect an increased permeability of pulmonary capillaries. ¤

Treatment ¤ Maintenance of adequate oxygen. ¤ Support of the hemodynamic function. ¤ Ribavirin is of some benefit.

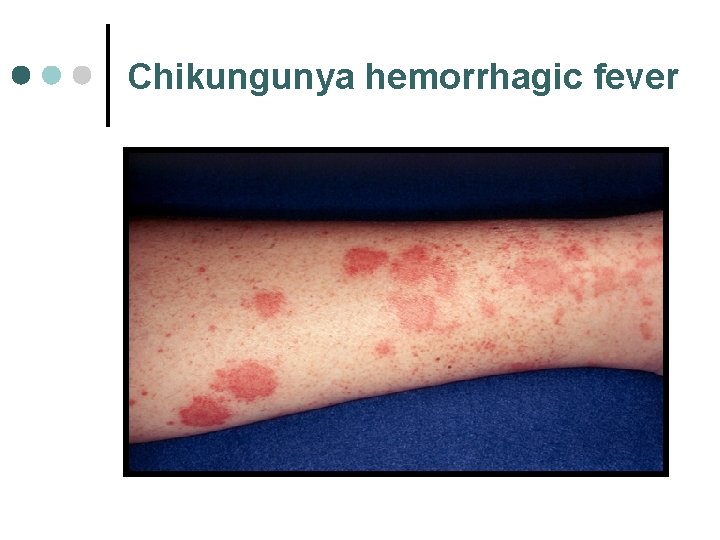
Chikungunya hemorrhagic fever First isolated in Tanzania in 1952, currently can be found in Africa, India, South-East Asia (Thailand, Vietnam, Singapore, Indonesia and Philipines). ¤ Is caused by an alphavirus, an enveloped singlestranded RNA virus. ¤ Is transmitted via mosquitoes (Aedes aegypti and Aedes africanus) ¤ Human is the only vertebrate host. ¤

Chikungunya hemorrhagic fever

Clinical manifestations Incubation period: 3 -12 days. ¤ Sudden onset – biphasic fever, severe joint pains, headache, conjunctivitis, maculopapular rash, anorexia and constipation. ¤ Recovery takes place within 6 -10 days. ¤ Hemorrrhagic complications are noted in patients in India and Asia. ¤ Chikungunya virus

Laboratory diagnosis ¤ Serological tests: - Immunofluorescent test - Hemagglutination – inhibition test Treatment Symptomatic treatment ¤ Vaccines not available as yet. ¤
 Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Application of visible light
Application of visible light Surah al an'am ayat 16 and 17 for dengue
Surah al an'am ayat 16 and 17 for dengue Surveilans terpadu adalah
Surveilans terpadu adalah Malaria and dengue
Malaria and dengue Grades of hemorrhagic shock
Grades of hemorrhagic shock Blood loss classification
Blood loss classification Types of haemorrhage
Types of haemorrhage Ischemic vs hemorrhagic stroke
Ischemic vs hemorrhagic stroke Hemorrhagic diathesis
Hemorrhagic diathesis Antihaemorrhagic vitamin
Antihaemorrhagic vitamin Necrotizing fasciitis hemorrhagic bullae
Necrotizing fasciitis hemorrhagic bullae Neurocritical care society
Neurocritical care society Hemorrhagic transformation mri
Hemorrhagic transformation mri Shock classes
Shock classes What is acute hemorrhagic pancreatitis
What is acute hemorrhagic pancreatitis Stages of hemorrhagic shock
Stages of hemorrhagic shock Hantavirus humans
Hantavirus humans Cchf
Cchf Historia natural de la enfermedad del dengue
Historia natural de la enfermedad del dengue Criterios diagnosticos dengue
Criterios diagnosticos dengue Dengue duo
Dengue duo Pathogenesis dengue fever
Pathogenesis dengue fever Etapas del dengue comezón
Etapas del dengue comezón Dengue monitoring chart
Dengue monitoring chart Dengue shock syndrome
Dengue shock syndrome Taksonomi virus dengue
Taksonomi virus dengue Haemaccel in dengue
Haemaccel in dengue Symptoms dengue
Symptoms dengue Abcd dengue
Abcd dengue Definición de capilar
Definición de capilar Dengue
Dengue Gonorrea mujer
Gonorrea mujer Dengue no brasil
Dengue no brasil Dengue
Dengue Measles
Measles Como curar el dengue rápido
Como curar el dengue rápido Acelulares
Acelulares Ibuprofen
Ibuprofen Todo pernilongo listrado é da dengue
Todo pernilongo listrado é da dengue Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Viral induced wheeze vs asthma
Viral induced wheeze vs asthma Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Pantropizm
Pantropizm