The leukaemias are a group of disorders characterized










- Slides: 34


The leukaemias are a group of disorders characterized by the accumulation of malignant white cells in the bone marrow and blood. Classification of leukaemia The main classification is into: acute and chronic leukaemias, which are further subdivided into lymphoid or myeloid.

�Acute leukaemias are usually aggressive diseases in which malignant transformation occurs in the haemopoietic stem cell or early progenitors. -Acute leukaemia is either acute myeloid leukaemia (AML) or acute lymphoblastic leukaemia (ALL).

PATHOGENESIS The principal pathogenic defect in acute leukemia is a block in differentiation. This block in differentiation “maturation arrest” stems from acquired maturation arrest mutations in specific transcription factors that regulate the differentiation of immature lymphoid or myeloid progenitors.

-Approximately 90% of ALL have chromosomal changes. Many of the chromosomal aberrations dysregulate the expression or function of transcription factors that control normal B- and T-cell development and lead to maturation arrest e. g. Hyperdiploidy , hypodiploidy.

-Most genetic aberrations in AML interfere with transcription factor activities required for normal myeloid cell differentiation. For example t (15; 17). Many AML ‘driver mutations’ have been identified, e. g. FLT 3 , NPM 1

These events cause accumulation in the bone marrow of early haemopoietic cells known as blast cells. -

These abnormal cells cause symptoms of leukaemia because of: 1. Bone marrow failure: blasts accumulating in the failure: marrow suppress the growth of normal hematopoietic cells (e. g. anaemia , neutropenia , thrombocytopenia). 2. Infiltration of organs (e. g. liver, spleen, lymph 2. Infiltration of organs nodes, meninges, brain, skin or testes).

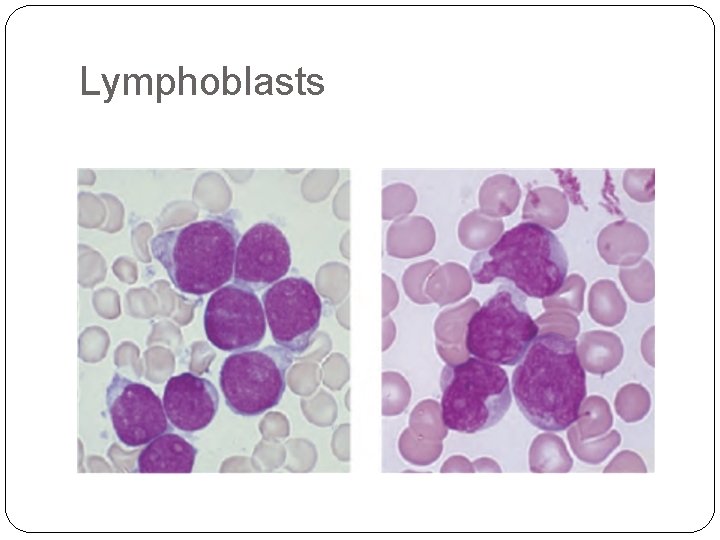
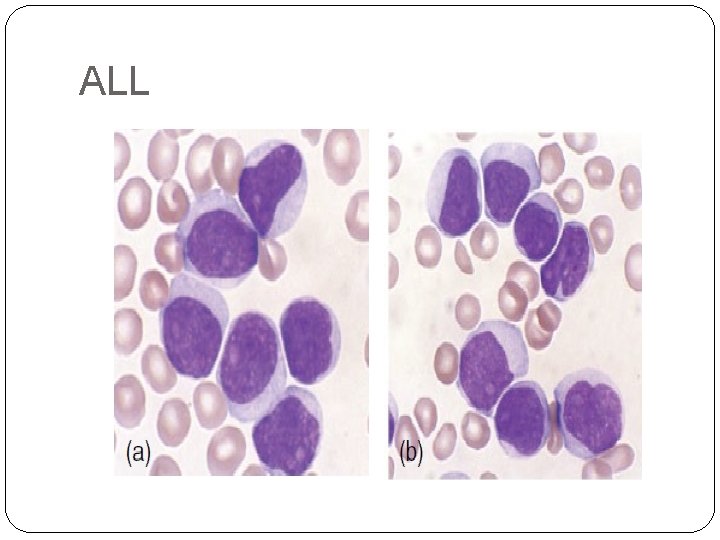
-Acute lymphoblastic leukaemia (ALL) is caused by an accumulation of lymphoblasts in the bone marrow. It is the most common malignant disease of childhood ( 75% of cases occur before the age of 6 years). Eighty five per cent of cases are of B‐cell lineage with the rest being of T‐cell lineage. -

