NEW MODEL SURFACES FOR MINERAL AEROSOLS ANILINELINKED OLEFINS


![Amide -linked terpene + 30 ppm ozone DESFG [a. u. ] CH 3 as Amide -linked terpene + 30 ppm ozone DESFG [a. u. ] CH 3 as](https://slidetodoc.com/presentation_image_h/2c72cc3eefec1ac47ed167f77e23e609/image-30.jpg)


- Slides: 49

NEW MODEL SURFACES FOR MINERAL AEROSOLS: ANILINELINKED OLEFINS ON SILICA STUDIED WITH BROADBAND VIBRATIONAL SFG Avram M. Buchbinder, GRACE Y. STOKES, JULIANNE M. GIBBS-DAVIS, KARL A. SCHEIDT, FRANZ M. GEIGER Northwestern University

I Atmospheric Chemistry Voges, A. B. , et al. J. Phys. Chem. C Feature Article (2007) Voges, A. B. , et al. J. Phys. Chem. B (2004) Schmidt, C. M. et al. J. Phys. Chem. C. (2007) Schmidt, C. M. et al. Langmuir (2006) II Geochemistry Mifflin, A. L. et al. J. Phys. Chem. B (2005) Mifflin, A. L. , et al. J. Phys. Chem. A (2003) Gibbs-Davis, J. L. , et al. J. Am. Chem. Soc. , (2007) Konek, C. T. et al. Elsevier series on "Developments in Earth and Environmental Sciences", in press (2007) Al-Abadleh, H. A. , et al. J. Phys. Chem. B (2005) Al-Abadleh, H. A. , et al. J. Am. Chem. Soc, (2004) Konek, C. T. , et al. J. Am. Chem. Soc, (2004) III Biophysics Stokes, G. Y. et al. J. Am. Chem. Soc. (2007) Boman, F. C. , et al. J. Am. Chem. Soc. (2005) Hayes, P. L. et al. J. Phys. Chem. C (2007) Mifflin, A. L. et al. J. Phys. Chem. B (2006) Konek, C. T. et al. J. Am. Chem. Soc. (2005)

I Atmospheric Chemistry Voges, A. B. , et al. J. Phys. Chem. C Feature Article (2007) Voges, A. B. , et al. J. Phys. Chem. B (2004) Schmidt, C. M. et al. J. Phys. Chem. C. (2007) Schmidt, C. M. et al. Langmuir (2006) II Geochemistry Mifflin, A. L. et al. J. Phys. Chem. B (2005) Mifflin, A. L. , et al. J. Phys. Chem. A (2003) Gibbs-Davis, J. L. , et al. J. Am. Chem. Soc. , (2007) Konek, C. T. et al. Elsevier series on "Developments in Earth and Environmental Sciences", in press (2007) Al-Abadleh, H. A. , et al. J. Phys. Chem. B (2005) Al-Abadleh, H. A. , et al. J. Am. Chem. Soc, (2004) Konek, C. T. , et al. J. Am. Chem. Soc, (2004) III Biophysics Stokes, G. Y. et al. J. Am. Chem. Soc. (2007) Boman, F. C. , et al. J. Am. Chem. Soc. (2005) Hayes, P. L. et al. J. Phys. Chem. C (2007) Mifflin, A. L. et al. J. Phys. Chem. B (2006) Konek, C. T. et al. J. Am. Chem. Soc. (2005)

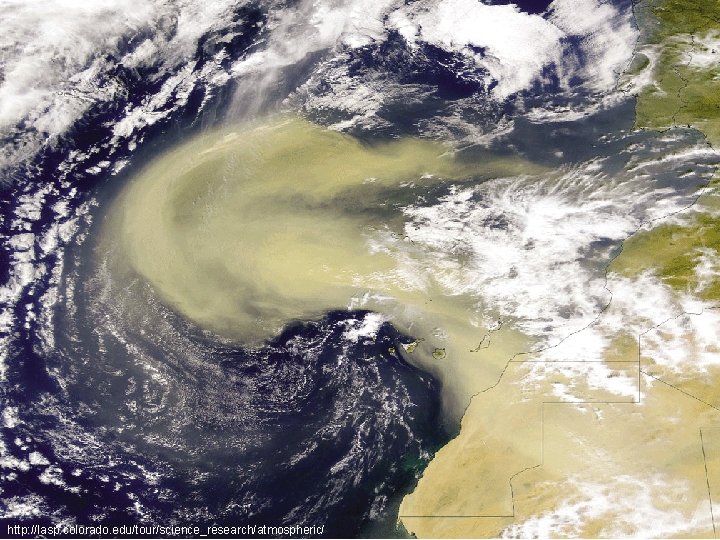
http: //lasp. colorado. edu/tour/science_research/atmospheric/

Terpenes http: //www. hermann-uwe. de/files/images/remarkable_forest. preview. jpg

Terpenes Biogenic VOC sources outweigh anthropogenic sources 5: 1 http: //www. hermann-uwe. de/files/images/remarkable_forest. preview. jpg Finlayson-Pitts, B. J. ; Pitts, J. N. Chemisry of the Upper and Lower Atmosphere; Academic Press: New York, 1999.

Terpenes Limonene Biogenic VOC sources outweigh anthropogenic sources 5: 1 http: //www. hermann-uwe. de/files/images/remarkable_forest. preview. jpg Finlayson-Pitts, B. J. ; Pitts, J. N. Chemisry of the Upper and Lower Atmosphere; Academic Press: New York, 1999.

