Organic Chemistry oh what fun Organic Chemistry What









- Slides: 29

Organic Chemistry …oh what fun…

Organic Chemistry What does it mean to be organic? p To be an organic compound means that you contain carbon p n … that’s it… if you contain carbon, you are organic p n n n There a few exceptions, but you don’t have to worry about those Carbon atoms comprise about 20% (by mass) of all animals Fossil Fuels Common products like plastic, adhesives, soaps, etc

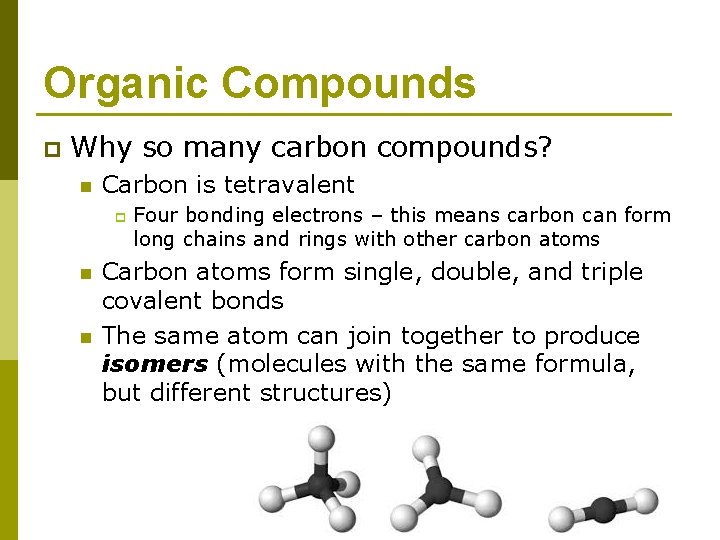
Organic Compounds p Why so many carbon compounds? n Carbon is tetravalent p n n Four bonding electrons – this means carbon can form long chains and rings with other carbon atoms Carbon atoms form single, double, and triple covalent bonds The same atom can join together to produce isomers (molecules with the same formula, but different structures)

Homologous Series p Homologous Series: n n n General formula can be written to represent all member of a series Each successive member of the series differs by a common structural unit The chemistry of any one member is similar to that of the other members p Similar preparation and similar chemical properties

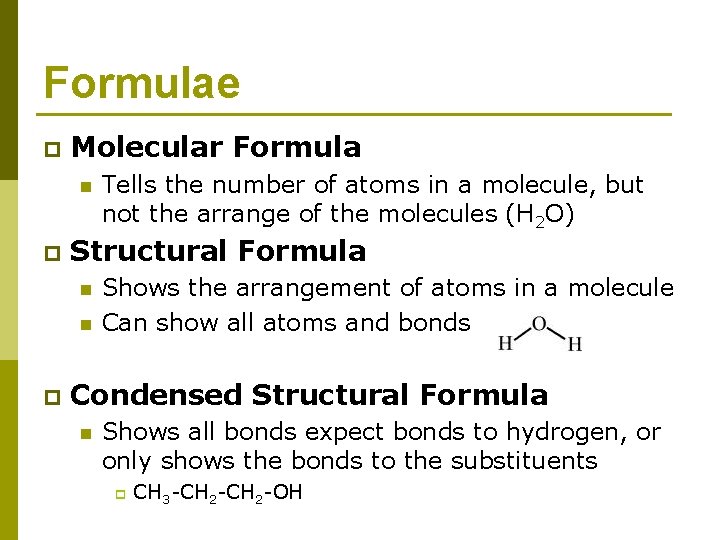
Formulae p Molecular Formula n p Structural Formula n n p Tells the number of atoms in a molecule, but not the arrange of the molecules (H 2 O) Shows the arrangement of atoms in a molecule Can show all atoms and bonds Condensed Structural Formula n Shows all bonds expect bonds to hydrogen, or only shows the bonds to the substituents p CH 3 -CH 2 -OH

Simplest Organic Compounds p Simplest organic compounds are hydrocarbons n Meaning that they only contain carbon and hydrogen