Lymphoblasts

ALL

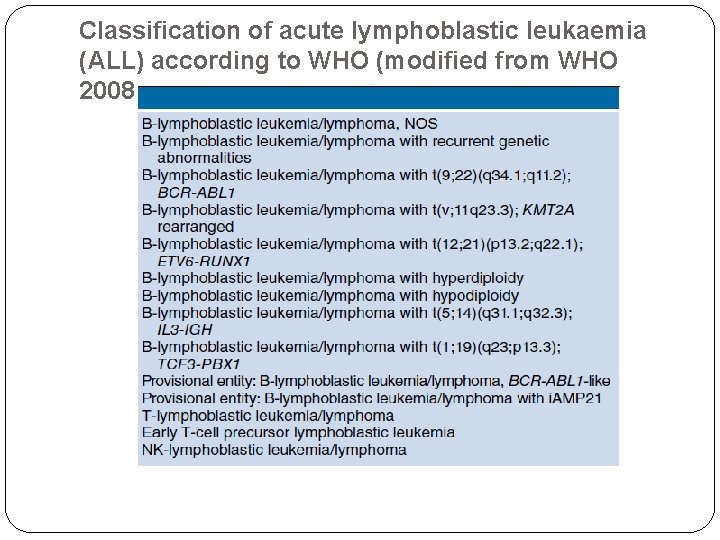
Classification of acute lymphoblastic leukaemia (ALL) according to WHO (modified from WHO 2008

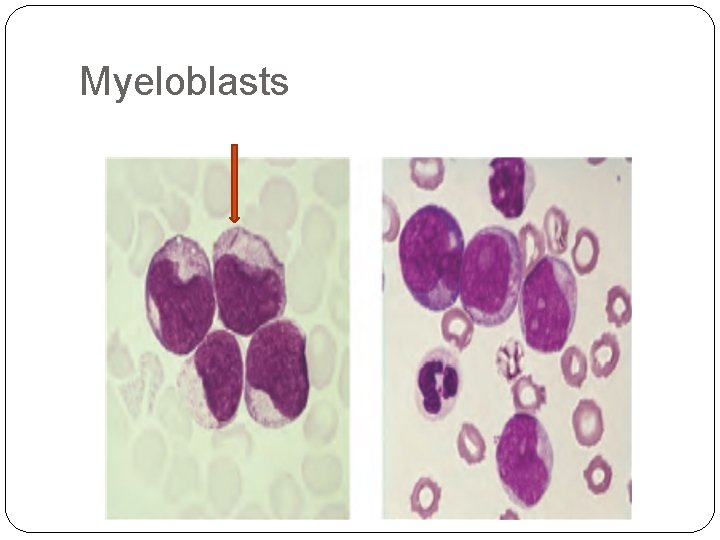
�Acute myeloid leukaemia(AML) primarily affects older adults; the median age is 50 years, it is caused by an accumulation of myeloid blasts( Myeloblasts). . .

�Auer rods, distinctive red-staining rodlike structures, may be present in myeloblasts. �Auer rods are specific for neoplastic myeloblasts and thus a helpful diagnostic clue when present.

Myeloblasts

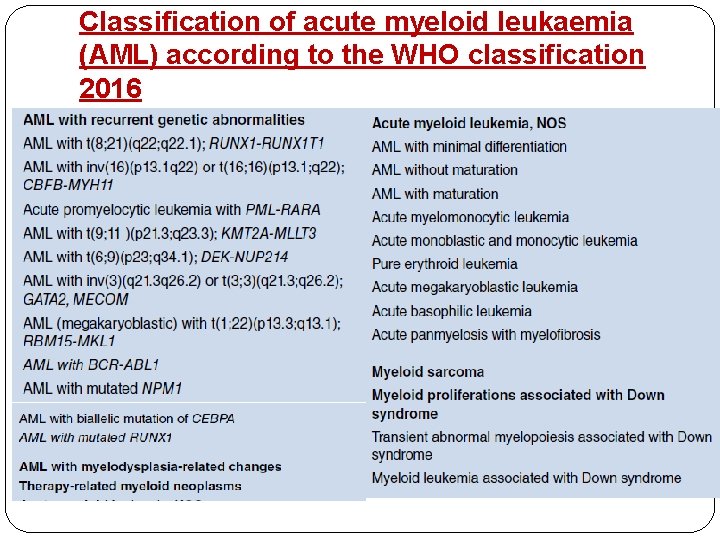
Classification of acute myeloid leukaemia (AML) according to the WHO classification 2016


