Multiple Myeloma Treatment Options for Newly Diagnosed The





- Slides: 51

Multiple Myeloma Treatment Options for Newly Diagnosed The American Perspective Rafat Abonour, M. D.

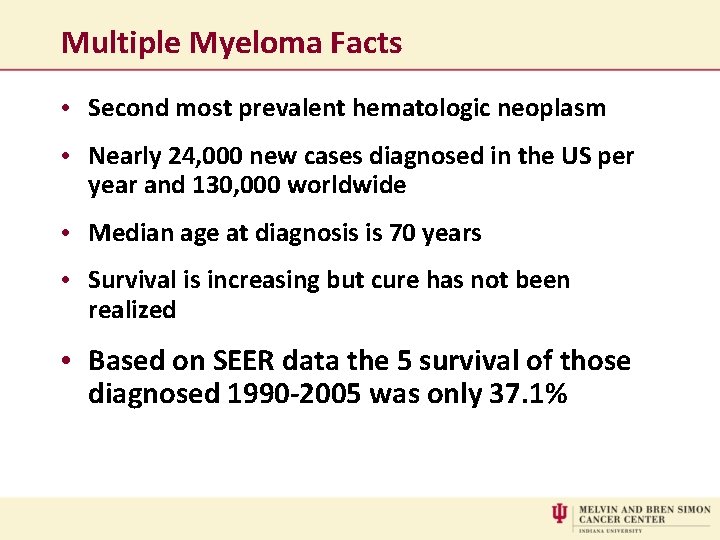
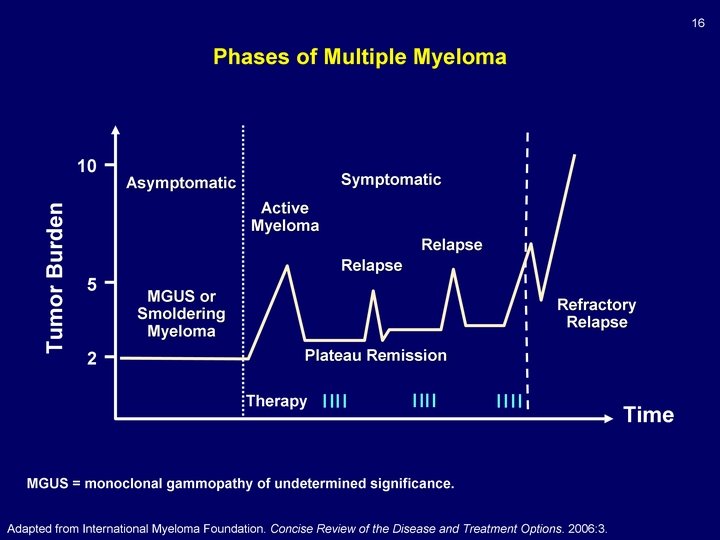
Multiple Myeloma Facts • Second most prevalent hematologic neoplasm • Nearly 24, 000 new cases diagnosed in the US per year and 130, 000 worldwide • Median age at diagnosis is 70 years • Survival is increasing but cure has not been realized • Based on SEER data the 5 survival of those diagnosed 1990 -2005 was only 37. 1%

Multiple Myeloma The First Recognized case Sarah Newberry, 1844. Fractures of femurs and right humerus BJH, 2000, 111, 1035 -44

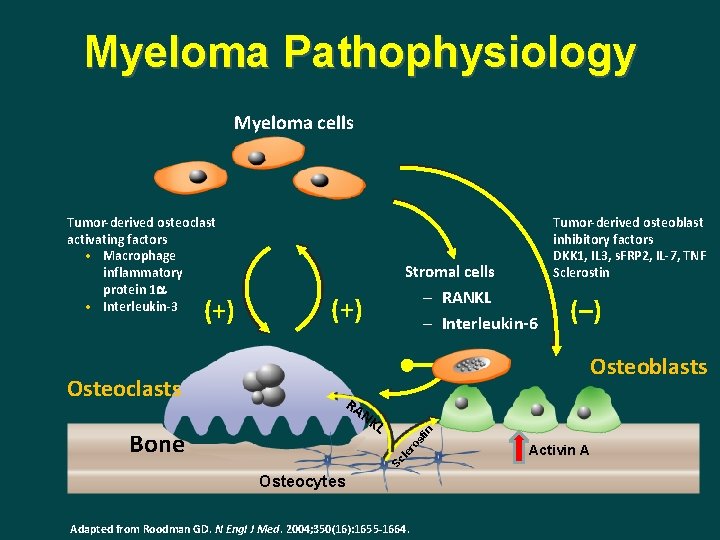
Myeloma Pathophysiology Myeloma cells (–) Osteoblasts RA NK in L st Bone (+) ro Osteoclasts – RANKL – Interleukin-6 le (+) Tumor-derived osteoblast inhibitory factors DKK 1, IL 3, s. FRP 2, IL-7, TNF Sclerostin Stromal cells Sc Tumor-derived osteoclast activating factors • Macrophage inflammatory protein 1 a • Interleukin-3 Osteocytes Adapted from Roodman GD. N Engl J Med. 2004; 350(16): 1655 -1664. Activin A


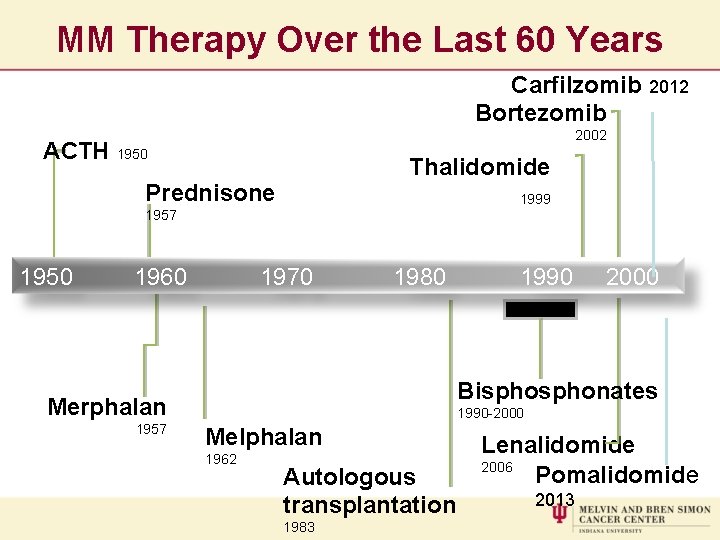
MM Therapy Over the Last 60 Years Carfilzomib 2012 Bortezomib 2002 ACTH 1950 Thalidomide Prednisone 1999 1957 1950 1960 1970 1980 1990 2000 Bisphonates Merphalan 1957 1990 -2000 Melphalan 1962 Autologous transplantation 1983 Lenalidomide 2006 Pomalidomide 2013

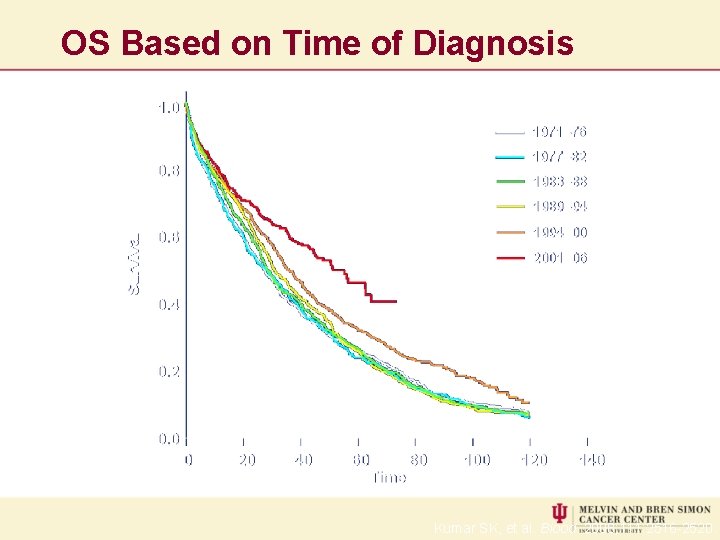
OS Based on Time of Diagnosis Kumar SK, et al. Blood. 2008; 111: 2516 -2520.