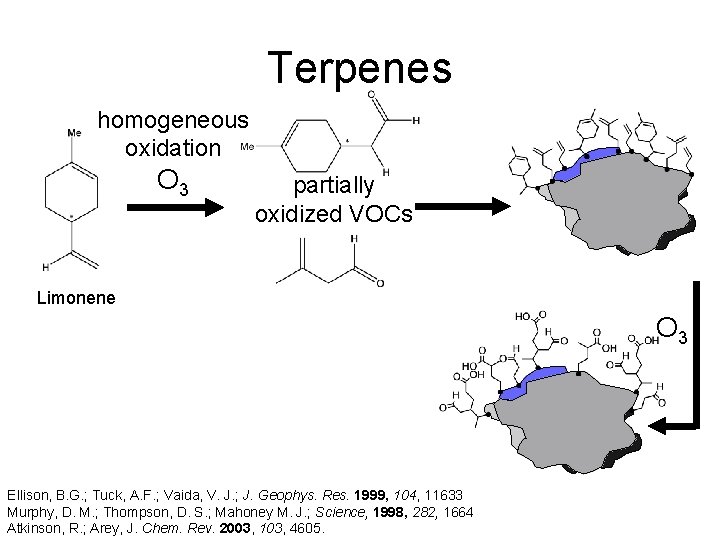
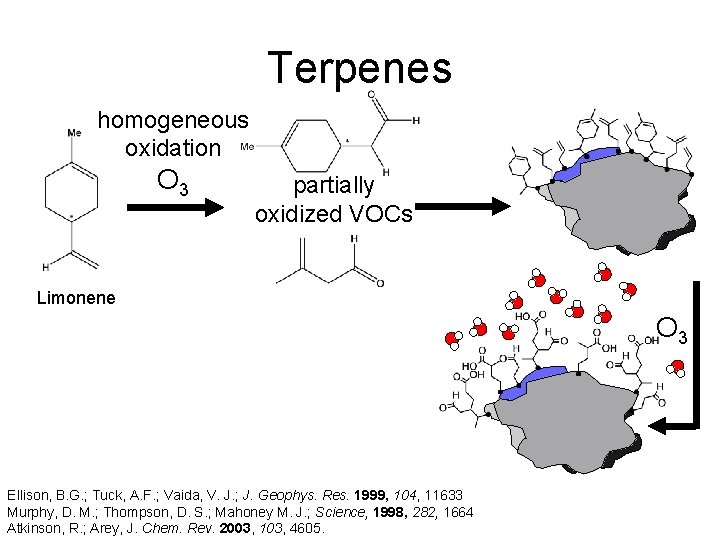
Terpenes homogeneous oxidation O 3 partially oxidized VOCs Limonene http: //www. hermann-uwe. de/files/images/remarkable_forest. preview. jpg Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605.

Terpenes homogeneous oxidation O 3 partially oxidized VOCs Limonene O 3 Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605.

Terpenes homogeneous oxidation O 3 partially oxidized VOCs Limonene O 3 Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605.

Terpenes Hydrophilic oxidized organic adlayer mediates cloud nucleation with consequences for radiative forcing “Aerosols… remain the dominant uncertainty in radiative forcing” Climate Change 2007 IPCC Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605. IPCC, 2007: Summary for Policymakers. In Climate Change 2007: The Physical science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S. , et al. Eds. ; Cambridge University Press: New York, 2007.

Terpenes Hydrophilic oxidized organic adlayer mediates cloud nucleation with consequences for radiative forcing “Aerosols… remain the dominant uncertainty in radiative forcing” Climate Change 2007 IPCC • How fast? Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605. IPCC, 2007: Summary for Policymakers. In Climate Change 2007: The Physical science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S. , et al. Eds. ; Cambridge University Press: New York, 2007.

Terpenes Hydrophilic oxidized organic adlayer mediates cloud nucleation with consequences for radiative forcing “Aerosols… remain the dominant uncertainty in radiative forcing” Climate Change 2007 IPCC • How fast? • How does molecular orientation, structure and variability control reactivity? Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605. IPCC, 2007: Summary for Policymakers. In Climate Change 2007: The Physical science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S. , et al. Eds. ; Cambridge University Press: New York, 2007.

Terpenes Hydrophilic oxidized organic adlayer mediates cloud nucleation with consequences for radiative forcing “Aerosols… remain the dominant uncertainty in radiative forcing” Climate Change 2007 IPCC • How fast? • How does molecular orientation, structure and variability control reactivity? • How can we address the complexity of the system? Ellison, B. G. ; Tuck, A. F. ; Vaida, V. J. ; J. Geophys. Res. 1999, 104, 11633 Murphy, D. M. ; Thompson, D. S. ; Mahoney M. J. ; Science, 1998, 282, 1664 Atkinson, R. ; Arey, J. Chem. Rev. 2003, 103, 4605. IPCC, 2007: Summary for Policymakers. In Climate Change 2007: The Physical science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S. , et al. Eds. ; Cambridge University Press: New York, 2007.

Previous models Oleic acid coating Moise, T. ; Rudich, Y. JPC A 2002, 106, 6469. Hearn, J. D. ; Lovett, A. J. ; Smith, G. D. PCCP 2005, 7, 501. Oleic Acid Languir-Blodgett film Voss, L. F. ; Bazerbashi, M. F. ; Beekman, C. P. ; Hadad, C. M. ; Allen, H. C. J. Geophys. Res. 2007, 112, D 06209 Voss, L. F. ; Hadad, C. M. ; Allen, H. C. J. Phys. Chem. B. 2006, 110, 19487 undec-10 -ene-1 -thiol SAM on gold Fiegland, L. R. ; Mc. Corn Saint Fleur, M. ; Morris, J. R. Langmuir 2005, 21, 2660. 7 -octenyltrichlorosilane, allyltrichlorosilane on silicon Dubowski, Y. ; Vieceli, J. ; Tobias, D. J. ; Gomez, A. ; Lin, A. ; Nizkorodov, S. A. ; Mc. Intire, T. M. ; Finlayson-Pitts, B. J. JPC A 2004, 108, 10473. 10 -undecenoic acid Langmuir-Blodgett film Elisason, T. L. ; Aloisio, A. ; Donaldson, D. J. ; Cziczo, D. J. ; Vaida, V. Atmos. Env. 2003, 37, 2207. Previous studies mainly straight-chain unsaturated systems High variability of reaction probability for terpenes in the gas phase; but chemical variability has not been addressed for heterogeneous reactions.

