Hemorrhagic Fevers n Sudden onset of flulike pyrexia































- Slides: 39


Hemorrhagic Fevers n Sudden onset of flu-like pyrexia, headache, myalgia. n Nausea, vomiting, diarrhea, skin rash. n Hemorrhagic manifestations (Petechiae, ecchymoses, epistaxis, gingival bleeding, G. I. bleeding) in previously immunological normal individuals. n Occur in sporadic or epidemic forms. n High mortality rate.

Causes 1. 2. 3. 4. 5. Viral: • • Primary viral Complicating viral • • • Epidemic louse borne typhus. Rochy mountain spotted fever. Mediterranean spotted fever. • • • Malignant malaria (capillaries of microcirculation) Visceral leishmaniasis African trypansomiasis Bacterial Leptospiral (Weil’s disease) Rickettsial: Parasitic:

Bacterial Meningoccocal meningitis Plague (Yersinia pestis) Typhoid fever. Septicemia (Staph, strept, pseudomonas and other gram negative bacilli). Pathogenesis: DIC A. Toxic theory (Toxic injury of vessel wall by endotoxin). B. Adhesive theory (Effect of toxin upon leucocytes and platelets resting aggregated trapped in small vessels). 1. 2. 3. 4.

Viral 1. Primary viral: A. B. C. 2. Arboviruses (Mosquito or tick borne, many mammals reservoir hosts) – – Dengue hgic fever (Flaviviridae) RVF (Bunyaviridae) Yellow fever (Flaviviridae) Greamean Congo Hgic fever (CCHF) – – – Argen. hgic fever Bolivian hgic fever Lassa fever Arena viruses (Direct contact secretions of tissues of infected animals or ingestion of food contaminated by animal excreta). – - Filoviridae: Marburg disease Ebola hgic fever Complicating viral A. B. C. D. E. Influenza Measles Herpes virus (IM, CMV, varicella) Hepatitis Rubella

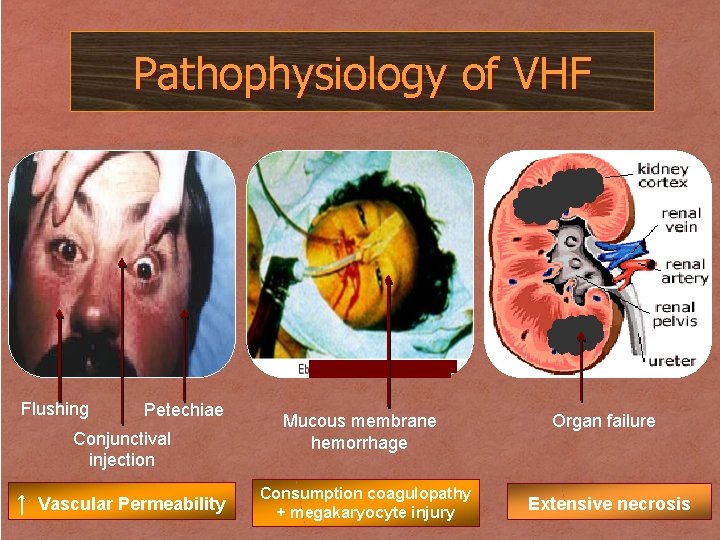
Pathophysiology of VHF Flushing Petechiae Conjunctival injection ↑ Vascular Permeability Mucous membrane hemorrhage Consumption coagulopathy + megakaryocyte injury Organ failure Extensive necrosis

Clinical Picture of VHF Secondary Primary exposure History Contact of travel with to an infected endemic cases area Direct Contact contact with infected with vectors primates

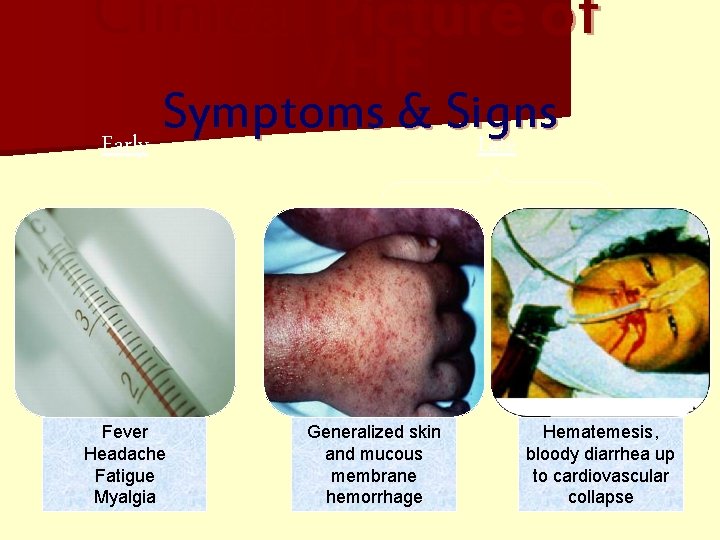
Clinical Picture of VHF Symptoms & Signs Early Late Fever Headache Fatigue Myalgia Generalized skin and mucous membrane hemorrhage Hematemesis, bloody diarrhea up to cardiovascular collapse