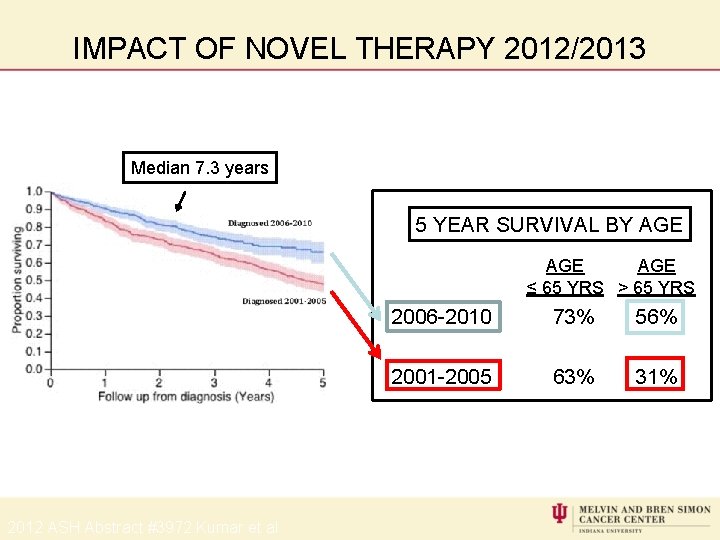
IMPACT OF NOVEL THERAPY 2012/2013 Median 7. 3 years 5 YEAR SURVIVAL BY AGE AGE ≤ 65 YRS > 65 YRS 2012 ASH Abstract #3972 Kumar et al 2006 -2010 73% 56% 2001 -2005 63% 31%

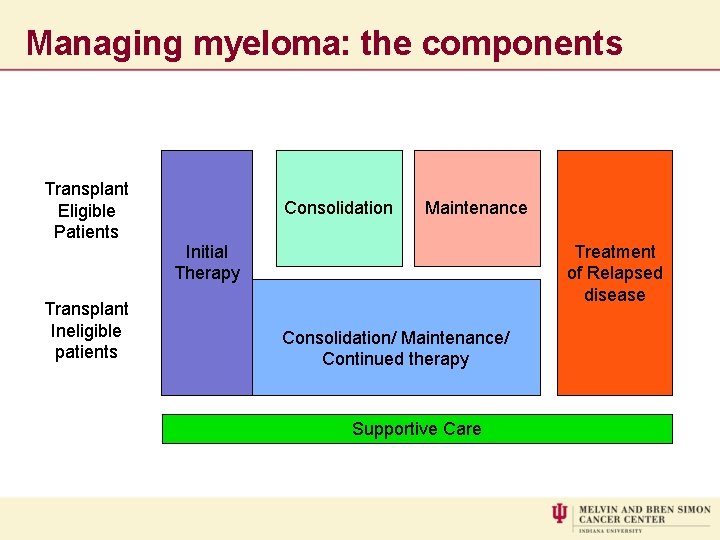
Managing myeloma: the components Transplant Eligible Patients Transplant Ineligible patients Consolidation Maintenance Initial Therapy Treatment of Relapsed disease Consolidation/ Maintenance/ Continued therapy Supportive Care

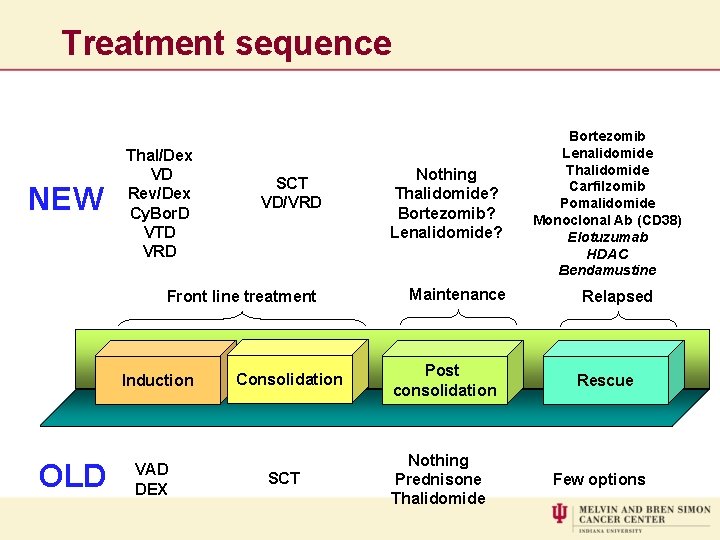
Treatment sequence NEW Thal/Dex VD Rev/Dex Cy. Bor. D VTD VRD SCT VD/VRD Front line treatment Induction OLD VAD DEX Consolidation SCT Nothing Thalidomide? Bortezomib? Lenalidomide? Maintenance Post consolidation Nothing Prednisone Thalidomide Bortezomib Lenalidomide Thalidomide Carfilzomib Pomalidomide Monoclonal Ab (CD 38) Elotuzumab HDAC Bendamustine Relapsed Rescue Few options

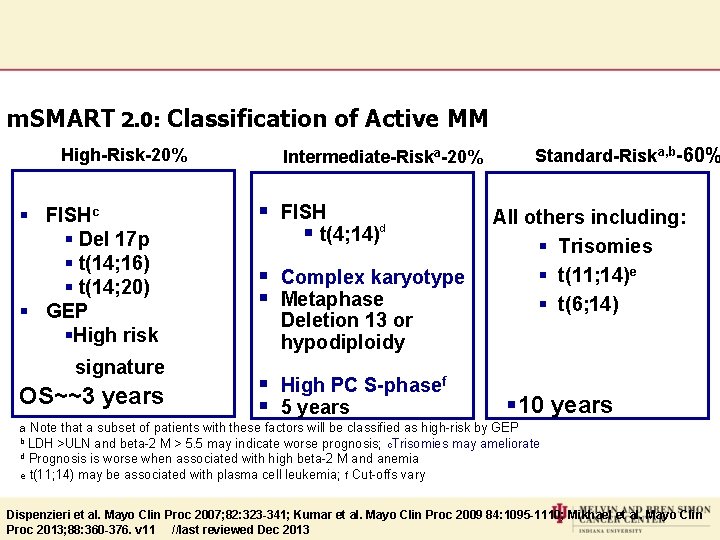
m. SMART 2. 0: Classification of Active MM High-Risk-20% § FISHc § Del 17 p § t(14; 16) § t(14; 20) § GEP §High risk signature OS~~3 years Standard-Riska, b-60% Intermediate-Riska-20% § FISH § t(4; 14)d § Complex karyotype § Metaphase Deletion 13 or hypodiploidy § High PC S-phasef § 5 years All others including: § Trisomies § t(11; 14)e § t(6; 14) § 10 years a Note that a subset of patients with these factors will be classified as high-risk by GEP b LDH >ULN and beta-2 M > 5. 5 may indicate worse prognosis; c. Trisomies may ameliorate d Prognosis is worse when associated with high beta-2 M and anemia e t(11; 14) may be associated with plasma cell leukemia; f Cut-offs vary Dispenzieri et al. Mayo Clin Proc 2007; 82: 323 -341; Kumar et al. Mayo Clin Proc 2009 84: 1095 -1110; Mikhael et al. Mayo Clin Proc 2013; 88: 360 -376. v 11 //last reviewed Dec 2013

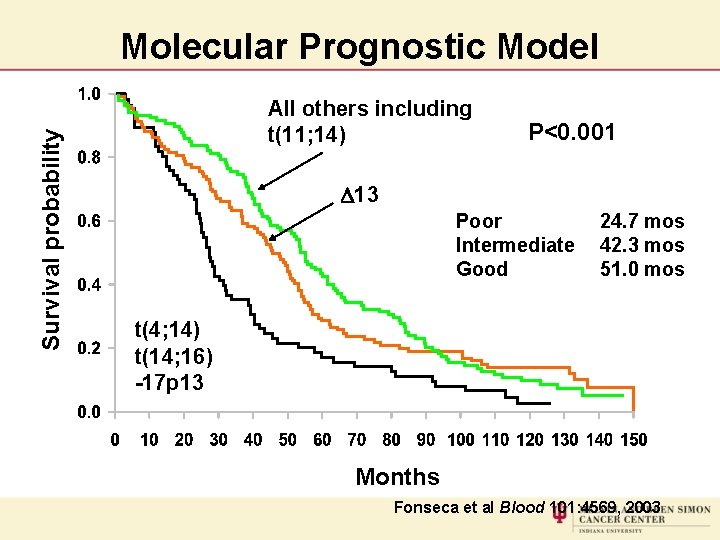
Survival probability Molecular Prognostic Model All others including t(11; 14) P<0. 001 D 13 Poor Intermediate Good 24. 7 mos 42. 3 mos 51. 0 mos t(4; 14) t(14; 16) -17 p 13 Months Fonseca et al Blood 101: 4569, 2003

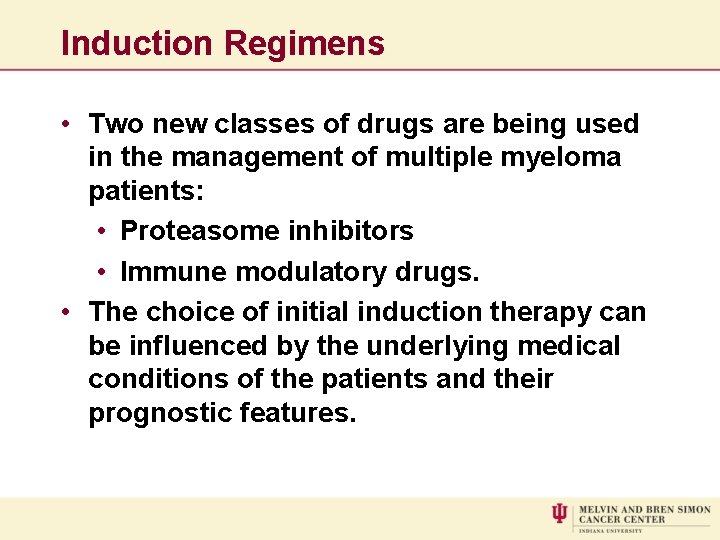
Induction Regimens • Two new classes of drugs are being used in the management of multiple myeloma patients: • Proteasome inhibitors • Immune modulatory drugs. • The choice of initial induction therapy can be influenced by the underlying medical conditions of the patients and their prognostic features.

