MINERAL CLASSIFICATION AND RELATED CONCEPTS Mineral Classification Mineral




















- Slides: 25

MINERAL CLASSIFICATION AND RELATED CONCEPTS

• Mineral Classification • Mineral Classes • minerals are divided into classes based on chemical composition and primarily on the main anion, anionic complex or oxyacid anion, or lack of an anion present--the main mineral classes are: • native elements ( monoelemental composition--lack of anion) • sulfides, sulfarsenides, sulfosalts ( main anion is S-2 or As-3) • oxides ( main anion is O-2) • hydroxides ( main anion complex is OH-1) • halides (main anion is Cl-1, F-1, Br-1 or I-1) • carbonates ( main anion is the oxyacid anion, CO 3 -3)

• borates ( the oxyacid anion, Bx. Oy-z) • nitrates ( the oxyacid anion, NO 3 -1) • phosphates ( oxyacid anion, PO 4 -3) • sulfates ( the oxyacid anion, SO 4 -2) • tungstates ( the oxyacid anion, WO 4 -2) • silicates ( the oxyacid anion, Six. Oy-z) • Mineral subclasses • some classes are subdivided based on special chemistry or structural features • native element subclasses • native metals--minerals with metallic bonds • native semimetals--those with primarily semimetallic bonds

• silicate subclasses--based on linkage structure of the silica tetrahedra • neso-, soro-, cyclo-, ino-, phylo-, tectosilicates) • Mineral Groups • classes or subclasses can be divided based on atomic structure and similar chemistry--examples are isomorphic (isostructural) groups or polymorphic groups • 1. isomorphic group • an assemblage of minerals with the same atomic structure but different chemical formulas • the equivalent elements in different minerals in the isomorphic group have the same CN

• Fe. CO 3 (siderite) and Ca. CO 3(calcite) belong to the same isomorphic group in the carbonate class because according to Paulings rules, both have 6 O around each Fe and Ca, 3 O around each C and 1 C and 2 Fe or Ca around each O • often isomorphs have basically the same or similar chemical, physical and crystallographic properties • some examples are: 1. in the oxide class--hematite group, spinel group, and rutile group; 2. in the carbonate class--calcite group and aragonite group; 3. in the sulfate class--barite group; 4. in the silicate subclasses--(nesosilicates)--garnet group etc.

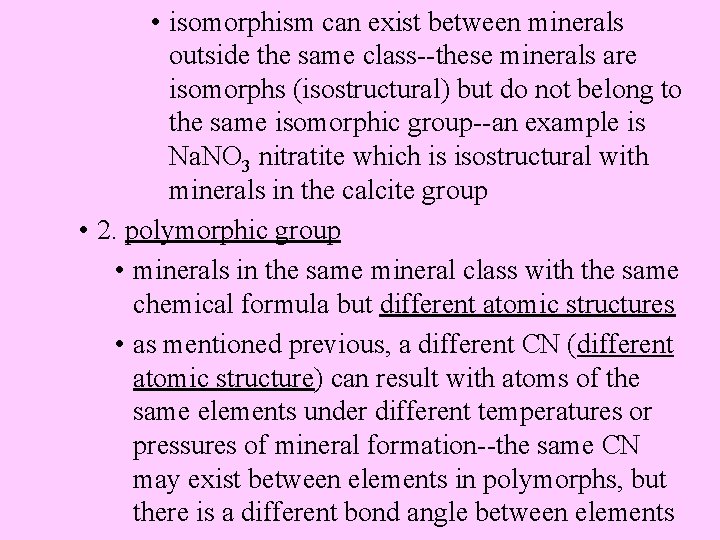
• isomorphism can exist between minerals outside the same class--these minerals are isomorphs (isostructural) but do not belong to the same isomorphic group--an example is Na. NO 3 nitratite which is isostructural with minerals in the calcite group • 2. polymorphic group • minerals in the same mineral class with the same chemical formula but different atomic structures • as mentioned previous, a different CN (different atomic structure) can result with atoms of the same elements under different temperatures or pressures of mineral formation--the same CN may exist between elements in polymorphs, but there is a different bond angle between elements

• the difference in atomic structures will often result in polymorphs forming in different crystal systems or crystal classes in the same crystal system • examples of some polymorphs are: • calcite and aragonite--Ca. CO 3 --calcite is hexagonal and aragonite is orthorhombic • pyrite and marcasite--Fe. S 2 --pyrite forms at a high temperature and is isometric while marcasite forms at a low temperature and is orthorhombic • quartz, tridymite , cristobalite, stishovite and coesite--Si. O 2 --quartz = low temp form and hexagonal, cristobalite = high temp form and tetragonal