Hydrocarbons p Alkanes – only single bonds b/w carbons n p Alkenes – at least 1 double bond b/w carbons n p General Formula: Cn. H 2 n+2 General Formula: Cn. H 2 n Alkynes – at least 1 triple bond b/w carbons n General Formula: Cn. H 2 n-2

Functional Groups An atom or group of atoms that always react a certain way p Adding a functional group to a hydrocarbon will always change the physical and chemical properties of that hydrocarbon p n And a functional group will always change these properties the same way

Functional Groups p p p p p Halide (Haloalkane) Alcohol Ether Aldehyde Ketone Organic acid Ester Amine Amide Carbonyl Group: carbon with a double-bonded oxygen

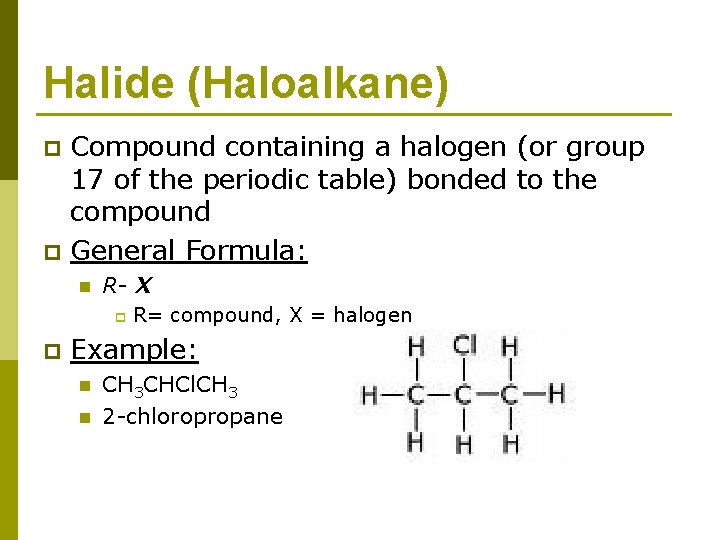
Halide (Haloalkane) p p Compound containing a halogen (or group 17 of the periodic table) bonded to the compound General Formula: n R- X p p R= compound, X = halogen Example: n n CH 3 CHCl. CH 3 2 -chloropropane

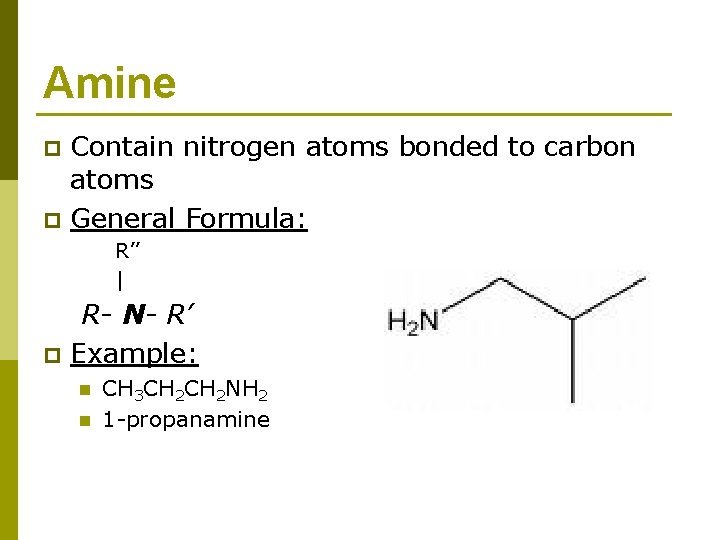
Amine p p Contain nitrogen atoms bonded to carbon atoms General Formula: R’’ | p R- N- R’ Example: n n CH 3 CH 2 NH 2 1 -propanamine