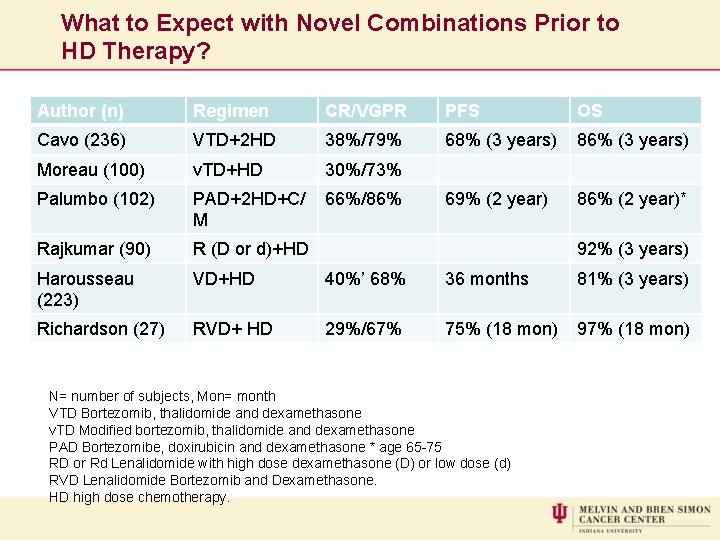
What to Expect with Novel Combinations Prior to HD Therapy? Author (n) Regimen CR/VGPR PFS OS Cavo (236) VTD+2 HD 38%/79% 68% (3 years) 86% (3 years) Moreau (100) v. TD+HD 30%/73% Palumbo (102) PAD+2 HD+C/ M 66%/86% 69% (2 year) 86% (2 year)* Rajkumar (90) R (D or d)+HD Harousseau (223) VD+HD 40%’ 68% 36 months 81% (3 years) Richardson (27) RVD+ HD 29%/67% 75% (18 mon) 97% (18 mon) 92% (3 years) N= number of subjects, Mon= month VTD Bortezomib, thalidomide and dexamethasone v. TD Modified bortezomib, thalidomide and dexamethasone PAD Bortezomibe, doxirubicin and dexamethasone * age 65 -75 RD or Rd Lenalidomide with high dose dexamethasone (D) or low dose (d) RVD Lenalidomide Bortezomib and Dexamethasone. HD high dose chemotherapy.

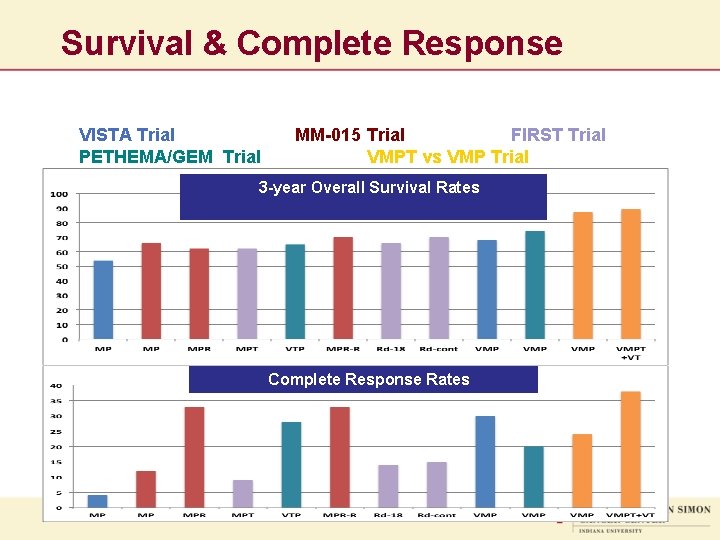
Survival & Complete Response VISTA Trial PETHEMA/GEM Trial MM-015 Trial FIRST Trial VMPT vs VMP Trial 3 -year Overall Survival Rates Complete Response Rates

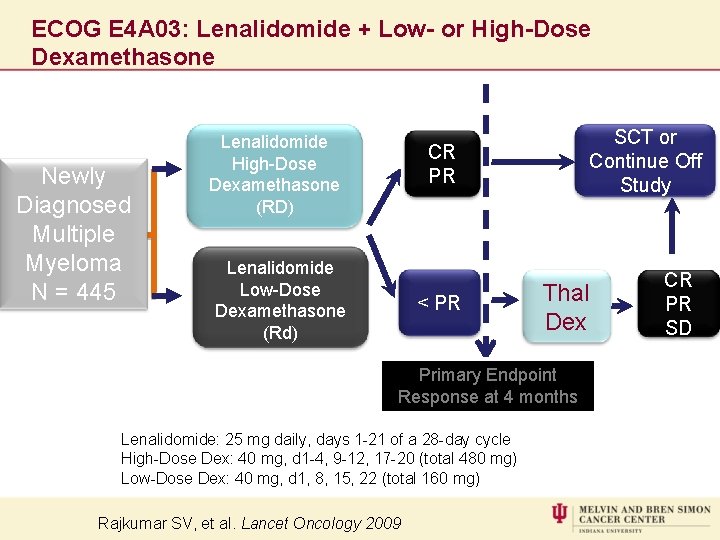
ECOG E 4 A 03: Lenalidomide + Low- or High-Dose Dexamethasone Newly Diagnosed Multiple Myeloma N = 445 Lenalidomide High-Dose 1: 1 Dexamethasone (RD) SCT or Continue Off 1: 1 Study CR PR Lenalidomide Low-Dose Dexamethasone (Rd) < PR Thal Dex Primary Endpoint Response at 4 months Lenalidomide: 25 mg daily, days 1 -21 of a 28 -day cycle High-Dose Dex: 40 mg, d 1 -4, 9 -12, 17 -20 (total 480 mg) Low-Dose Dex: 40 mg, d 1, 8, 15, 22 (total 160 mg) Rajkumar SV, et al. Lancet Oncology 2009 CR PR SD

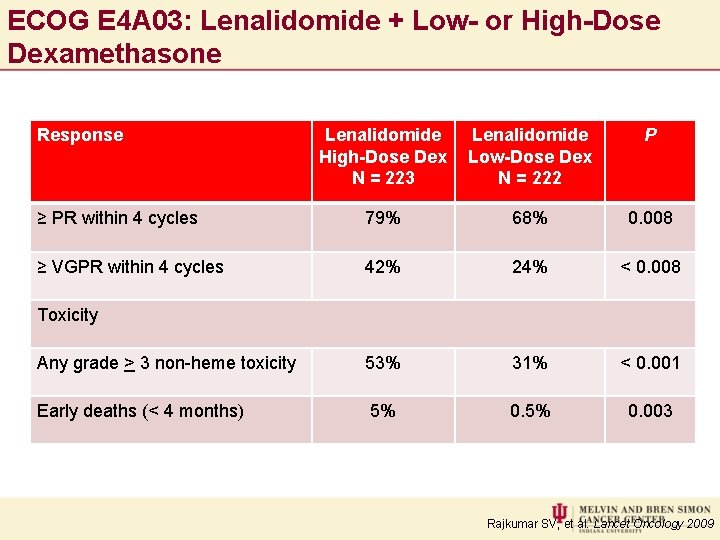
ECOG E 4 A 03: Lenalidomide + Low- or High-Dose Dexamethasone Response Lenalidomide High-Dose Dex N = 223 Lenalidomide Low-Dose Dex N = 222 P ≥ PR within 4 cycles 79% 68% 0. 008 ≥ VGPR within 4 cycles 42% 24% < 0. 008 Any grade > 3 non-heme toxicity 53% 31% < 0. 001 Early deaths (< 4 months) 5% 0. 003 Toxicity Rajkumar SV, et al. Lancet Oncology 2009

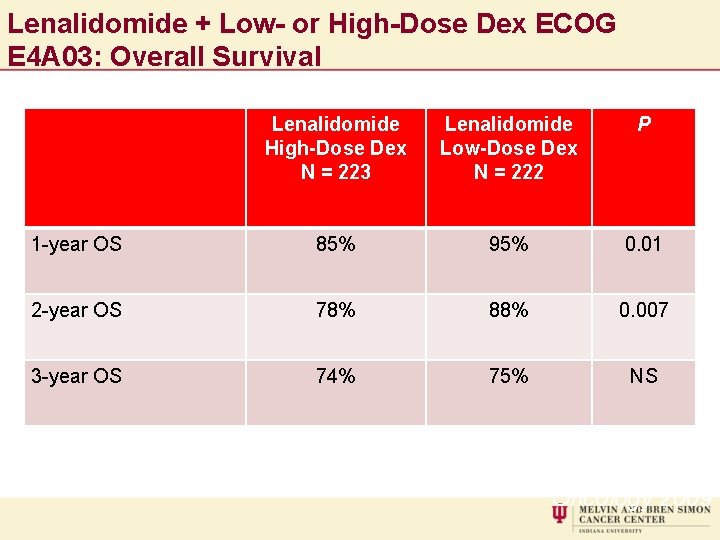
Lenalidomide + Low- or High-Dose Dex ECOG E 4 A 03: Overall Survival Lenalidomide High-Dose Dex N = 223 Lenalidomide Low-Dose Dex N = 222 P 1 -year OS 85% 95% 0. 01 2 -year OS 78% 88% 0. 007 3 -year OS 74% 75% NS Rajkumar SV, et al. Lancet Oncology 2009