Diversity-oriented surface functionalization strategies electrophilic/nucleophilic • Address chemical diversity and complexity – study any organic moiety at will • Covalently couple analyte to surface to avoid volatilization • Use a standardized “linker” approach to allow for synthetic versatility Zhang, F. ; Srinivasan, M. P. Langmuir, 2004, 20, 2309 Voges, A. B. ; Al-Abadleh, H. A. ; Musorrafiti, M. J. ; Bertin, P. A. ; Nguyen, S. T. ; Geiger, F. M. J. Phys. Chem. B 2004, 108, 18675. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

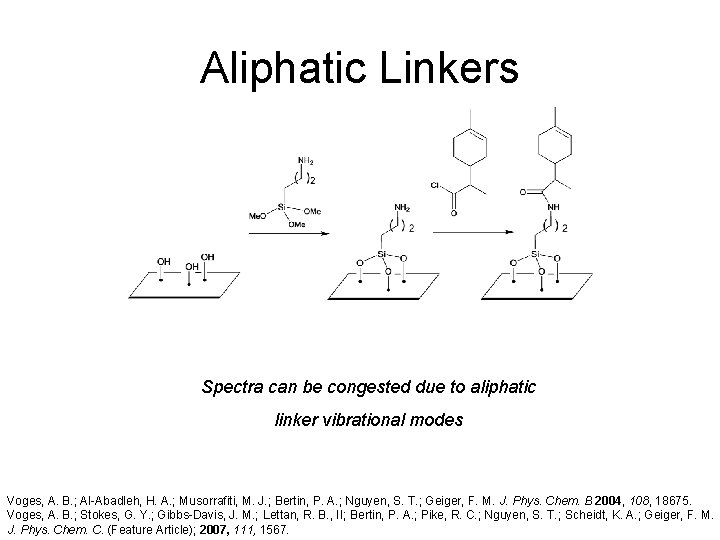
Aliphatic Linkers Spectra can be congested due to aliphatic linker vibrational modes Voges, A. B. ; Al-Abadleh, H. A. ; Musorrafiti, M. J. ; Bertin, P. A. ; Nguyen, S. T. ; Geiger, F. M. J. Phys. Chem. B 2004, 108, 18675. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

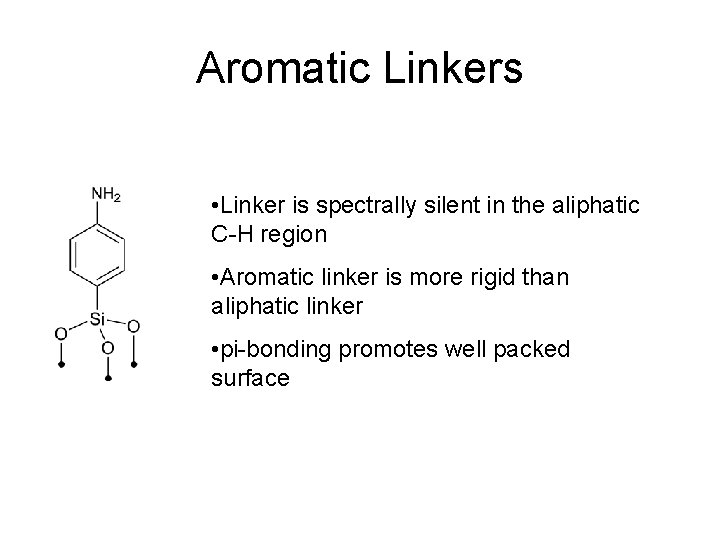
Aromatic Linkers • Linker is spectrally silent in the aliphatic C-H region • Aromatic linker is more rigid than aliphatic linker • pi-bonding promotes well packed surface

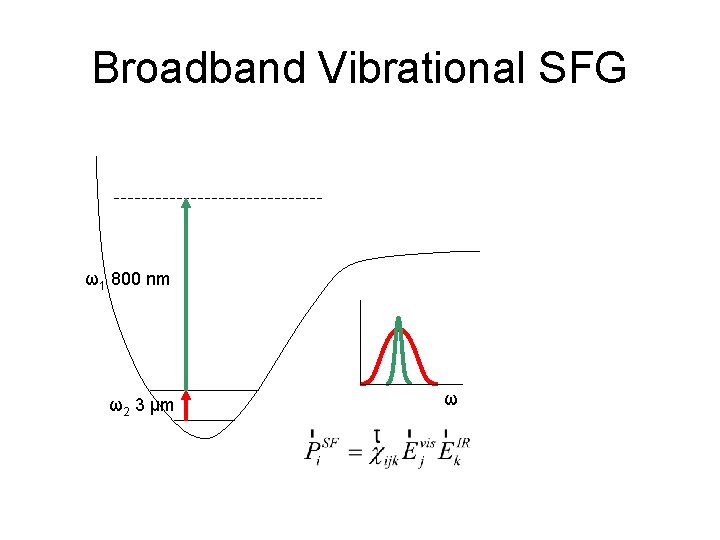
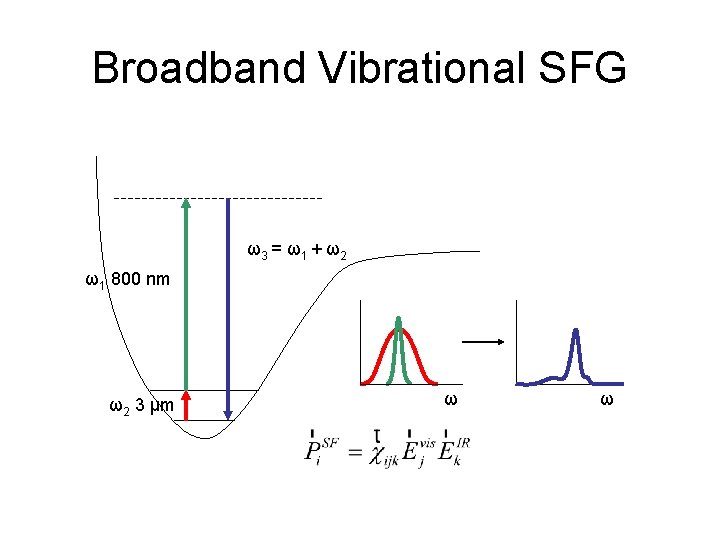
Broadband Vibrational SFG ω3 = ω1 + ω2 ω1 800 nm ω2 3 μm ω ω