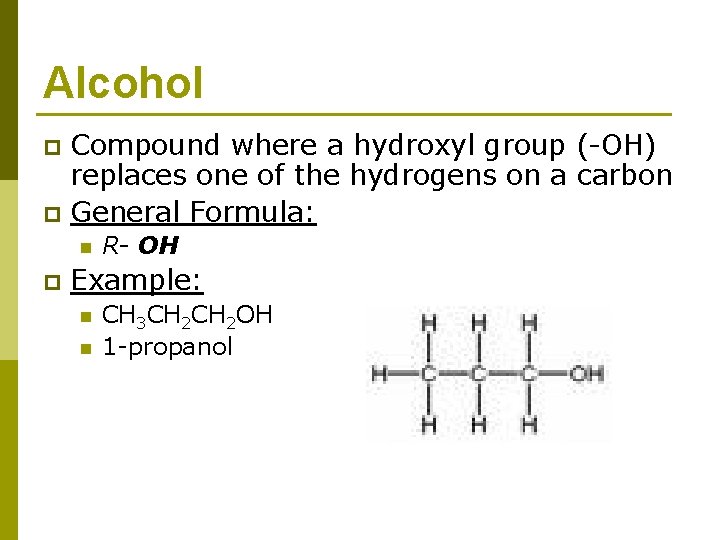
Alcohol Compound where a hydroxyl group (-OH) replaces one of the hydrogens on a carbon p General Formula: p n p R- OH Example: n n CH 3 CH 2 OH 1 -propanol

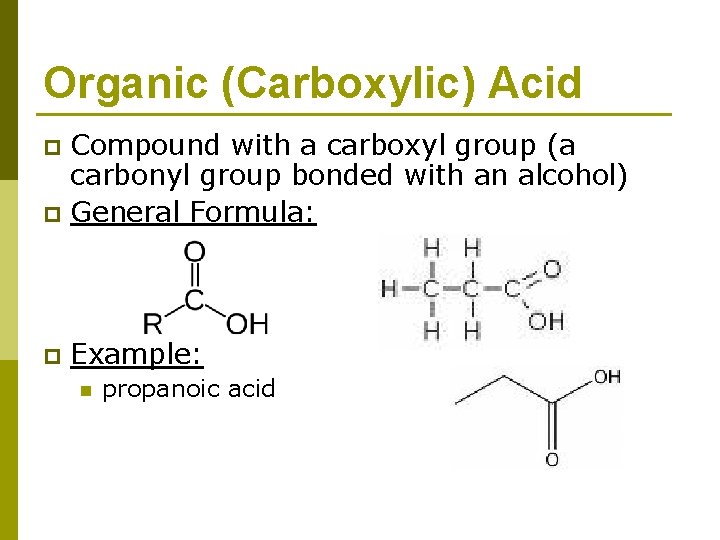
Organic (Carboxylic) Acid Compound with a carboxyl group (a carbonyl group bonded with an alcohol) p General Formula: p p Example: n propanoic acid

Physical Properties of Organic Compounds Colour: colourless p State @ Room Temp: p n n Hydrocarbons: gases then liquids, then solids as molar mass increases Haloalkanes, alcohols, carboxylic acids and amines: liquids then solids as molar mass increases

Physical Properties of Organic Compounds p Solubility in Water: n n Hydrocarbons and Haloalkanes insoluble (nonpolar) Alcohols, carboxylic acids and amines: Lower molar mass members are soluble in water (polar) p Higher molar masses insoluble § The more carbons, the less polar it becomes p

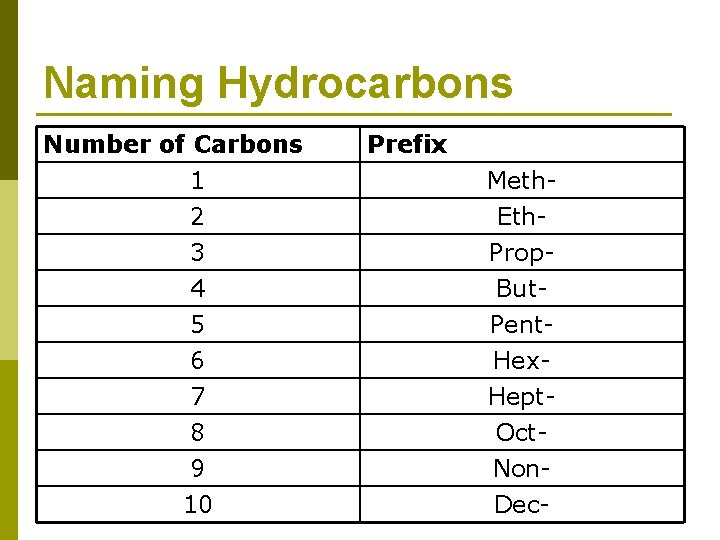
Naming Hydrocarbons p There is very simple nomenclature for hydrocarbons n First of all, we’ll start with naming straight chains 1. 2. Each chain that contains only single bonds ends with –ane Count the carbons and add the correct the prefix for that number of carbons. § What are the prefixes?