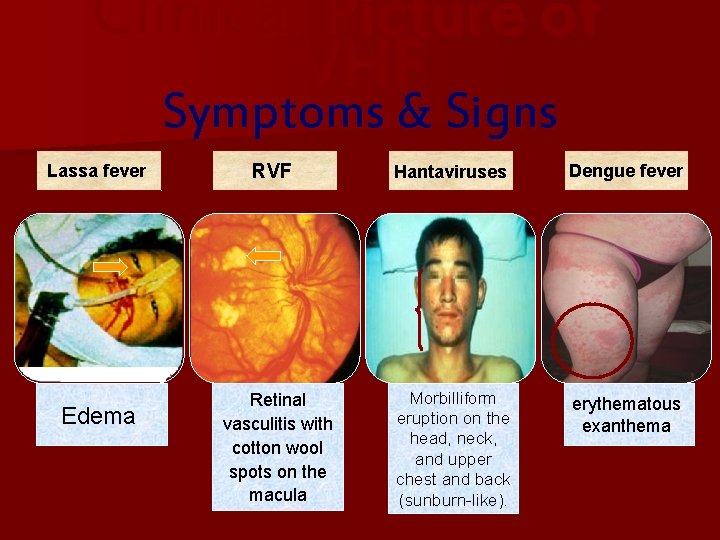
Clinical Picture of VHF Symptoms & Signs Lassa fever Edema RVF Retinal vasculitis with cotton wool spots on the macula Hantaviruses Dengue fever Morbilliform eruption on the head, neck, and upper chest and back (sunburn-like). erythematous exanthema

Arthropode- borne hemorrhagic fevers Yellow fever: Organism: Yellow fever virus is a member of Flaviviridae genus (Flavi means “yellow”). It is one of the major antigenic type only, so that solid immunity occur after an infection. ·Geographical distribution: It is particularly endemic in tropical Americas and Sub-Saharan Africa (Western and central Africa) ·Arthropod vector: Aedes aegypti mosquito (Urban yellow fever) and Forest mosquitoes (Sylvan yellow fever) ·

Arthropode- borne hemorrhagic fevers A. Yellow fever Ø Main reservoir: Humans (Urban yellow fever) and monkeys (Sylvan yellow fever) Ø Incubation period: 3 -6 days Ø Clinical features: Most infected individuals suffer only mild illness with fever and malaise. Ø About 15% develop a serious illness manifested by three phases: Ø The first is an acute phase of fever, headache, myalgia, nausea and vomiting, few physical specific signs are apparent at this stage, except for Faget’s sign, a relative bradycardia with fever.

Arthropode- borne hemorrhagic fevers Yellow fever n The second phase is a period of “remission” in which the fever remits for 1 to 2 days. n During the third phase a period of intoxication fever recurs, accompanied by jaundice and haemoohagic manifestations “Black vomit” refers to the massive hematemesis that can occur, 50% mortality can often be related to liver failure, myocarditis, encephalopathy and acute renal failure that occur during this phase.