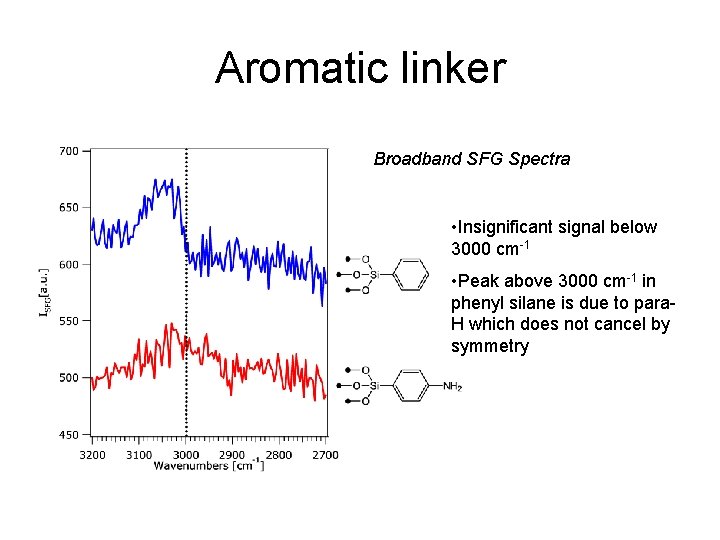
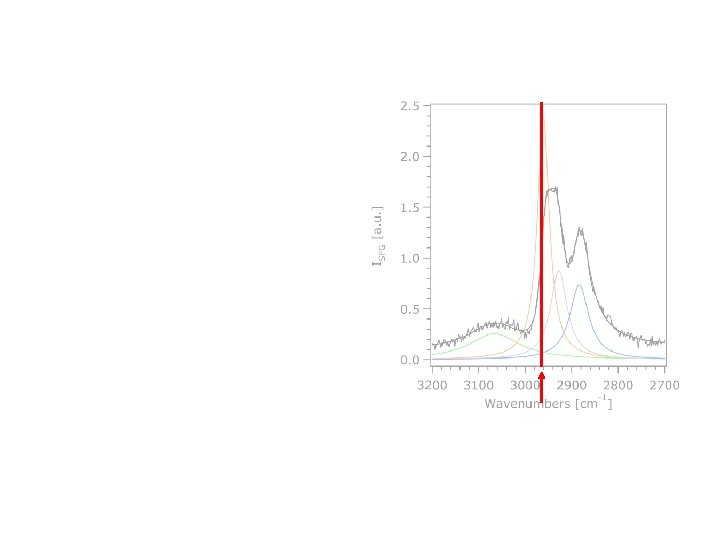
Aromatic linker Broadband SFG Spectra • Insignificant signal below 3000 cm-1 • Peak above 3000 cm-1 in phenyl silane is due to para. H which does not cancel by symmetry

Tailor-made organic surfaces for addressing chemical complexity and chemical diversity 2003 2002 2001 2004 2005 2006 2007

2003 2002 2001 2004 2005 Taylor-made surface functionalization for: Atmospheric chemistry Biochemical sensing Geochemistry Catalysis 2006 Molecular electronics 2007 2006 2007

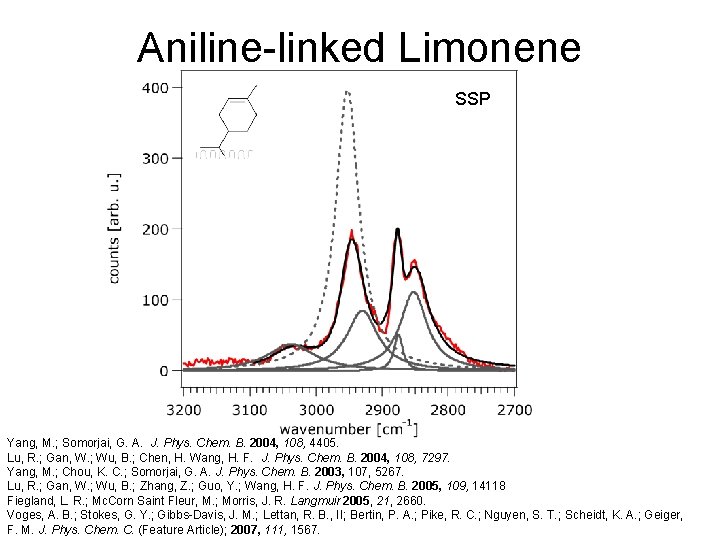
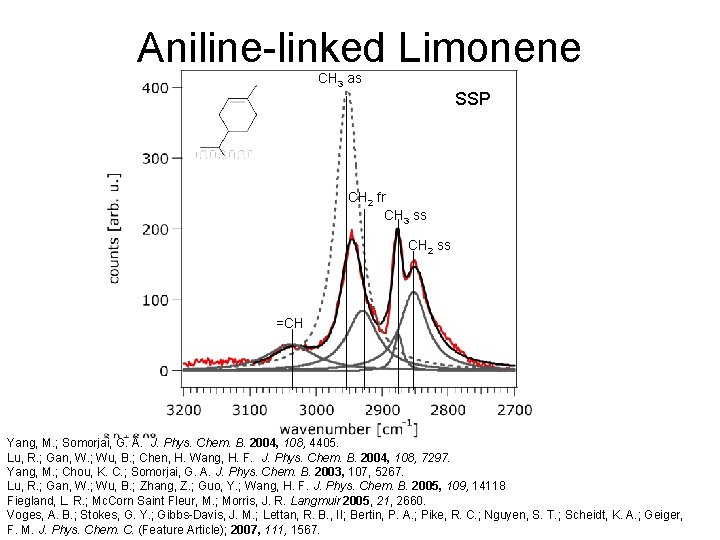
Aniline-linked Limonene SSP