Naming Hydrocarbons Number of Carbons 1 2 3 4 5 6 7 8 9 10 Prefix Meth. Eth. Prop. But. Pent. Hex. Hept. Oct. Non. Dec-

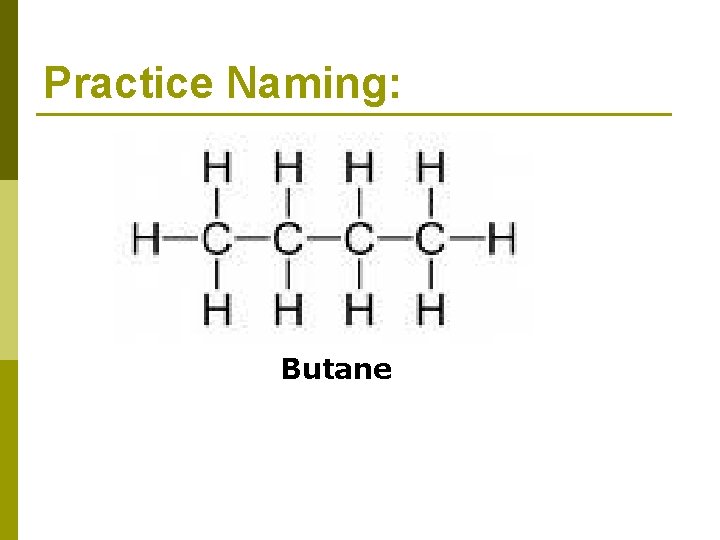
Practice Naming: Butane

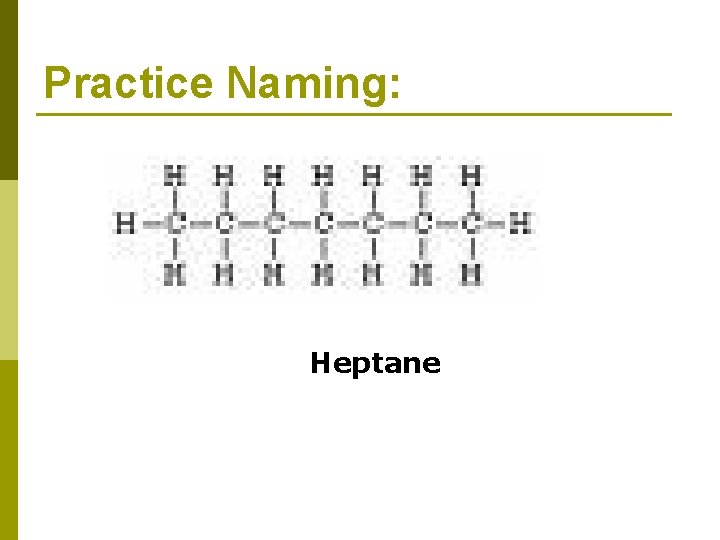
Practice Naming: Heptane

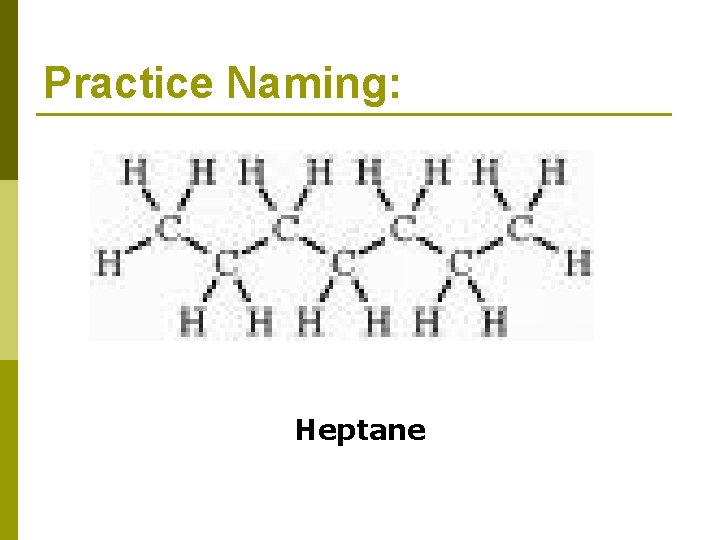
Practice Naming: Heptane

Different Diagrams p There are different ways to represent carbon chains. n 1) there is the standard way where you draw in every