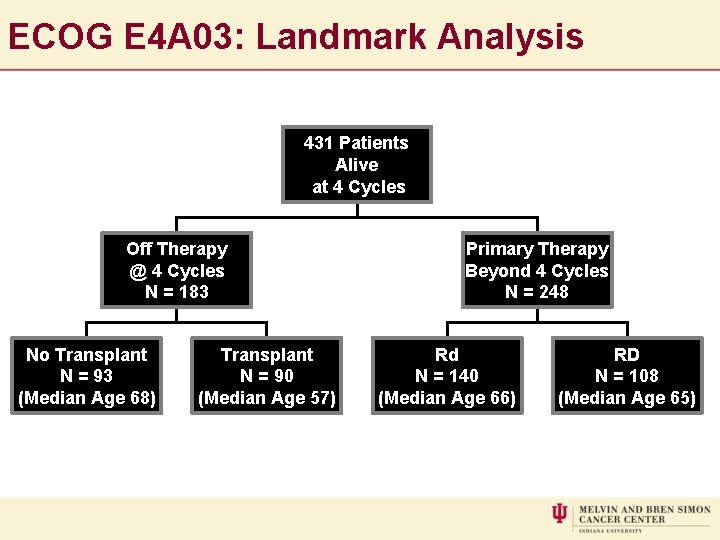
ECOG E 4 A 03: Landmark Analysis 431 Patients Alive at 4 Cycles Off Therapy @ 4 Cycles N = 183 No Transplant N = 93 (Median Age 68) Transplant N = 90 (Median Age 57) Primary Therapy Beyond 4 Cycles N = 248 Rd N = 140 (Median Age 66) Median Follow Up: 36 Months RD N = 108 (Median Age 65)

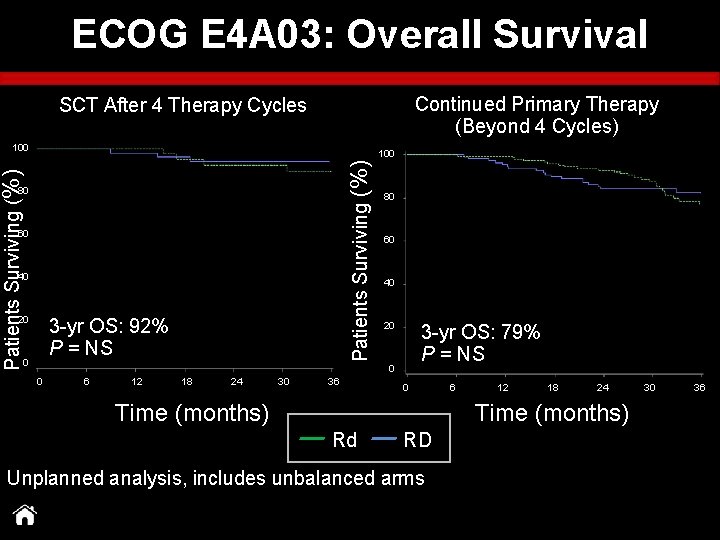
ECOG E 4 A 03: Overall Survival Continued Primary Therapy (Beyond 4 Cycles) SCT After 4 Therapy Cycles 100 Patients Surviving (%) 100 80 60 40 20 0 3 -yr OS: 92% P = NS 80 60 40 20 0 0 6 12 18 24 30 36 P = NS 3 -yr OS: 79% P = NS 0 6 12 18 24 30 36 Time (months) Rd RD Unplanned analysis, includes unbalanced arms

Stringent complete response (s. CR) in patients with newly diagnosed multiple myeloma (NDMM) treated with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (DEX) AJ Jakubowiak, 1 K Griffith, 2 D Dytfeld, 3 DH Vesole, 4 S Jagannath, 5 T Anderson, 2 B Nordgren, 2 K Detweiler-Short, 2 D Lebovic, 2 K Stockerl-Goldstein, 6 T Jobkar, 2 S Wear, 7 A Al-Zoubi, 2 A Ahmed, 2 M Mietzel, 2 D Couriel, 2 M Kaminski, 2 M Hussein, 8 H Yeganegi, 9 R Vij 6 1 University of Chicago, IL; 2 University of Michigan Comprehensive Cancer Center, Ann Arbor, MI; 3 Poznan University of Medical Sciences, Poznan, Poland; 4 John Theurer Cancer Center, Hackensack, NJ; 5 Mount Sinai Medical Center, New York, NY; 6 Washington University School of Medicine, St. Louis, MO; 7 Multiple Myeloma Research Consortium, Norwalk, CT; 8 Celgene, Inc, Summit, NJ; 9 Onyx Pharmaceuticals, South San Francisco, CA

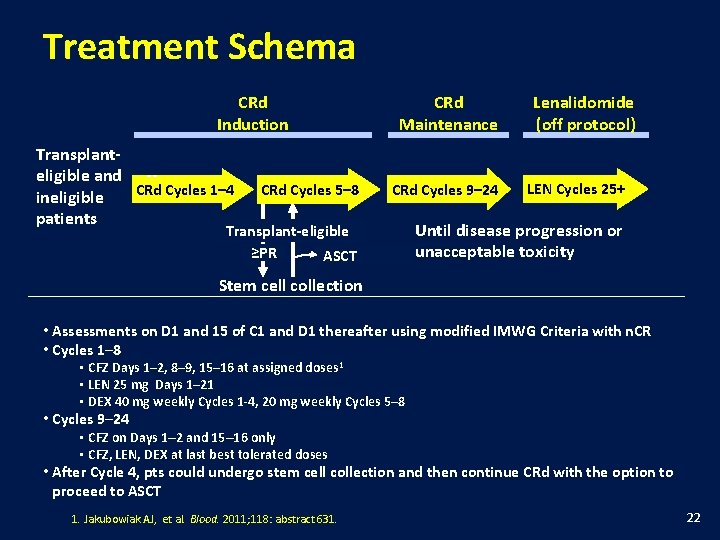
Treatment Schema CRd Induction Transplanteligible and -CRd Cycles 1– 4 ineligible patients CRd Cycles 5– 8 CRd Maintenance CRd Cycles 9– 24 Transplant-eligible ≥PR ASCT Lenalidomide (off protocol) LEN Cycles 25+ Until disease progression or unacceptable toxicity Stem cell collection • Assessments on D 1 and 15 of C 1 and D 1 thereafter using modified IMWG Criteria with n. CR • Cycles 1– 8 • CFZ Days 1– 2, 8– 9, 15– 16 at assigned doses 1 • LEN 25 mg Days 1– 21 • DEX 40 mg weekly Cycles 1 -4, 20 mg weekly Cycles 5– 8 • Cycles 9– 24 • CFZ on Days 1– 2 and 15– 16 only • CFZ, LEN, DEX at last best tolerated doses • After Cycle 4, pts could undergo stem cell collection and then continue CRd with the option to proceed to ASCT 1. Jakubowiak AJ, et al. Blood. 2011; 118: abstract 631. 22

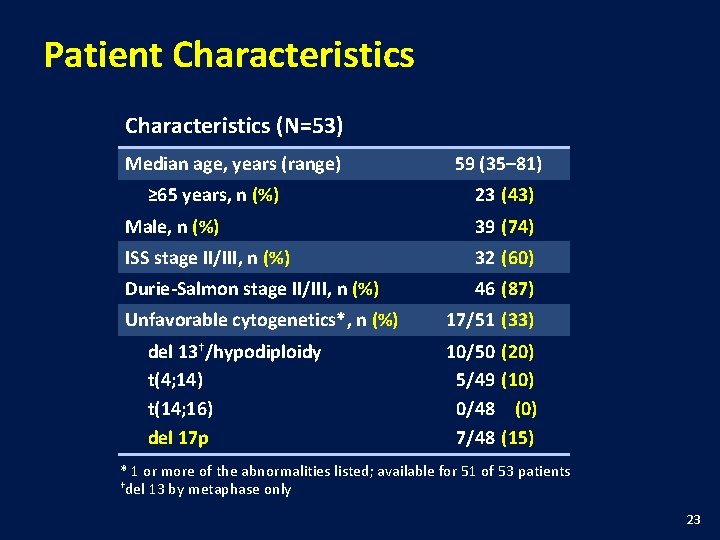
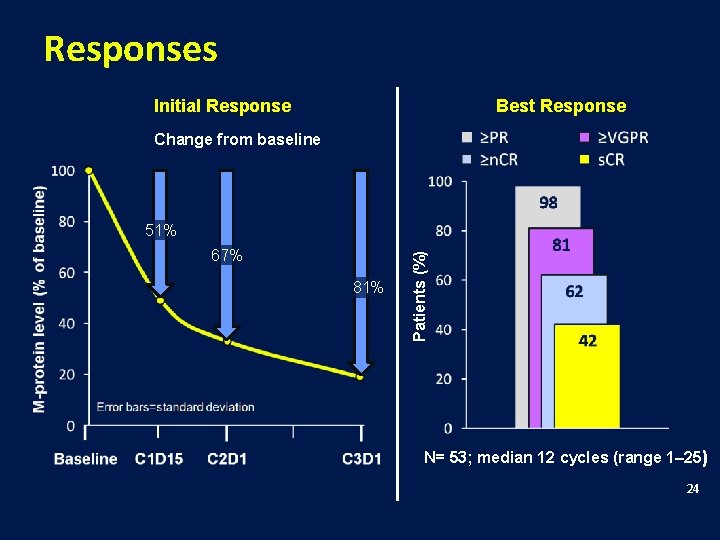
Patient Characteristics (N=53) Median age, years (range) ≥ 65 years, n (%) 59 (35– 81) 23 (43) Male, n (%) 39 (74) ISS stage II/III, n (%) 32 (60) Durie-Salmon stage II/III, n (%) 46 (87) Unfavorable cytogenetics*, n (%) del 13†/hypodiploidy t(4; 14) t(14; 16) del 17 p 17/51 (33) 10/50 (20) 5/49 (10) 0/48 (0) 7/48 (15) * 1 or more of the abnormalities listed; available for 51 of 53 patients †del 13 by metaphase only 23