Aniline-linked Limonene SSP

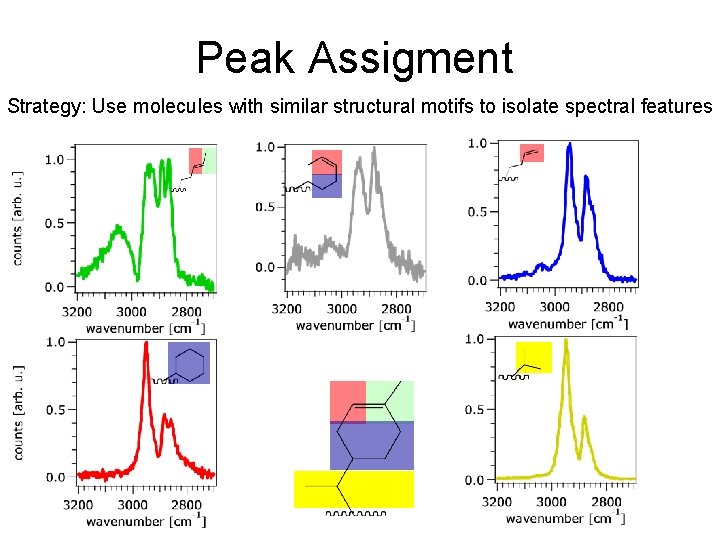
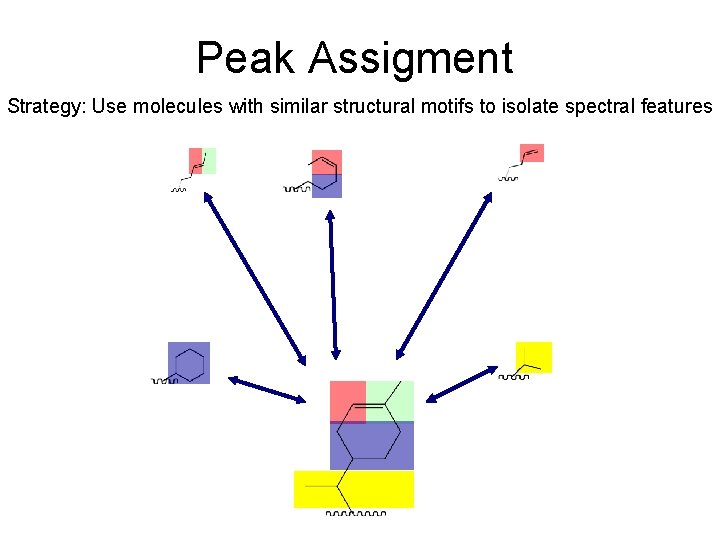
Peak Assigment Strategy: Use molecules with similar structural motifs to isolate spectral features

Aniline-linked Limonene SSP Yang, M. ; Somorjai, G. A. J. Phys. Chem. B. 2004, 108, 4405. Lu, R. ; Gan, W. ; Wu, B. ; Chen, H. Wang, H. F. J. Phys. Chem. B. 2004, 108, 7297. Yang, M. ; Chou, K. C. ; Somorjai, G. A. J. Phys. Chem. B. 2003, 107, 5267. Lu, R. ; Gan, W. ; Wu, B. ; Zhang, Z. ; Guo, Y. ; Wang, H. F. J. Phys. Chem. B. 2005, 109, 14118 Fiegland, L. R. ; Mc. Corn Saint Fleur, M. ; Morris, J. R. Langmuir 2005, 21, 2660. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

Aniline-linked Limonene CH 3 as SSP CH 2 fr CH 3 ss CH 2 ss =CH Yang, M. ; Somorjai, G. A. J. Phys. Chem. B. 2004, 108, 4405. Lu, R. ; Gan, W. ; Wu, B. ; Chen, H. Wang, H. F. J. Phys. Chem. B. 2004, 108, 7297. Yang, M. ; Chou, K. C. ; Somorjai, G. A. J. Phys. Chem. B. 2003, 107, 5267. Lu, R. ; Gan, W. ; Wu, B. ; Zhang, Z. ; Guo, Y. ; Wang, H. F. J. Phys. Chem. B. 2005, 109, 14118 Fiegland, L. R. ; Mc. Corn Saint Fleur, M. ; Morris, J. R. Langmuir 2005, 21, 2660. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