Diagnosis of Yellow fever n n n n Laboratory findings include: 1. Leucopenia, thrombocytopenia and abnormal coagulation parameters. 2. Leucocytosis can evolve, as can elevated transaminases, hyperbilirubinemmia and hypoglycemia, all indicating incipient liver failure. 3. Nephrotic range proteinuria and renal failure can occur. Yellow fever infection can be detected early in serum or blood using Ig. M antibody capture ELISA or PCR, Ig. M specific ELISA appears by the end of the first week. A four fold or greater rise in titre in serum plaque neutralizing antibody, complement fixation or haemagglutination inhibition antibodies is also diagnostic but requires paired acute and convalescent sera. Cell culture can detect virus in acute serum. Pathologic examination and liver with viral isolation provides a postmortem diagnosis

Treatment of Yellow fever Ø Ø Ø No effective antiviral agent is available. All treatment is supportive. Index patients should be protected from mosquito bites for 5 days after illness to avoid spread. The best preventive measure against yellow fever is the live attenuated 17 D vaccine. A single subcutaneous injection is immunogenic in 90% of recipients and probably offers life long duration of immunity, but 10 year boosting is suggested if travel to an endemic area is anticipated. Immunization during pregnancy should be postponed unless absolutely necessary, although it poses only a small risk to the fetus. Patients with symptomatic human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS) should not receive the live vaccine. Infants younger than 9 to 12 months of age should not receive the vaccine because vaccine strain encephalitis has been described.

Dengue fever n Transmission by Aedes aegypti of Dengue Virus

Clinical Characteristics of Dengue Fever n Headache n Muscle and joint pain n Nausea/vomiting n Rash n Hemorrhagic manifestations

Hemorrhagic Manifestations of Dengue n Skin hemorrhages: petechiae, purpura, ecchymoses n Gingival bleeding n Nasal bleeding n Gastro-intestinal bleeding: hematemesis, melena, hematochezia n Hematuria n Increased menstrual flow

Danger Signs in Dengue Hemorrhagic Fever n Abdominal pain - intense and sustained n Persistent vomiting n Abrupt change from fever to hypothermia, with sweating and prostration n Restlessness or somnolence

Laboratory Tests in Dengue Fever n Clinical laboratory tests – CBC--WBC, platelets, hematocrit – Albumin – Liver function tests – Urine--check for microscopic hematuria n Dengue-specific – Virus isolation – Serology tests

Treatment of Dengue Fever n Fluids n Rest n Antipyretics (avoid aspirin and nonsteroidal anti-inflammatory drugs( n Monitor blood pressure, hematocrit, platelet count, level of consciousness

Rift Vally Fever n n n 1. 2. Ø Ø Organism: Rift valley fever virus is a Phlebovirus (Bunyaviridae) Geographical distribution: Africa and Middle East. Arthropod vector: Aedes sp. Mosquitoes Main reservoir: Sheep, cattle and goats Clinical features: Early clinical features: mild viral illness can occur with recovery and immunity. Acute onset of fever, headache, back pain, myalgia, nausea and epigastric pain. Advanced clinical features: Severe hemorrhage (epistaxis and gastrointestinal), icterus, hepatic necrosis, anuria, disseminated intravascular coagulation (DIC) and shock. Retinal vasculitis and encephalitis may occur but these patients don’t develop hemorrhage.

Ø Diagnosis of Rift Vally fever Culture: virus isolation from the blood or liver via cell culture or mouse inoculation. Ø Antibodies: IFA testing of acute serum after 3 -4 days of illness. Treatment: Ø No specific effective treatment Ø Experimental inactivation vaccine (U. S Army availability) Ø Ribavirin effective in non-human primates and mice.

Non Arthropode borne hemorrhagic fevers Lassa fever n Organism: Lassa fever virus is an Arenavirus n Transmission routes: Rodent to human via direct contact and by aersolization and body fluids. n Geographical distribution: The disease was first described in patients from Lassa in Nigeria. It has occurred in patients in various western and central African countries n Main reservoir: Multimammate rat (Mastomys natalensis). The virus is excreted in the urine and saliva of infected rat, contaminate water which subsequently may infect human. Also the virus is present in excreta and blood of man, and person to person transmission may occur.

Clinical picture of Lassa fever Clinical features: after an inoculation period 5 to 21 days, the acute phase of Lassa fever usually lasts between 1 to 4 weeks. In contrast to other hemorrhagic fever, its onset is characteristically insidious featuring fever, severe sore throat and headache, back pain and abdominal pain. Ø The late phase of disease involves its hemorrhagic manifestations (gastrointestinal) with death occurring from hypovolemic shock secondary to hemorrhage. Other late features are reactive bradycardia, pleural, pericardial effusion, pneuminitis, encephalopathy, facial edema, and endothelial and platelet dysfunction. DIC, however, doesn’t occur.