Responses Initial Response Best Response Change from baseline 67% 81% Patients (%) 51% N= 53; median 12 cycles (range 1– 25) 24

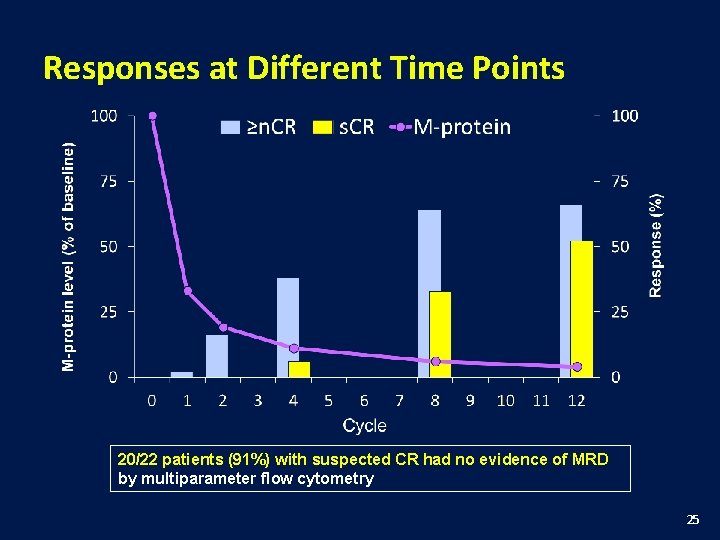
Responses at Different Time Points 20/22 patients (91%) with suspected CR had no evidence of MRD by multiparameter flow cytometry 25

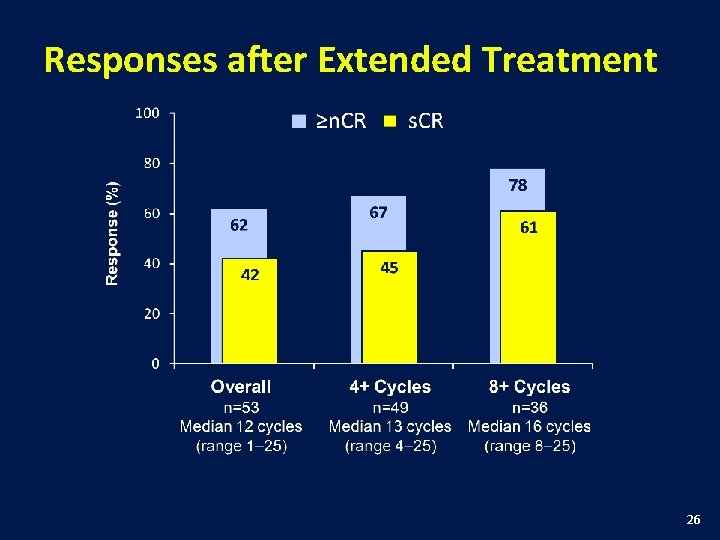
Responses after Extended Treatment 26

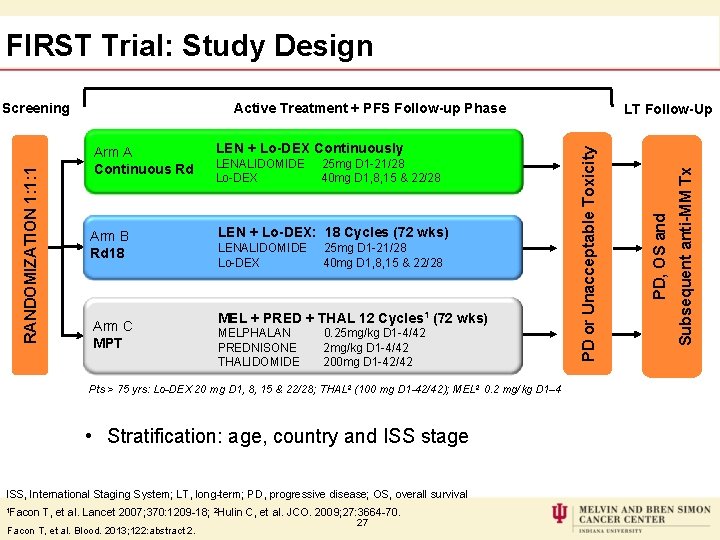
FIRST Trial: Study Design Active Treatment + PFS Follow-up Phase Arm B Rd 18 LEN + Lo-DEX: 18 Cycles (72 wks) Arm C MPT LENALIDOMIDE 25 mg D 1 -21/28 Lo-DEX 40 mg D 1, 8, 15 & 22/28 MEL + PRED + THAL 12 Cycles 1 (72 wks) MELPHALAN 0. 25 mg/kg D 1 -4/42 PREDNISONE 2 mg/kg D 1 -4/42 THALIDOMIDE 200 mg D 1 -42/42 Pts > 75 yrs: Lo-DEX 20 mg D 1, 8, 15 & 22/28; THAL 2 (100 mg D 1 -42/42); MEL 2 0. 2 mg/kg D 1– 4 • Stratification: age, country and ISS stage ISS, International Staging System; LT, long-term; PD, progressive disease; OS, overall survival 1 Facon T, et al. Lancet 2007; 370: 1209 -18; 2 Hulin C, et al. JCO. 2009; 27: 3664 -70. Facon T, et al. Blood. 2013; 122: abstract 2. 27 Subsequent anti-MM Tx LEN + Lo-DEX Continuously PD, OS and Arm A Continuous Rd LT Follow-Up PD or Unacceptable Toxicity RANDOMIZATION 1: 1: 1 Screening

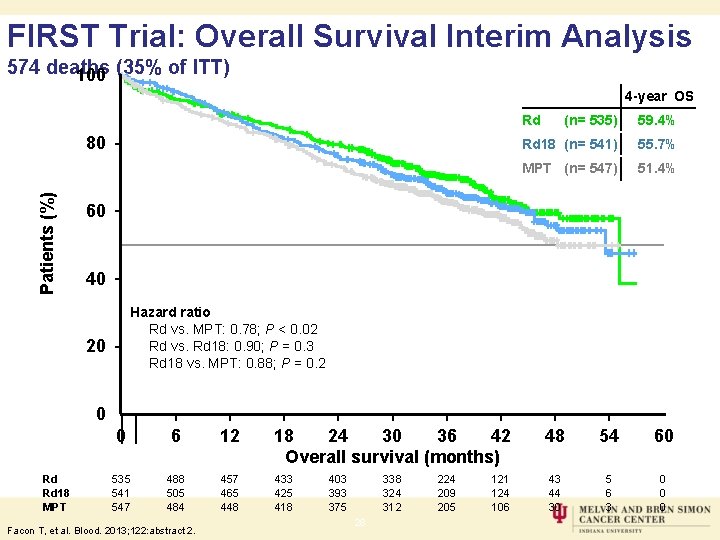
FIRST Trial: Overall Survival Interim Analysis 574 deaths 100 (35% of ITT) 4 -year OS Rd Patients (%) 80 (n= 535) 59. 4% Rd 18 (n= 541) 55. 7% MPT (n= 547) 51. 4% 60 40 Hazard ratio Rd vs. MPT: 0. 78; P < 0. 02 Rd vs. Rd 18: 0. 90; P = 0. 3 Rd 18 vs. MPT: 0. 88; P = 0. 2 20 0 Rd Rd 18 MPT 0 6 12 18 24 30 36 42 Overall survival (months) 535 541 547 488 505 484 457 465 448 433 425 418 Facon T, et al. Blood. 2013; 122: abstract 2. 403 393 375 338 324 312 28 224 209 205 121 124 106 48 54 60 43 44 30 5 6 3 0 0 0

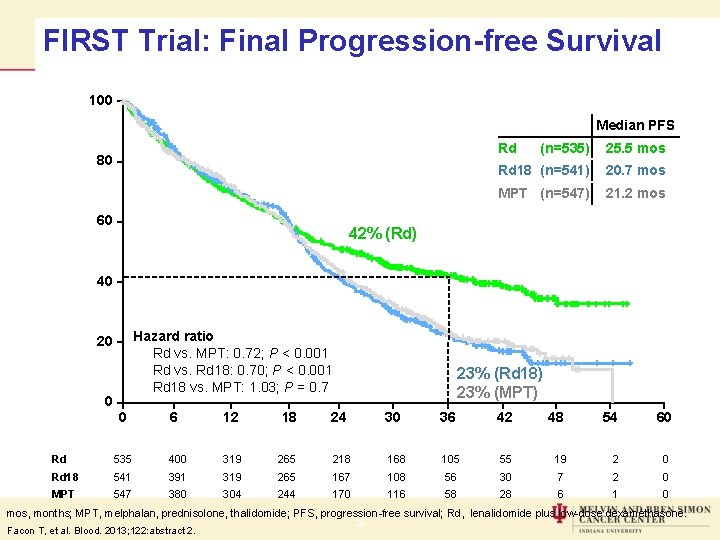
FIRST Trial: Final Progression-free Survival 100 Median PFS Rd Patients (%) 80 60 (n=535) 25. 5 mos Rd 18 (n=541) 20. 7 mos MPT (n=547) 21. 2 mos 42% (Rd) 40 Hazard ratio Rd vs. MPT: 0. 72; P < 0. 001 Rd vs. Rd 18: 0. 70; P < 0. 001 Rd 18 vs. MPT: 1. 03; P = 0. 7 20 0 0 6 12 18 23% (Rd 18) 23% (MPT) 24 30 36 42 48 54 60 Time (months) Rd 535 400 319 265 218 168 105 55 19 2 0 Rd 18 541 391 319 265 167 108 56 30 7 2 0 MPT 547 380 304 244 170 116 58 28 6 1 0 mos, months; MPT, melphalan, prednisolone, thalidomide; PFS, progression-free survival; Rd, lenalidomide plus low-dose dexamethasone. Facon T, et al. Blood. 2013; 122: abstract 2. 29

