Minerals Chapter 3 1 What is a mineral


















- Slides: 20

Minerals Chapter 3

1. What is a mineral? A. A naturally formed, inorganic (nonliving) solid that has a definite crystalline structure. B. Examples: halite (table salt), graphite (pencil lead), aluminum, copper and iron (pots and pans).

Identifying Minerals A. B. C. D. E. F. G. Color Luster Streak Cleavage and fracture Hardness Density We identify minerals by looking at their physical properties.

2. Mineral Color A. Minerals can be identified by color. B. BUT, sometimes they can change color if exposed to water, air or heat. C. EXAMPLES: Quartz – rock crystal (colorless), smoky quartz (brown), citrine (yellow), amethyst (violet), rose quartz (pink)

Quartz Examples

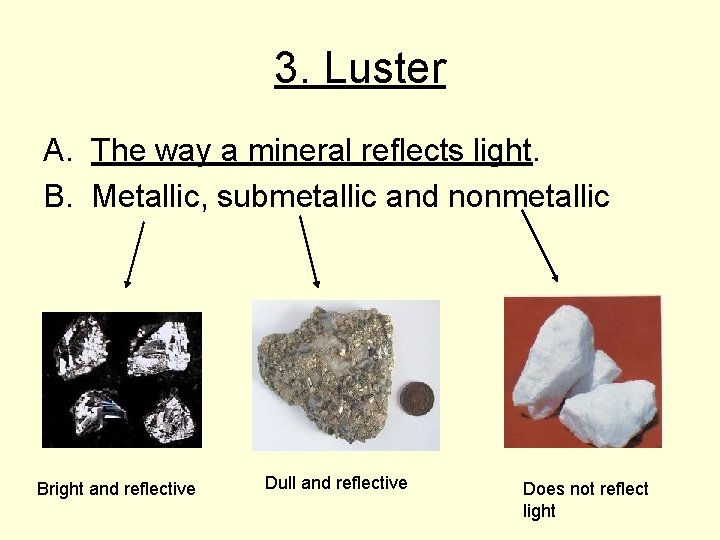
3. Luster A. The way a mineral reflects light. B. Metallic, submetallic and nonmetallic Bright and reflective Dull and reflective Does not reflect light

4. Streak A. The color of the powder of a mineral.

5. Cleavage A. The splitting of a mineral along smooth, flat surfaces.

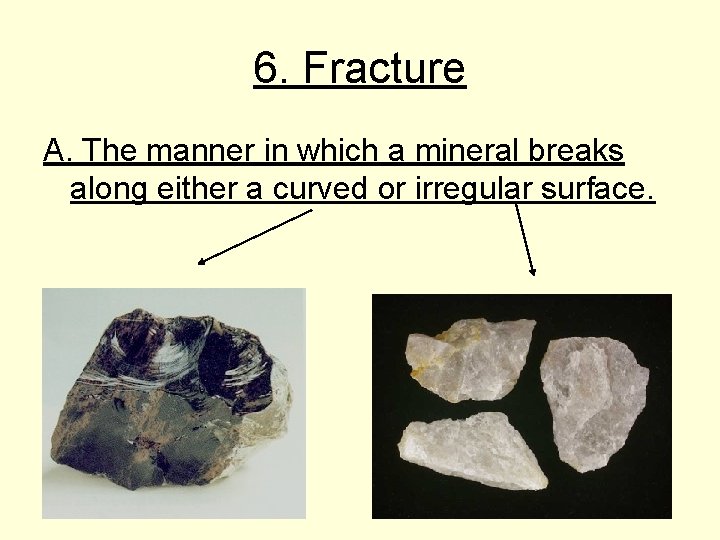
6. Fracture A. The manner in which a mineral breaks along either a curved or irregular surface.

7. Hardness A. The measure of the ability of a mineral to resist scratching. B. Moh’s Hardness Scale determines hardness of minerals. C. Scale 1 -10: 1 being the softest (Talc) and 10 being the hardest (Diamond)


Special Properties of Minerals A. Some minerals have very unique characteristics that make them easily identifiable. B. Fluorescence C. Chemical Reaction D. Optical Properties E. Magnetism F. Taste G. Radioactivity

8. Fluorescence A. Fluorescence is a phenomenon that causes a substance to "glow".

9. Chemical Reaction A. When weak acid is placed on minerals they fizz or become bubbly.

10. Optical Properties A. Placing the mineral over an image causes the image to change.

11. Magnetism A. Mineral is a natural magnet.

12. Taste A. Halite has a salty taste. Sodium chloride, also known as salt, common salt, table salt, or halite, is an ionic compound with the formula Na. Cl. It has over 14, 000 uses, and is probably used in greater quantities and for more applications than any other chemical.

13. Radioactivity A. Minerals that contain radium or uranium can be detected with a Geiger Counter.

What is radioactivity? • Radioactivity is the spontaneous emission of energy from unstable atoms. • Atoms are found in all natural matter. • There are stable atoms, which remain the same forever, and unstable atoms, which break down or 'decay' into new atoms. • These unstable atoms are said to be 'radioactive', because they emit radioactivity from the nucleus as they decay.

Links • http: //mjksciteachingideas. com/minerals. ht ml