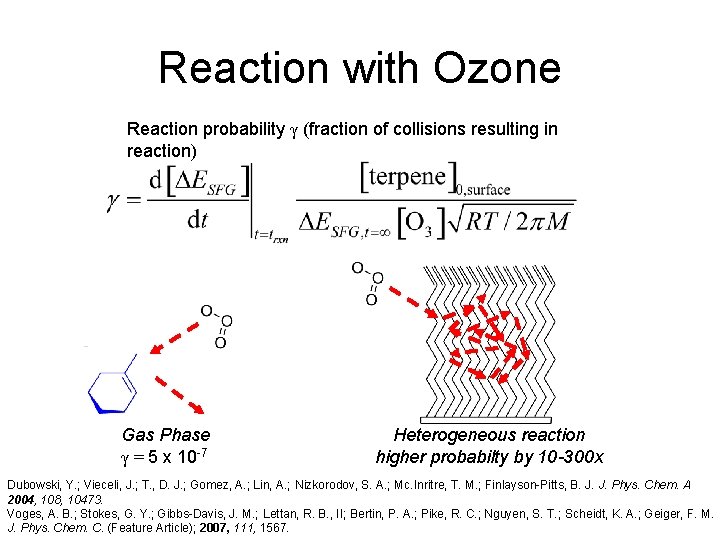
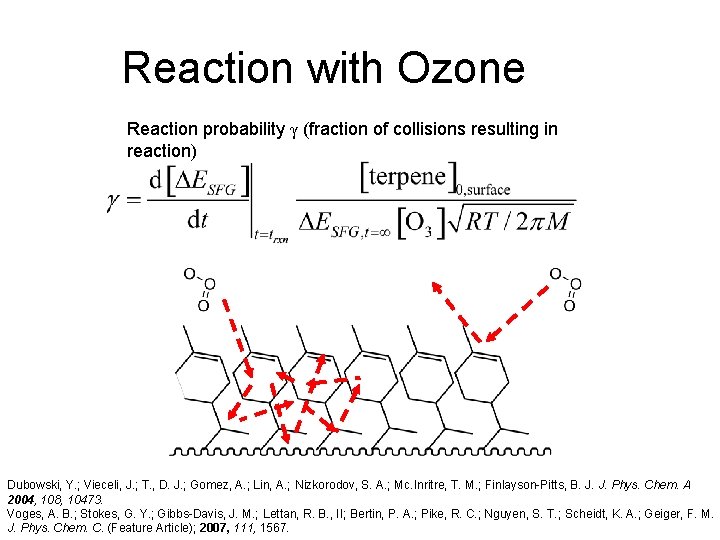
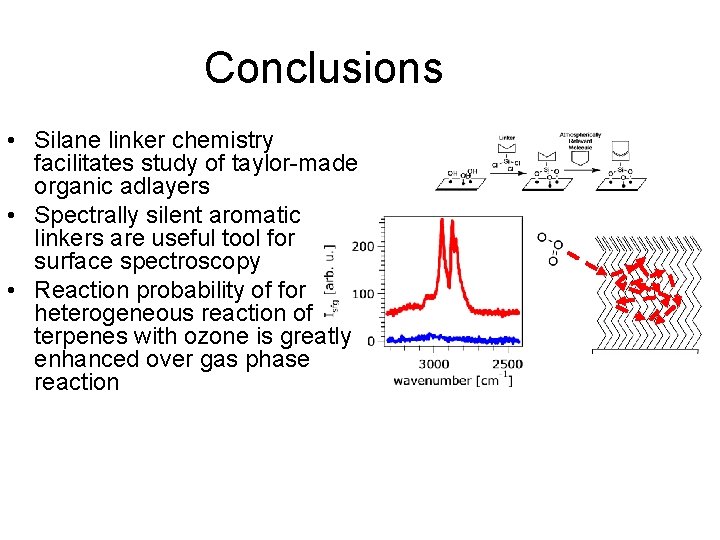
Reaction with Ozone Reaction probability γ (fraction of collisions resulting in reaction) Gas Phase γ = 5 x 10 -7 Heterogeneous reaction higher probabilty by 10 -300 x Dubowski, Y. ; Vieceli, J. ; T. , D. J. ; Gomez, A. ; Lin, A. ; Nizkorodov, S. A. ; Mc. Inritre, T. M. ; Finlayson-Pitts, B. J. J. Phys. Chem. A 2004, 108, 10473. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

Reaction with Ozone Reaction probability γ (fraction of collisions resulting in reaction) Dubowski, Y. ; Vieceli, J. ; T. , D. J. ; Gomez, A. ; Lin, A. ; Nizkorodov, S. A. ; Mc. Inritre, T. M. ; Finlayson-Pitts, B. J. J. Phys. Chem. A 2004, 108, 10473. Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.
![Amide linked terpene 30 ppm ozone DESFG a u CH 3 as Amide -linked terpene + 30 ppm ozone DESFG [a. u. ] CH 3 as](https://slidetodoc.com/presentation_image_h/2c72cc3eefec1ac47ed167f77e23e609/image-30.jpg)
Amide -linked terpene + 30 ppm ozone DESFG [a. u. ] CH 3 as 2961 cm -1 0. 5 0. 0 -0. 5 -1. 0 CH 3 as signal decreases as it changes orientation during reaction -1. 5 -2. 0 0 50 100 150 Time [min] Assume first order kinetics Initial reaction probability γ = 10 -5 Voges, A. B. ; Stokes, G. Y. ; Gibbs-Davis, J. M. ; Lettan, R. B. , II; Bertin, P. A. ; Pike, R. C. ; Nguyen, S. T. ; Scheidt, K. A. ; Geiger, F. M. J. Phys. Chem. C. (Feature Article); 2007, 111, 1567.