• coesite = stable (forms) at very high pressures is monoclinic and is associated with meteor impact; stishovite = associated with rocks from Mars • kinds of polymorphism • 1. based on reversible vs irreversible changes • reversible (enantiotropic)--quartz = tridymite equilibrium at 867 degrees and 1 atm. Or graphite = diamond • irreversible (monotropy)--marcasite = pyrite but not pyrite = marcasite • 2. based on change or reconstitution nature of the atomic structure

• reconstructive change--during the change there is the breaking of atomic bonds and a reassembling of structural units--involves a lot of energy and change is not readily reversed and is sluggish--quartz = tridymite = cristobalite • displacive change--in the change, atomic bonds are not broken and the original structure is maintained--only a slight displacement of atoms results in different bond angles between atoms-instantaneous change involving little energy-high quartz = low quartz

• ordered-disordered change--in which the more disordered form will have more symmetry-microcline = ordered and orthoclase = disordered form • other groupings • minerals grouped based on the same general or empirical formula such as the pyroxenes (XYZ 2 O 6), amphiboles (W 0 -1 X 2 Y 5 Z 8 O 22(OH, F)2), and micas • Mineral Series • classes and groups can be subdivided into mineral series in which solid solution is most prominently displayed

• solid solution series • different specimens of the same mineral can vary in composition in which each specimen is comprised of a mixture of “end member minerals” caused by ionic substitution (proxying) between some cations in the “end members” during mineral formation • the degree of proxying of elements into a mineral structure depends largely on the temperature of formation of the mineral • Plagioclase series • end members are Ca. Al 2 Si 2 O 8 (anorthite)(An) and Na. Al. Si 3 O 8 (albite)(Ab) where there is proxying between Na and Ca, and Al and Si

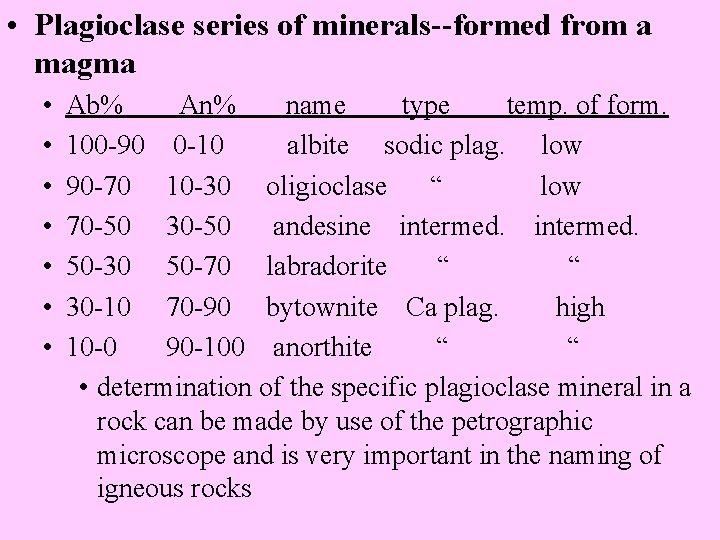
• Plagioclase series of minerals--formed from a magma • • Ab% An% name type temp. of form. 100 -90 0 -10 albite sodic plag. low 90 -70 10 -30 oligioclase “ low 70 -50 30 -50 andesine intermed. 50 -30 50 -70 labradorite “ “ 30 -10 70 -90 bytownite Ca plag. high 10 -0 90 -100 anorthite “ “ • determination of the specific plagioclase mineral in a rock can be made by use of the petrographic microscope and is very important in the naming of igneous rocks

• Olivine series--(Fe, Mg)2 Si. O 4 • end members are fayalite, Fe 2 Si. O 4 and forsterite, Mg 2 Si. O 4 in which there is proxying between Fe and Mg concentrations as a function of temperature • Enstatite-ferosilite series--(Fe, Mg) Si. O 3 • end members are enstatite, Mg. Si. O 3 and ferosilite, Fe. Si. O 3 in which Fe and Mg concentrations proxy as a function of temperature of mineral formation

• Mineral Varieties • 1. Chemical rich minerals • are those which can be expressed by an adjective denoting an unusual large amounts of chemical constituents