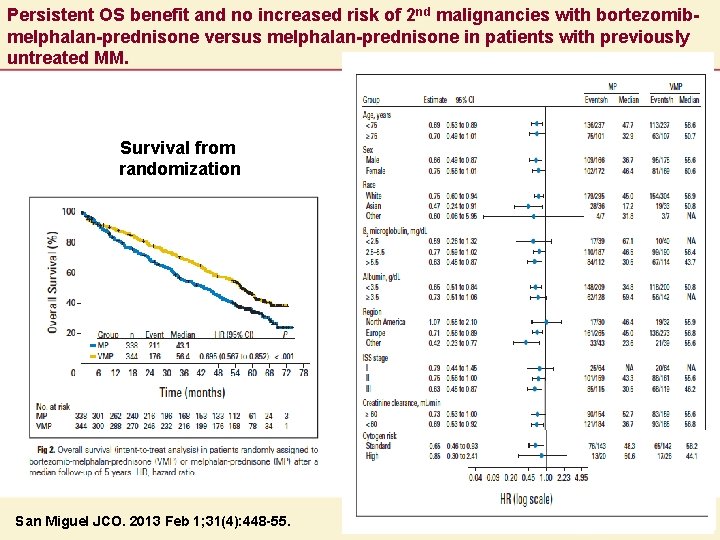
Persistent OS benefit and no increased risk of 2 nd malignancies with bortezomibmelphalan-prednisone versus melphalan-prednisone in patients with previously untreated MM. Survival from randomization San Miguel JCO. 2013 Feb 1; 31(4): 448 -55.

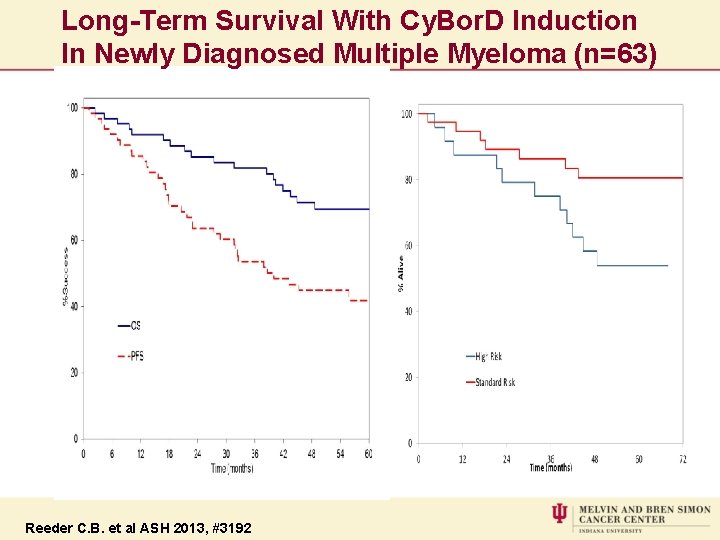
Long-Term Survival With Cy. Bor. D Induction In Newly Diagnosed Multiple Myeloma (n=63) Reeder C. B. et al ASH 2013, #3192

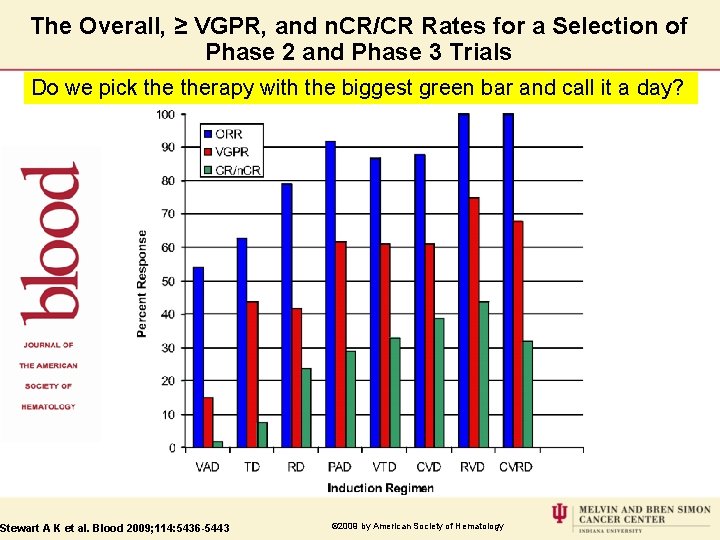
The Overall, ≥ VGPR, and n. CR/CR Rates for a Selection of Phase 2 and Phase 3 Trials Do we pick therapy with the biggest green bar and call it a day? Stewart A K et al. Blood 2009; 114: 5436 -5443 © 2009 by American Society of Hematology

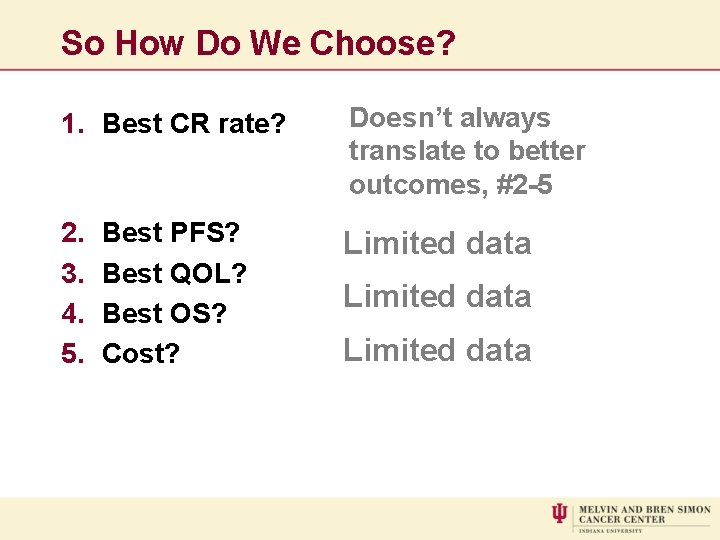
So How Do We Choose? 1. Best CR rate? Doesn’t always translate to better outcomes, #2 -5 2. 3. 4. 5. Limited data Best PFS? Best QOL? Best OS? Cost? Limited data

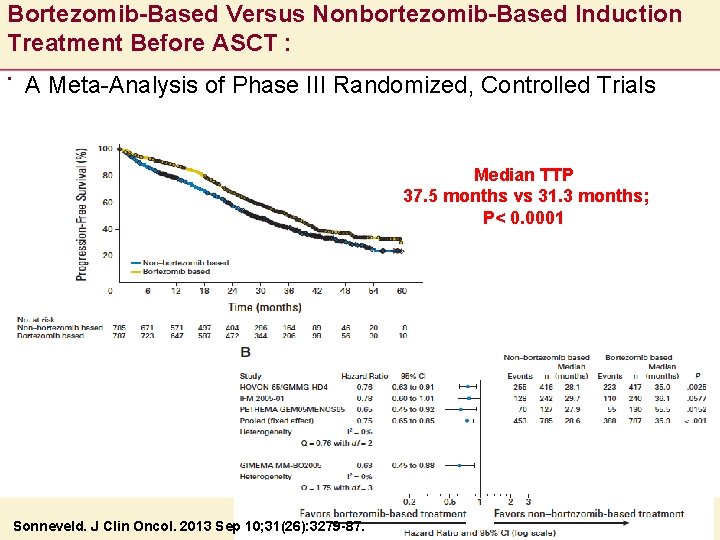
Bortezomib-Based Versus Nonbortezomib-Based Induction Treatment Before ASCT : . A Meta-Analysis of Phase III Randomized, Controlled Trials Median TTP 37. 5 months vs 31. 3 months; P< 0. 0001 Sonneveld. J Clin Oncol. 2013 Sep 10; 31(26): 3279 -87.