Future work – Accessibility Gas Phase Reaction Surface Reaction Determine how accessibility and orientation govern reactivity for various terpenes

Chiral Atmospheric Surface Chemistry? H H O O H In gas phase ozone can collide from any direction, so the reaction is not diasteriomerically specific. H On a surface ozone can only collide from the top and the reaction may be diasteriomerically specific for bound molecules. This specificity will also depend on surface residence time. H H O O H H

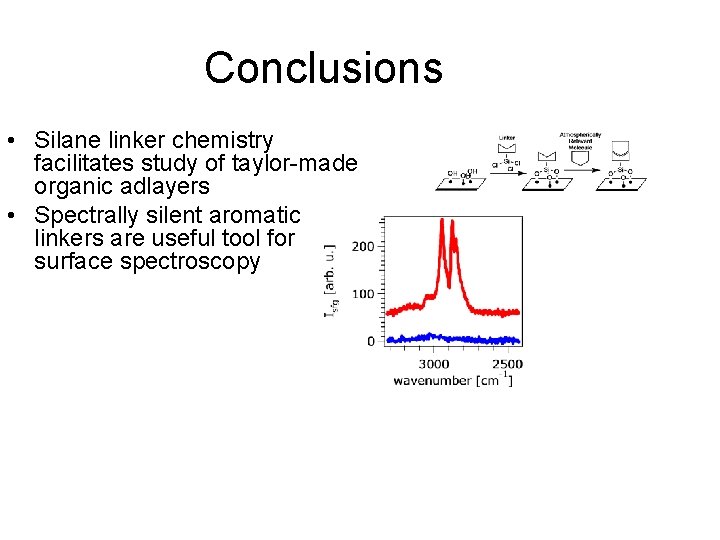
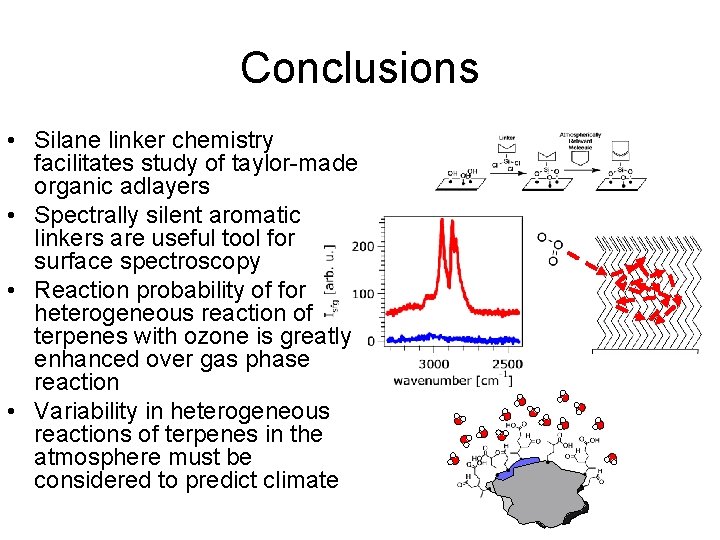
Conclusions • Silane linker chemistry facilitates study of taylor-made organic adlayers • Spectrally silent aromatic linkers are useful tool for surface spectroscopy • Reaction probability of for heterogeneous reaction of terpenes with ozone is greatly enhanced over gas phase reaction • Heterogeneous reactions of terpenes in the atmosphere must be considered in order to accurately predict climate

Conclusions • Silane linker chemistry facilitates study of taylor-made organic adlayers • Spectrally silent aromatic linkers are useful tool for surface spectroscopy • Reaction probability of for heterogeneous reaction of terpenes with ozone is greatly enhanced over gas phase reaction • Heterogeneous reactions of terpenes in the atmosphere must be considered in order to accurately predict climate

Conclusions • Silane linker chemistry facilitates study of taylor-made organic adlayers • Spectrally silent aromatic linkers are useful tool for surface spectroscopy • Reaction probability of for heterogeneous reaction of terpenes with ozone is greatly enhanced over gas phase reaction • Heterogeneous reactions of terpenes in the atmosphere must be considered in order to accurately predict climate

Conclusions • Silane linker chemistry facilitates study of taylor-made organic adlayers • Spectrally silent aromatic linkers are useful tool for surface spectroscopy • Reaction probability of for heterogeneous reaction of terpenes with ozone is greatly enhanced over gas phase reaction • Variability in heterogeneous reactions of terpenes in the atmosphere must be considered to predict climate

Acknowledgements Prof. Franz Geiger Prof. Karl Scheidt Grace Stokes Dr. Julianne Gibbs-Davis Dr. Andrea Voges Funding: NASA Earth and Space Sciences Predoctoral Fellowship (GYS) NASA Earth Systems Sciences Predoctoral Fellowship (ABV) Camille & Henry Dreyfus Postdoctoral Fellowship in Environmental Chem. (JGD) Sloan Fellowship (FMG) NSF EAR Atmospheric Sciences Program DOE BES Geochemistry Program Northwestern University International Institute for Nanotechnology Northwestern University Institute for Catalysis and Energy Processes



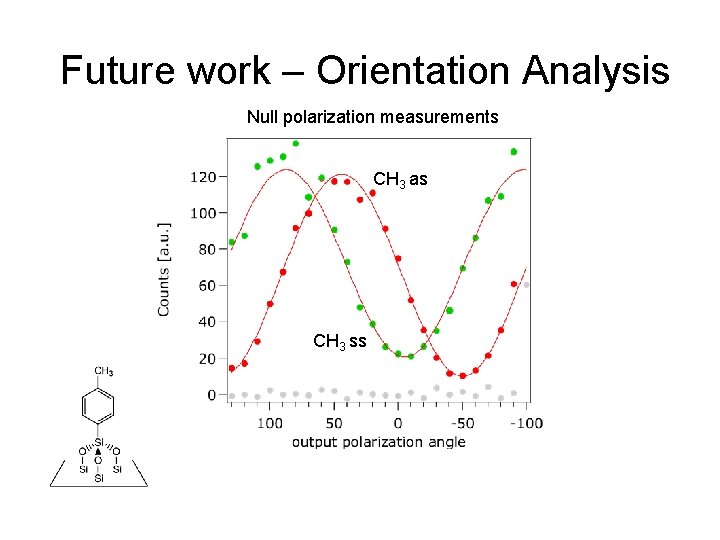
Future work – Orientation Analysis Null polarization measurements CH 3 as CH 3 ss