hydrogen atom n 2) You can simply draw in dashes and not label the ‘H’ s because we know what they are

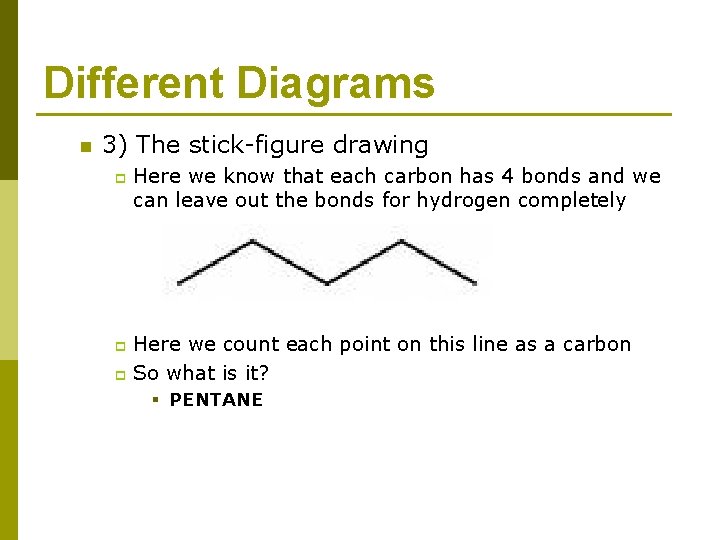
Different Diagrams n 3) The stick-figure drawing p Here we know that each carbon has 4 bonds and we can leave out the bonds for hydrogen completely Here we count each point on this line as a carbon p So what is it? p § PENTANE

Naming Branched Alkanes: p What do you do if it’s not a straight chain?