Clinical Features of Acute Leukemias: • Abrupt, stormy onset. Most patients present for medical attention within 3 months of the onset of symptoms. •

• -Clinical signs and symptoms related to suppressed marrow function, function including fatigue (due to anemia), fever (reflecting infections resulting from neutropenia), and bleeding (petechiae, ecchymoses, epistaxis, gum bleeding) secondary to thrombocytopenia

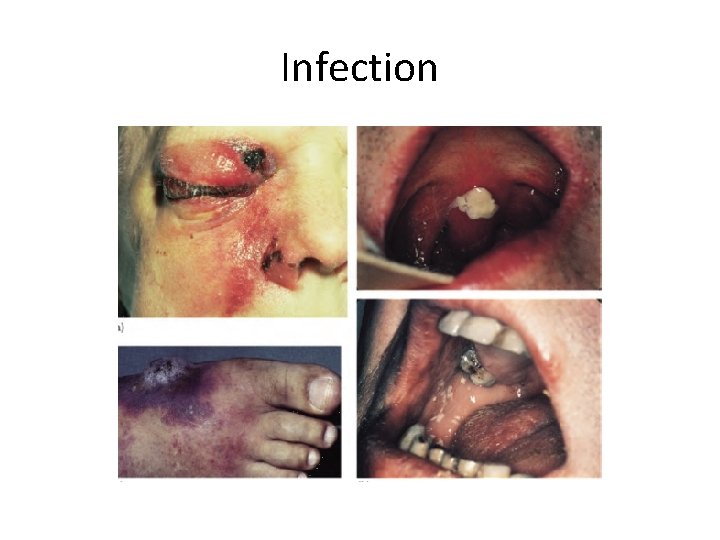
Infection

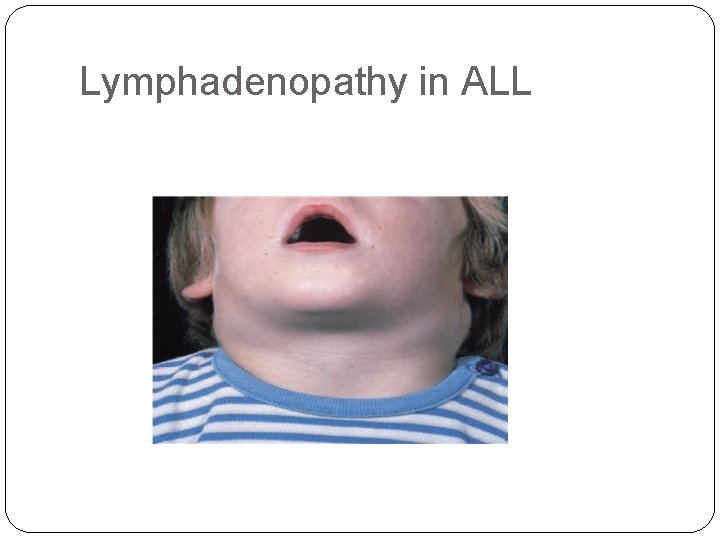
Bone pain and tenderness, resulting from marrow expansion and infiltration of the subperiosteum - --Generalized lymphadenopathy, splenomegaly, and hepatomegaly due to dissemination of the leukemic cells. These are more pronounced in ALL than in AML.