• some examples of modifiers are: • aluminian = Al-rich • calcian = Ca-rich • chromian = Cr-rich • ferroan = Fe+2 -rich, ferrian = Fe+3 -rich • magnesian = Mg-rich • manganoan = Mn-rich • examples of specific mineral variety names are: manganoan aegerine, ferrian diopside or magnesian augite • 2. Crystalline variety types • based on crystal size, color or appearance as in quartz

Coarsely crystalline quartz varieties rock crystals--colorless amethyst--purple rose quartz--rose

Coarsely crystalline quartz varieties smoky quartz--smoky yellow to brown citrine-- clear yellow rutilated quartz-with rutile inclusions

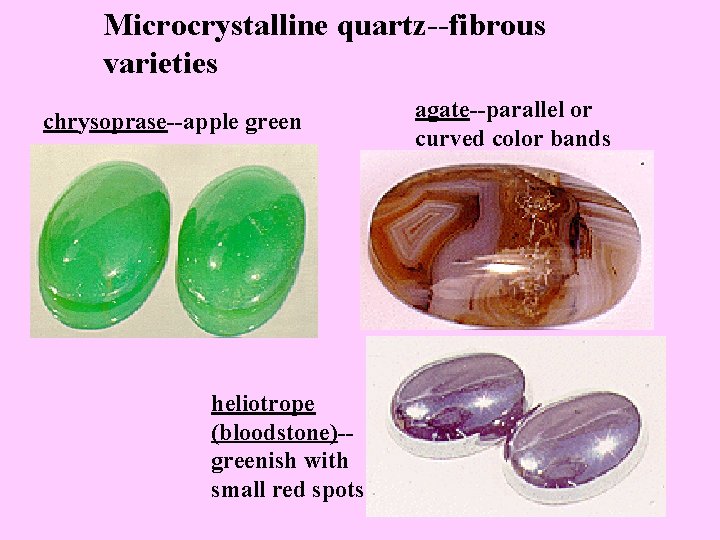
Microcrystalline quartz--fibrous varieties chrysoprase--apple green heliotrope (bloodstone)-greenish with small red spots agate--parallel or curved color bands

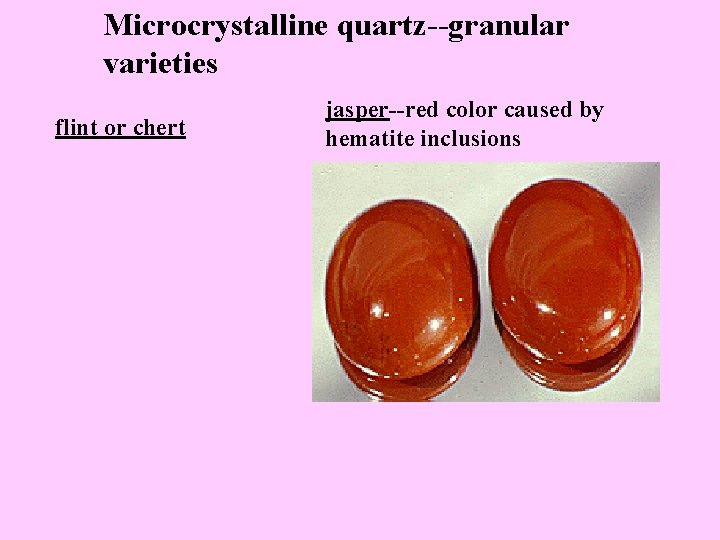
Microcrystalline quartz--granular varieties flint or chert jasper--red color caused by hematite inclusions

Related Topics • Exsolution • the association of similar composition minerals formed instantaneously from a cooling magma resulting in stringers of one mineral appearing in the other more abundant mineral • this occurs if both minerals have equivalent cations close to ionic substitution at high temperature but not at the actual solidification temperature of both minerals • examples are perthites and antiperthites ( macro-, microand crypto-) • perthite is albite (Ab, Na. Al. Si 3 O 8) stringers in orthoclase (Or, KAl. Si 3 O 8) • antiperthite is Or stringers in Ab

• Pseudomorphism • the shape of mineral B displayed by mineral A--the existance of a mineral with the outward form of another • a false form • kinds of pseudomorphism • 1. encrustation • one mineral is deposited over crystals of another as quartz (a hexagonal mineral) encrusting cubes of fluorite (isometric mineral)--the more solubilized fluorite can be dissolved leaving a cast of its form in the quartz • 2. alteration • a mineral can be chemically altered (weathered)