Clinical Picture of Lassa fever n The mortality rate of clinically apparent Lassa fever is estimated at 15%. n A mortality rate of 80% is associated with an elevated Aspartate aminotransferase (AST) (> 150 IU/L) and high level of viremia (> 103 TCID 50 /ml). Also there is an increased mortality rate in pregnancy.

Diagnosis of Lassa fever Ø Ø 1. 2. 3. 4. Clinical suspicion for patients in epidemic with the clinical triad of pharyngitis, retosternal pain and proteinuria. Lassa Fever can be serologically diagnosed in several ways. Using acute serum via the ELISA test can be diagnostic of either Ig. G or Ig. M antibodies. Detection of four fold or greater rise in Ig. G serum titre, using paired acute and convalescent titre can be diagnostic. Antigen can be detected by RT-PCR of acute serum. Isolation of virus in cell culture is possible if the patient is acutely ill.

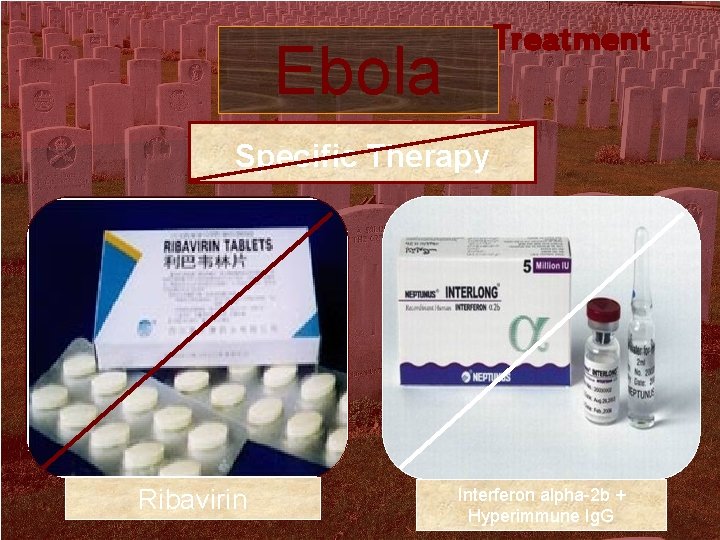
Treatment of Lassa fever n n n Patients treated with I. V or oral ribavirin with the first 6 days of fever had a statistically significant decrease in mortality rate. Ribavirin Treatment 30 mg/kg I. V. loading dose Then 16 mg / kg I. V. 6 hr. for 4 days Then 8 mg/ Kg I. V. 8 hr. for 6 days (Total treatment time 10 days)

Other non-Arthropode borne hemorrhagic fevers. § Marburg disease § Ebola disease

Ebola Marburg Filoviridae

Ebola Host & Reservoir ? No Known Reservoir Chimpanzee Monkey Bat Non-Human Reservoir. Host Gorilla

Ebola Sex Primary exposure Secondary exposure Male Female

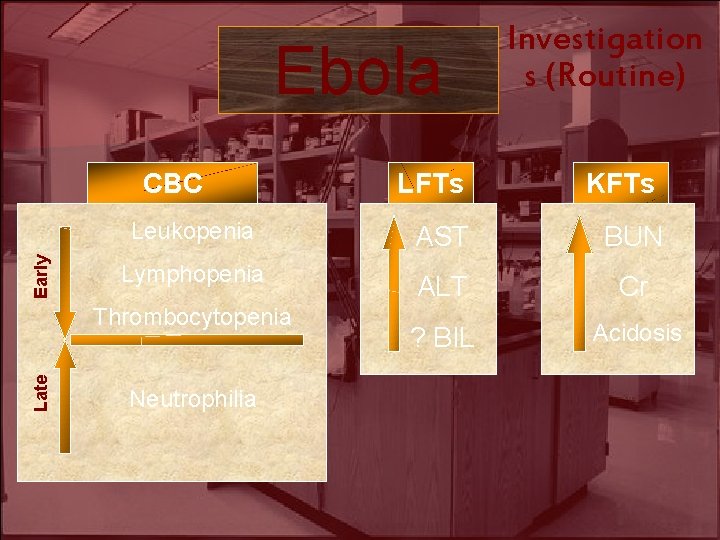
Ebola Early CBC LFTs KFTs Leukopenia AST BUN Lymphopenia ALT Cr ? BIL Acidosis Thrombocytopenia Late Investigation s (Routine) Neutrophilia

Ebola Tissue culture Investigation s (Specific)