Lymphadenopathy in ALL

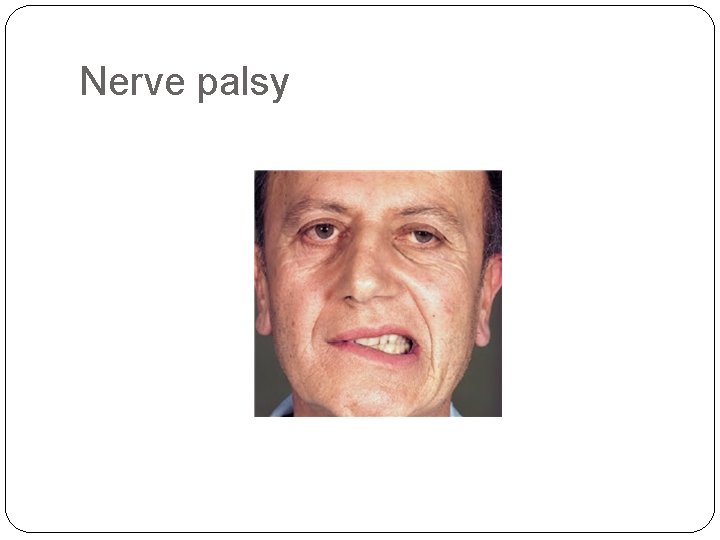
• Central nervous system manifestations, including headache, vomiting, and nerve palsies resulting from meningeal spread. These are more common in children than in adults and in ALL than in AML. ALL - Granulocytic sarcoma as a discrete tissue mass can be seen in AML.

Nerve palsy

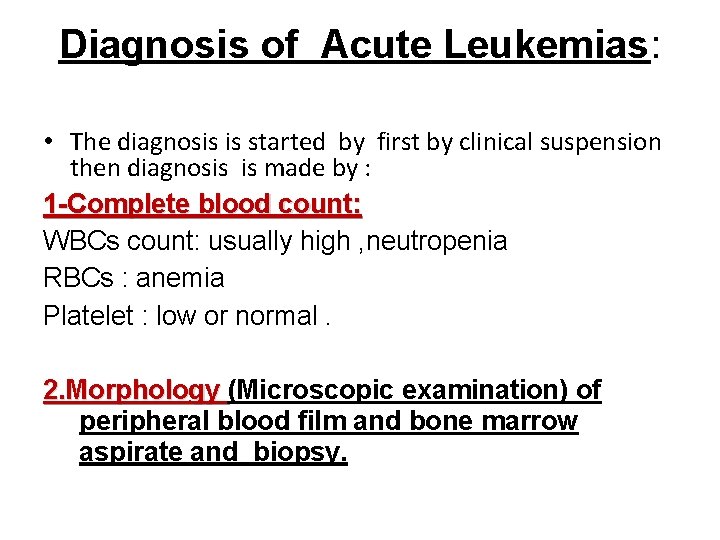
Diagnosis of Acute Leukemias: • The diagnosis is started by first by clinical suspension then diagnosis is made by : 1 -Complete blood count: WBCs count: usually high , neutropenia RBCs : anemia Platelet : low or normal. 2. Morphology (Microscopic examination) of 2. Morphology peripheral blood film and bone marrow aspirate and biopsy.

�The diagnosis of acute leukemia rests on the presence of over 20% of blast cells in the bone marrow at clinical presentation. However, it can be diagnosed with less than 20% blasts if specific leukaemia‐associated cytogenetic or molecular genetic abnormalities are present. �

3. Immunophenotyping (flow cytometry) : Immunophenotyping is very useful in distinguishing ALL from AML, subtyping of them. The typical ‘myeloid immunophenotype’ is CD 13, CD 33+ CD 117, and Td. T−. The lymphoid phenotype is : CD 10+ , CD 19+, c. CD 22+ for B-ALL, CD 2+ , c. CD 3+, CD 7+ for T-ALL


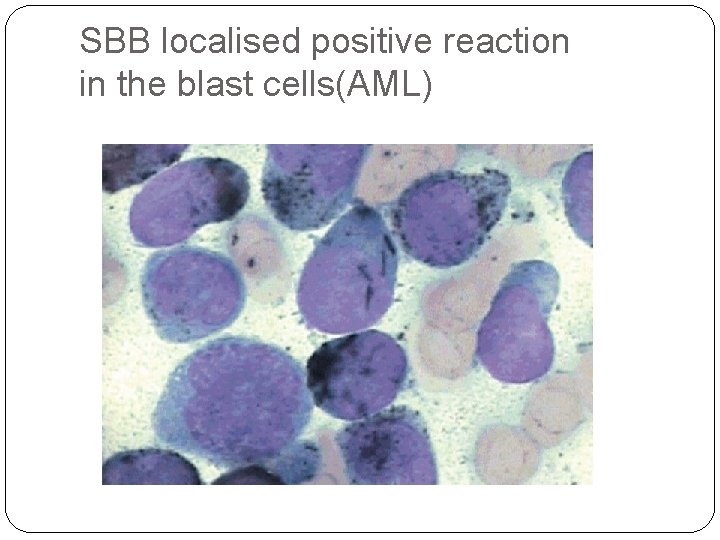
4. Cytochemical analysis: (performed in some countries instead of immunophenotyping) e. g. Myeloperoxidase and Sudan black are positive in AML cases. 5. Cytogenetic and molecular studies: to identify leukaemia specific mutations , translocations, chromosomal abnormalities