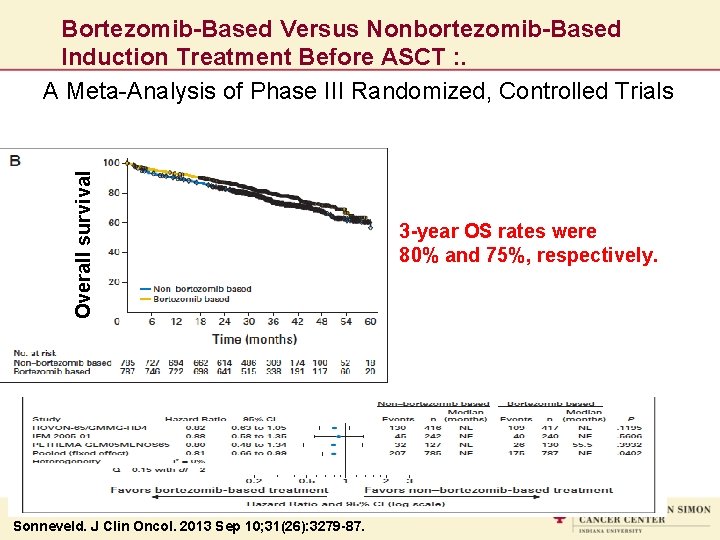
Overall survival Bortezomib-Based Versus Nonbortezomib-Based Induction Treatment Before ASCT : . A Meta-Analysis of Phase III Randomized, Controlled Trials Sonneveld. J Clin Oncol. 2013 Sep 10; 31(26): 3279 -87. 3 -year OS rates were 80% and 75%, respectively.

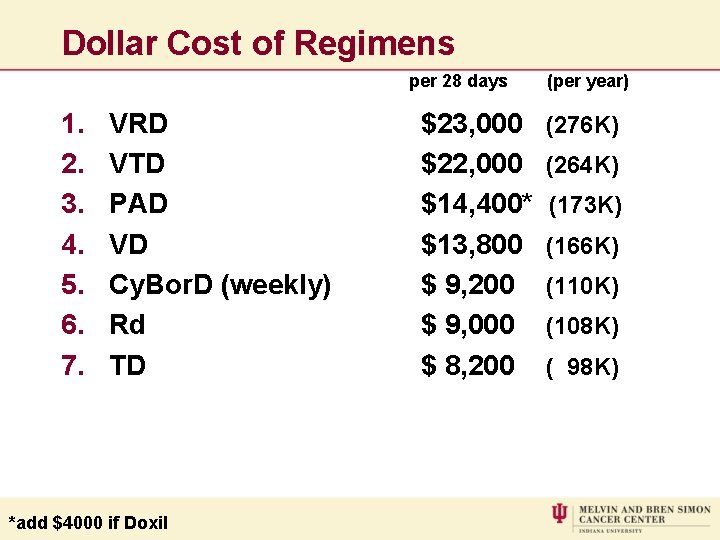
Dollar Cost of Regimens per 28 days 1. 2. 3. 4. 5. 6. 7. VRD VTD PAD VD Cy. Bor. D (weekly) Rd TD *add $4000 if Doxil $23, 000 $22, 000 $14, 400* $13, 800 $ 9, 200 $ 9, 000 $ 8, 200 (per year) (276 K) (264 K) (173 K) (166 K) (110 K) (108 K) ( 98 K)

How Deep a Response Required before ASCT?

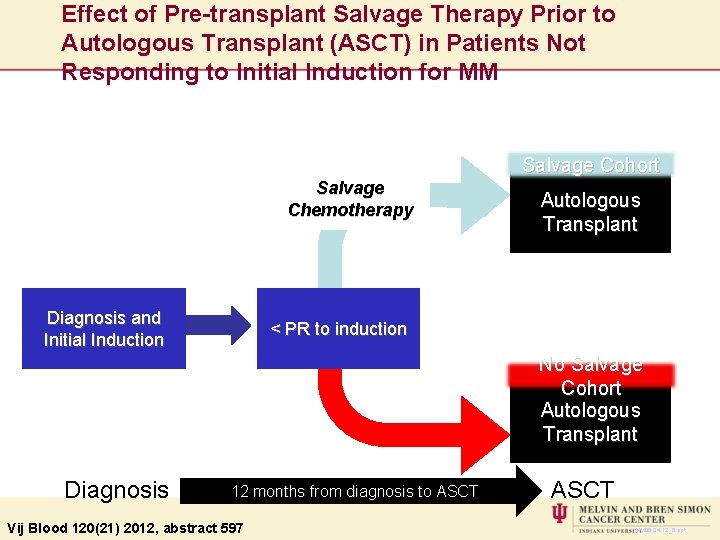
Effect of Pre-transplant Salvage Therapy Prior to Autologous Transplant (ASCT) in Patients Not Responding to Initial Induction for MM Salvage Cohort Salvage Chemotherapy Diagnosis and Initial Induction Autologous Transplant < PR to induction No Salvage Cohort Autologous Transplant Diagnosis 12 months from diagnosis to ASCT Vij Blood 120(21) 2012, abstract 597 ASCT MM 06 -04 -12_6. ppt

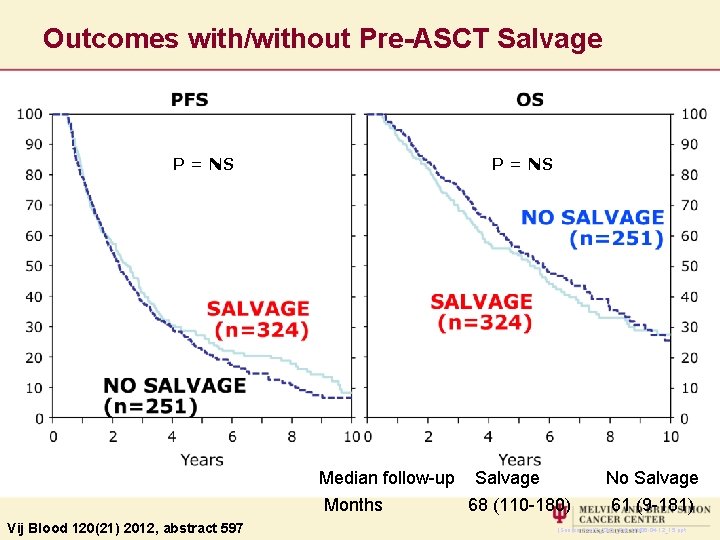
Outcomes with/without Pre-ASCT Salvage P = NS Vij Blood 120(21) 2012, abstract 597 P = NS Median follow-up Salvage No Salvage Months 68 (110 -180) 61 (9 -181) (Source: Txz 12_23 & _24) MM 06 -04 -12_15. ppt

CONSOLIDATION • Historically • 4 cycles of VAD (limited to 4 due to cardio toxicity) • ASCT as consolidation • No furtherapy later • Now • Why limited to 4 cycles? • Why limited to pre transplant?

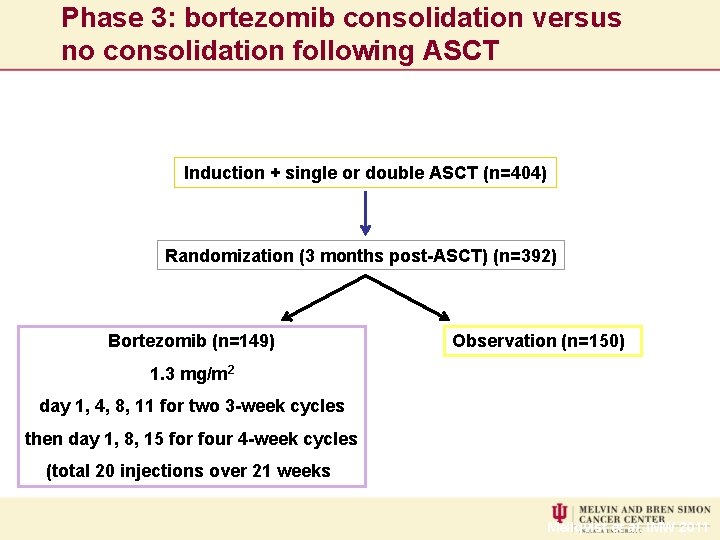
Phase 3: bortezomib consolidation versus no consolidation following ASCT Induction + single or double ASCT (n=404) Randomization (3 months post-ASCT) (n=392) Bortezomib (n=149) Observation (n=150) 1. 3 mg/m 2 day 1, 4, 8, 11 for two 3 -week cycles then day 1, 8, 15 for four 4 -week cycles (total 20 injections over 21 weeks) Mellqvist et al. IMW 2011

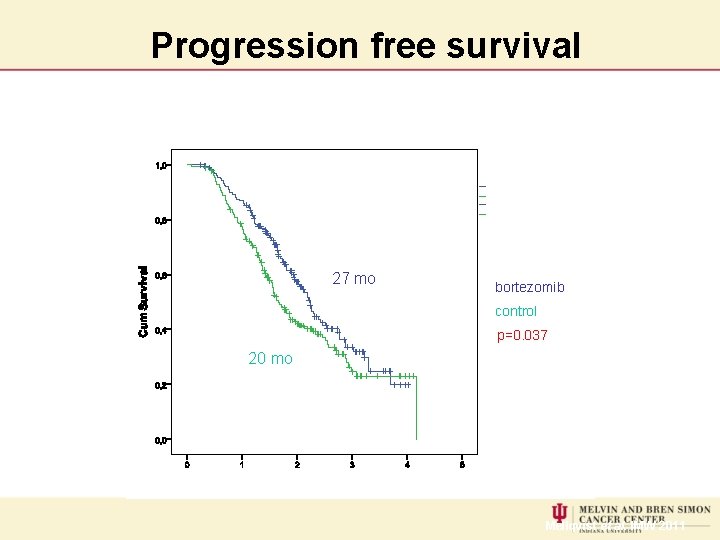
Progression free survival 27 mo bortezomib control p=0. 037 20 mo Mellqvist et al. IMW 2011