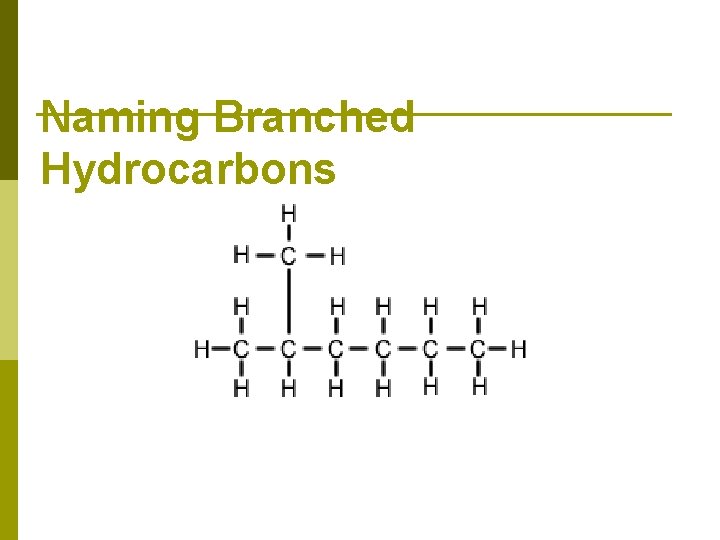
Naming Branched Hydrocarbons

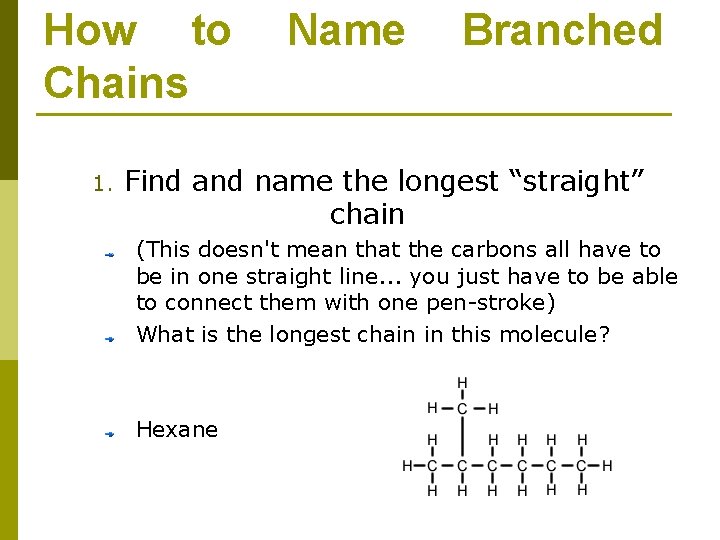
How to Chains 1. Name Branched Find and name the longest “straight” chain (This doesn't mean that the carbons all have to be in one straight line. . . you just have to be able to connect them with one pen-stroke) What is the longest chain in this molecule? Hexane

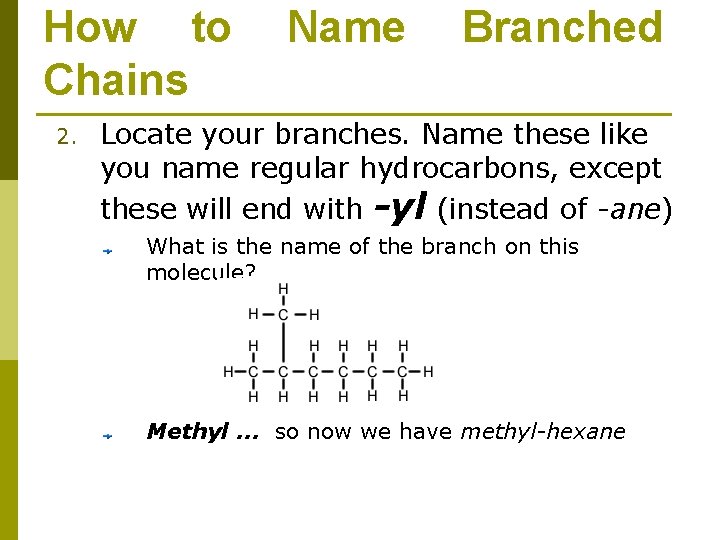
How to Chains 2. Name Branched Locate your branches. Name these like you name regular hydrocarbons, except these will end with -yl (instead of -ane) What is the name of the branch on this molecule? Methyl … so now we have methyl-hexane

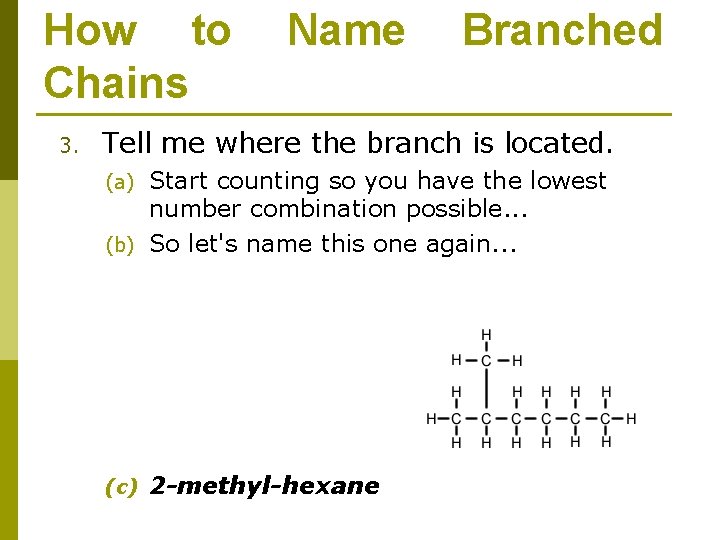
How to Chains 3. Name Branched Tell me where the branch is located. Start counting so you have the lowest number combination possible. . . (b) So let's name this one again. . . (a) (c) 2 -methyl-hexane