SBB localised positive reaction in the blast cells(AML)

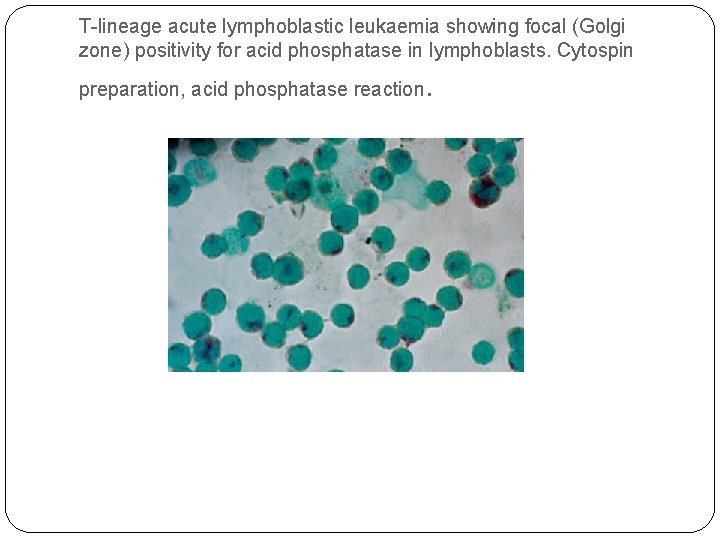
T-lineage acute lymphoblastic leukaemia showing focal (Golgi zone) positivity for acid phosphatase in lymphoblasts. Cytospin preparation, acid phosphatase reaction .


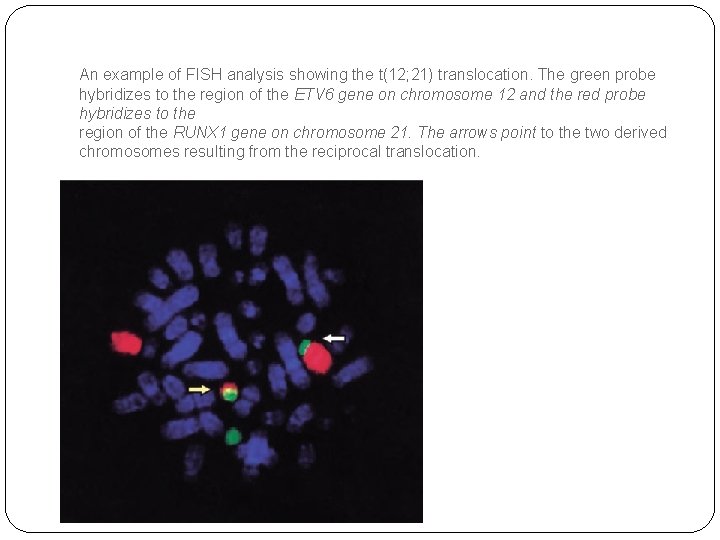
An example of FISH analysis showing the t(12; 21) translocation. The green probe hybridizes to the region of the ETV 6 gene on chromosome 12 and the red probe hybridizes to the region of the RUNX 1 gene on chromosome 21. The arrows point to the two derived chromosomes resulting from the reciprocal translocation.

 Insidan region jh
Insidan region jh Psychological disorders characterized by inflexible
Psychological disorders characterized by inflexible 101012 bằng
101012 bằng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worm breton
Tư thế worm breton Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te Hát lên người ơi
Hát lên người ơi điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Hệ hô hấp
Hệ hô hấp Công thức tiính động năng
Công thức tiính động năng So nguyen to
So nguyen to đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Cái miệng nó xinh thế
Cái miệng nó xinh thế Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Tư thế ngồi viết
Tư thế ngồi viết