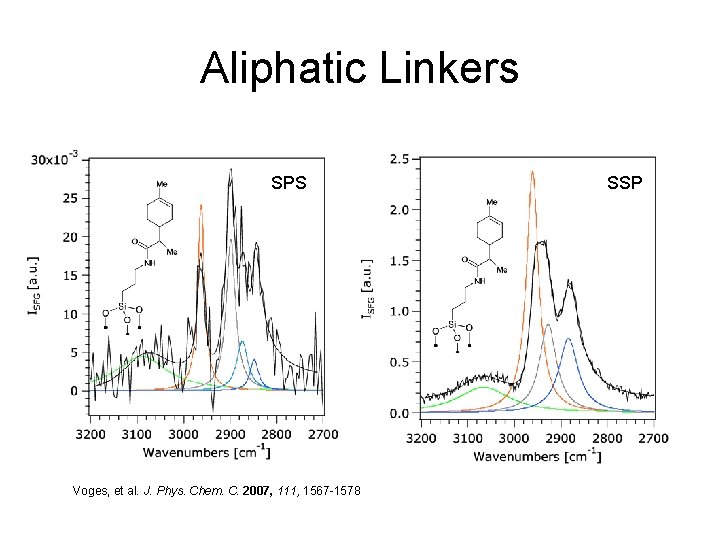
Aliphatic Linkers SPS Voges, et al. J. Phys. Chem. C. 2007, 111, 1567 -1578 SSP

Aliphatic Linkers CH 3 as CH 2 fr CH 2 ss (linker) CH 2 fr CH 2 ss (ring) CH 2 ss

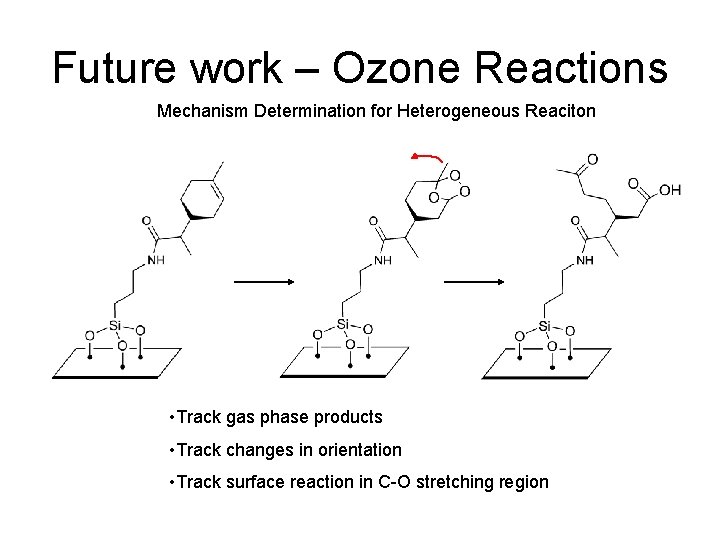
Future work – Ozone Reactions Mechanism Determination for Heterogeneous Reaciton • Track gas phase products • Track changes in orientation • Track surface reaction in C-O stretching region


Peak Assigment Strategy: Use molecules with similar structural motifs to isolate spectral features

Aromatic Linkers

Broadband Vibrational SFG ω2 3 μm ω

Broadband Vibrational SFG ω1 800 nm ω2 3 μm ω

Broadband Vibrational SFG ω3 = ω1 + ω2 ω1 800 nm ω2 3 μm ω ω
 The splitting of a mineral along smooth flat surfaces
The splitting of a mineral along smooth flat surfaces Aerosols is pressurised dosage form
Aerosols is pressurised dosage form Types of aerosols
Types of aerosols Kontinuitetshantering
Kontinuitetshantering Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidböcker
Tidböcker Sura för anatom
Sura för anatom Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Hur skriver man en debattartikel
Hur skriver man en debattartikel Autokratiskt ledarskap
Autokratiskt ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Publik sektor
Publik sektor Bo bergman jag fryser om dina händer
Bo bergman jag fryser om dina händer Presentera för publik crossboss
Presentera för publik crossboss Argument för teckenspråk som minoritetsspråk
Argument för teckenspråk som minoritetsspråk Vem räknas som jude
Vem räknas som jude Treserva lathund
Treserva lathund Epiteltyper
Epiteltyper Bästa kameran för astrofoto
Bästa kameran för astrofoto Cks
Cks Lågenergihus nyproduktion
Lågenergihus nyproduktion Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referat mall
Referat mall Redogör för vad psykologi är
Redogör för vad psykologi är Stål för stötfångarsystem
Stål för stötfångarsystem Tack för att ni har lyssnat
Tack för att ni har lyssnat Borra hål för knoppar
Borra hål för knoppar Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Variansen formel
Variansen formel Tack för att ni har lyssnat
Tack för att ni har lyssnat Steg för steg rita
Steg för steg rita Ledningssystem för verksamhetsinformation
Ledningssystem för verksamhetsinformation Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling effekt
Toppslätskivling effekt Mästar lärling modellen
Mästar lärling modellen Egg för emanuel
Egg för emanuel