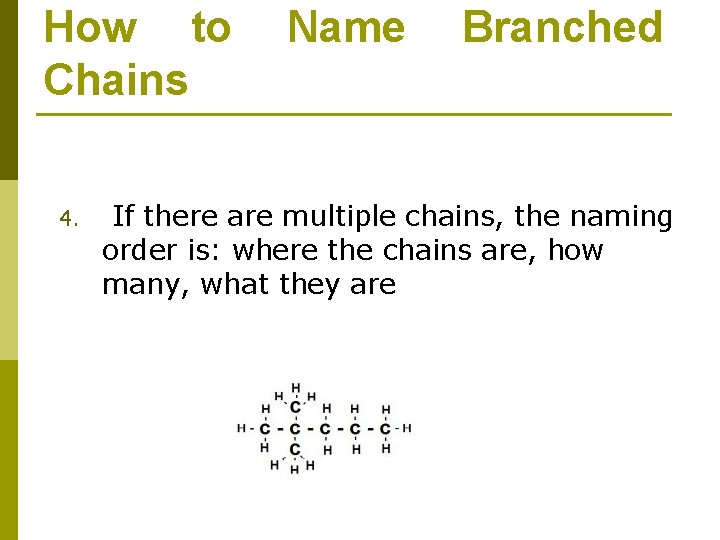
How to Chains 4. Name Branched If there are multiple chains, the naming order is: where the chains are, how many, what they are

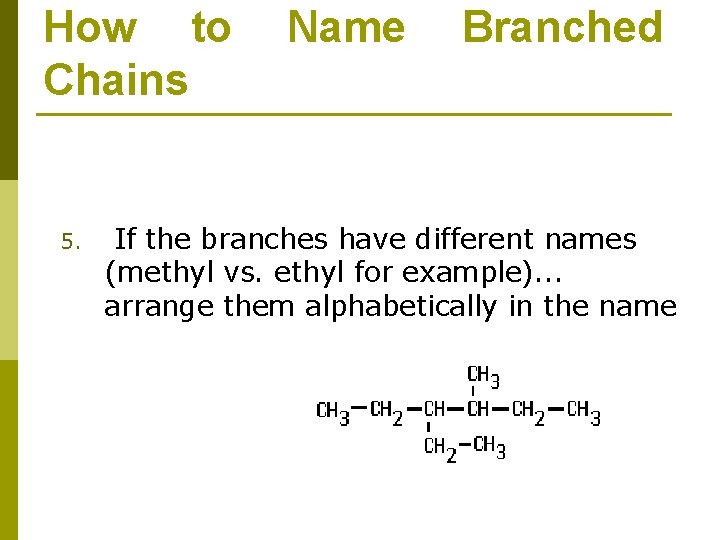
How to Chains 5. Name Branched If the branches have different names (methyl vs. ethyl for example). . . arrange them alphabetically in the name
 Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Transition state energy diagram
Transition state energy diagram Alkyl group example
Alkyl group example M+1 peak
M+1 peak Organic chemistry
Organic chemistry Organic chemistry chapter 1
Organic chemistry chapter 1 Entane
Entane Eth meth prop but
Eth meth prop but Enols and enolates organic chemistry
Enols and enolates organic chemistry Organic chemistry
Organic chemistry Ario acidity
Ario acidity Organic chemistry
Organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Nomenclature of ethers
Nomenclature of ethers What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry But prop
But prop Soap organic chemistry
Soap organic chemistry Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Carbohydrates organic chemistry
Carbohydrates organic chemistry Radicals
Radicals Organic chemistry chapter 9
Organic chemistry chapter 9 C-c-c-c-c chemistry
C-c-c-c-c chemistry Organic chemistry
Organic chemistry Organic chemistry lab report format
Organic chemistry lab report format Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report Chemistry
Chemistry Ee organic chemistry
Ee organic chemistry Organic chemistry
Organic chemistry