• to result in an external layer of another mineral on the weathered mineral • the weathering or alteration of anhydrite, Ca. SO 4 to gypsum, Ca. SO 4 • H 2 O; galena, Pb. S to anglesite, Pb. SO 4; pyrite, Fe. S 2 or siderite, Fe. CO 3 to limonite, Fe. O(OH) • n. H 2 O • in the above examples gypsum is said to be a pseudomorph after anhydrite, anglesite the same after galena and limonite the same after pyrite or siderite • 3. substitution (replacement) • involves a gradual removal of the original mineral and a simultaneous molecule for molecule replacement by another • silica replacing fluorite; silica replacement wood

• Mineraloids (Gels) • substances resembling minerals but have no ordered atomic arrangement • cannot belong to any of the crystal classes of the crystal systems because they are amorphous • are usually products of chemical weathering and are often in mammillary, botryoidal, or stalactitic masses as opal or limonite • Metamict Structure • minerals whose atomic structure has been altered by radiation • the original ordered atomic arrangement may be restored by exposure to elevated temperatures with an emission of light causing the mineral to appear incandescent

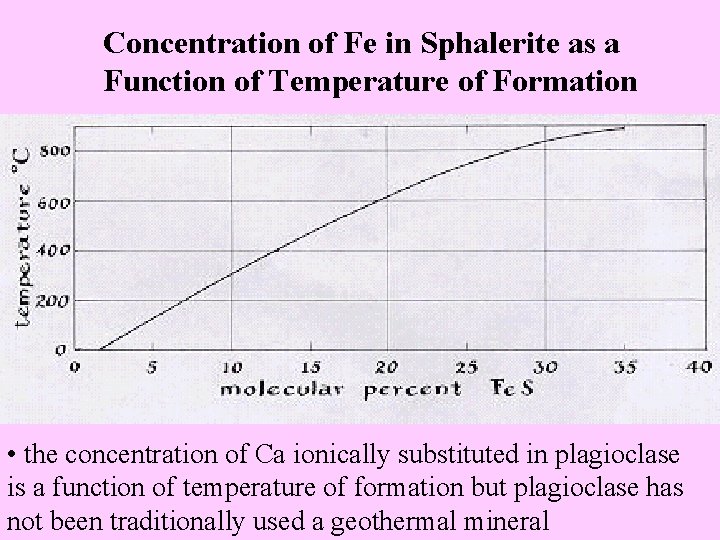
• examples are some specimens of allanite and zircon • Geologic Thermometry ( Geothermometry) • is the determination of the temperature of formation of a mineral and associated minerals by knowing the concentration of proxyed element in a mineral through ionic substitution • the temperature of formation of sphalerite and associated minerals occurring with it can be established by determining the concentration of ionically substituted Fe in the mineral, (Fe, Zn)S • the darker or higher red color of sphalerite indicates a higher Fe concentration while a clear or yellow color represents a lower Fe and higher Zn concentration • the following table explains the above

Concentration of Fe in Sphalerite as a Function of Temperature of Formation • the concentration of Ca ionically substituted in plagioclase is a function of temperature of formation but plagioclase has not been traditionally used a geothermal mineral
 Two types of fitness components
Two types of fitness components Health-related skill
Health-related skill Interpersonal attraction
Interpersonal attraction Principles of mineral processing
Principles of mineral processing Basic concepts in data classification
Basic concepts in data classification Basic concepts of classification in data mining
Basic concepts of classification in data mining Data and process modeling
Data and process modeling Describe data and process modeling concepts and tools
Describe data and process modeling concepts and tools Mineral resources and petroleum authority of mongolia
Mineral resources and petroleum authority of mongolia Metamorphic
Metamorphic Mineral resources and mining chapter 13
Mineral resources and mining chapter 13 Preparation of emulsion
Preparation of emulsion Mineral exploration and mining active reading
Mineral exploration and mining active reading Portfolio committee on mineral resources and energy
Portfolio committee on mineral resources and energy Criterion related validity predictive and concurrent
Criterion related validity predictive and concurrent Bipolar and other related disorders
Bipolar and other related disorders Bipolar and other related disorders
Bipolar and other related disorders Sixth commandment catholic
Sixth commandment catholic The problem of concept drift: definitions and related work
The problem of concept drift: definitions and related work Competitive advantage in diversified companies
Competitive advantage in diversified companies Are thermal and electrical conductivity related
Are thermal and electrical conductivity related Performance related pay advantages and disadvantages essay
Performance related pay advantages and disadvantages essay Neurotic stress-related and somatoform disorders
Neurotic stress-related and somatoform disorders National programs
National programs National child policy 1974
National child policy 1974 How are weight and gravity related
How are weight and gravity related