Treatment Ebola Supportive Therapy Nutrition Electrolytes Specific Therapy Intravascular volume

Treatment Ebola Specific Therapy Ribavirin Interferon alpha-2 b + Hyperimmune Ig. G

Ebola Prophylaxis Under Research Isolation Strict barrier nursing Safety Bury NP GP Announcement

Marburg virus disease n n n n 1 n 1967, in Germany and Yugoslavia, 31 cases with 7 deaths with traced to direct contact with blood, organs or tissue cell culture from a batch of green monkeys in Uganda. Outbreaks in 1975 in south Africa and in 1980 in Kenya. Clinically: High fever, myalgia, vomiting, diarrhea, jaundice, maculopapular rash and bleeding tendency. The patient may develop unilateral uveitis or orchitis or pancreatitis. Diagnosis: Leucopenia, thrombocytopenia, raised SGOT and SGPT and serum amylase. Inoculation of virus containing suspension into mice, guina pigs or monkeys. IIFT. P. M. diagnosis by electomicroscopy or IF of liver specimens. Therapy: Symptomatic, antibiotics for sec. infections. Fresh blood, vitamin K, PPSB (prothrobink proconvertin, stuart factor and antihaemophilic globin B). Auganda: Low morbidity and low mortality Angola (2005): High mortality rate (120 deaths mostly in children <5 years).

Diagnosis VHF 1. 2. 3. 4. 5. History of travel to endemic area or arthropod bites or contact wild animals. Occupation as health care or agriculture in endemic area. Immune status as moderate microorganisms in immunocompromised patients lead to fulminating infections resembling VHF Flu-like character of VHF seldomly aid clinical diagnosis. Suggestive signs: Rashes, bleeding tendencies, shock and evidence of hepatic impairment. Lab. : CBP, blood film for malaria and acute African trypanomiasis. Electron microscopy and virus isolation (tissue culture or animal inoculations) may be performed on blood samples, throat washings, urine, biopsy specimens and PM materials. Serology for specific antibodies.

Thank you
 Shock class
Shock class Ischemic vs hemorrhagic stroke
Ischemic vs hemorrhagic stroke Clinical manifestation of epistaxis
Clinical manifestation of epistaxis Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Baby temperature chart
Baby temperature chart Acute pancreatitis pathophysiology
Acute pancreatitis pathophysiology Hemorrhagic diathesis
Hemorrhagic diathesis Icp monitoring
Icp monitoring Blood clot size of clenched fist in ml
Blood clot size of clenched fist in ml Stages of hemorrhagic shock
Stages of hemorrhagic shock Antihaemorrhagic vitamin
Antihaemorrhagic vitamin Hemorrhagic transformation mri
Hemorrhagic transformation mri Types of haemorrhage
Types of haemorrhage Hemorrhagic fever with renal syndrome
Hemorrhagic fever with renal syndrome Necrotizing fasciitis hemorrhagic bullae
Necrotizing fasciitis hemorrhagic bullae Abrupt stormy onset
Abrupt stormy onset Onset coda and nucleus
Onset coda and nucleus Onset coda and nucleus
Onset coda and nucleus Onset nucleus coda examples
Onset nucleus coda examples Anaphylaxis onset
Anaphylaxis onset Voice onset time
Voice onset time Onset and coda examples
Onset and coda examples Normal labour
Normal labour Marginalization in probability
Marginalization in probability Lateinon
Lateinon Insulin day supply cheat sheet
Insulin day supply cheat sheet Onset reim beispiel
Onset reim beispiel Onset nukleus koda
Onset nukleus koda Webtopings
Webtopings Nph onset and peak
Nph onset and peak Hindi syllable structure
Hindi syllable structure Onset offset trial
Onset offset trial Nph onset and peak
Nph onset and peak Phonteic alphabet
Phonteic alphabet Syllable structure cvc examples
Syllable structure cvc examples Early onset scoliosis classification
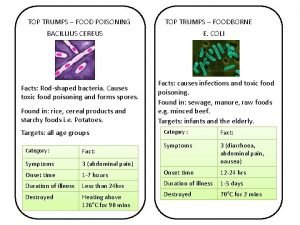
Early onset scoliosis classification Food poisoning onset
Food poisoning onset Food poisoning onset
Food poisoning onset Sudden successive flights of bullets
Sudden successive flights of bullets Loss of head due to sudden contraction of pipe *
Loss of head due to sudden contraction of pipe *