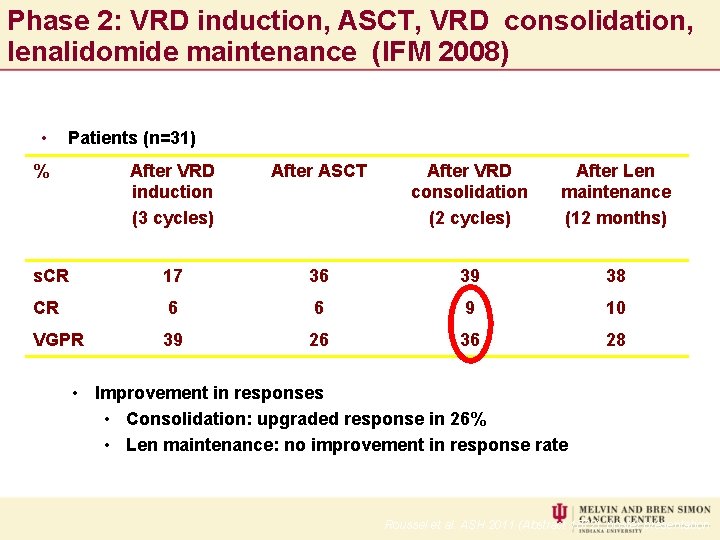
Phase 2: VRD induction, ASCT, VRD consolidation, lenalidomide maintenance (IFM 2008) • Patients (n=31) % After VRD induction (3 cycles) After ASCT After VRD consolidation (2 cycles) After Len maintenance (12 months) s. CR 17 36 39 38 CR 6 6 9 10 VGPR 39 26 36 28 • Improvement in responses • Consolidation: upgraded response in 26% • Len maintenance: no improvement in response rate Roussel et al. ASH 2011 (Abstract 1872), poster presentation

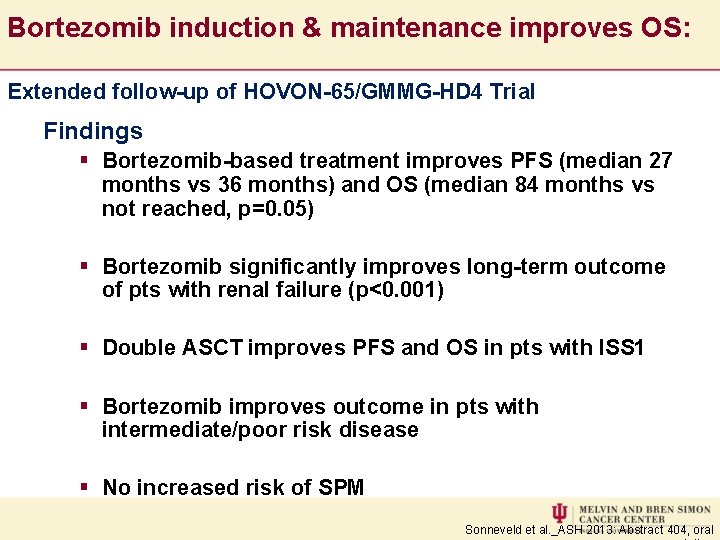
Bortezomib induction & maintenance improves OS: Extended follow-up of HOVON-65/GMMG-HD 4 Trial Findings § Bortezomib-based treatment improves PFS (median 27 months vs 36 months) and OS (median 84 months vs not reached, p=0. 05) § Bortezomib significantly improves long-term outcome of pts with renal failure (p<0. 001) § Double ASCT improves PFS and OS in pts with ISS 1 § Bortezomib improves outcome in pts with intermediate/poor risk disease § No increased risk of SPM Sonneveld et al. _ASH 2013: Abstract 404, oral

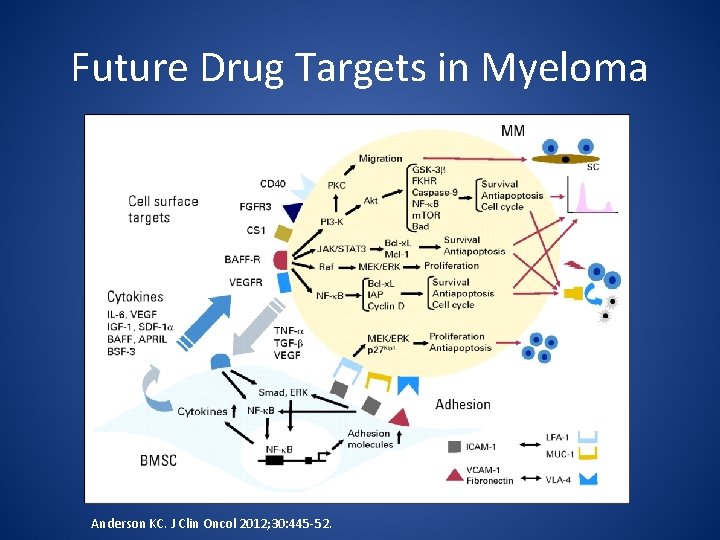
Future Drug Targets in Myeloma Anderson KC. J Clin Oncol 2012; 30: 445 -52.

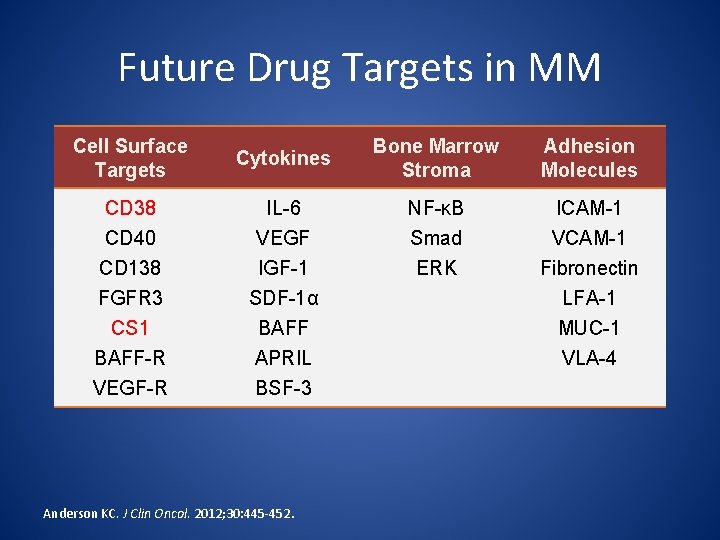
Future Drug Targets in MM Cell Surface Targets Cytokines Bone Marrow Stroma Adhesion Molecules CD 38 CD 40 IL-6 VEGF NF-κB Smad ICAM-1 VCAM-1 CD 138 FGFR 3 CS 1 BAFF-R VEGF-R IGF-1 SDF-1α BAFF APRIL BSF-3 ERK Fibronectin LFA-1 MUC-1 VLA-4 Anderson KC. J Clin Oncol. 2012; 30: 445 -452.

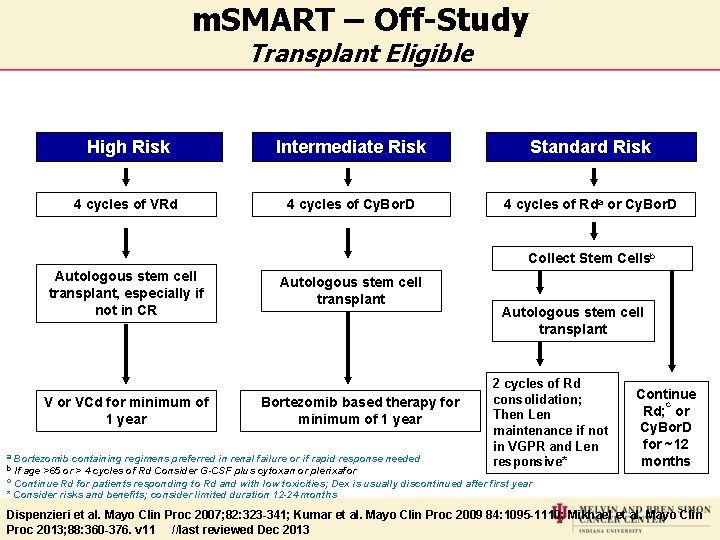
m. SMART – Off-Study Transplant Eligible High Risk Intermediate Risk Standard Risk 4 cycles of VRd 4 cycles of Cy. Bor. D 4 cycles of Rda or Cy. Bor. D Collect Stem Cellsb Autologous stem cell transplant, especially if not in CR V or VCd for minimum of 1 year Autologous stem cell transplant Bortezomib based therapy for minimum of 1 year a Bortezomib containing regimens preferred in renal failure or if rapid response b If age >65 or > 4 cycles of Rd Consider G-CSF plus cytoxan or plerixafor needed Autologous stem cell transplant 2 cycles of Rd consolidation; Then Len maintenance if not in VGPR and Len responsive* Continue c Rd; or Cy. Bor. D for ~12 months c Continue Rd for patients responding to Rd and with low toxicities; Dex is usually discontinued after first year * Consider risks and benefits; consider limited duration 12 -24 months Dispenzieri et al. Mayo Clin Proc 2007; 82: 323 -341; Kumar et al. Mayo Clin Proc 2009 84: 1095 -1110; Mikhael et al. Mayo Clin Proc 2013; 88: 360 -376. v 11 //last reviewed Dec 2013

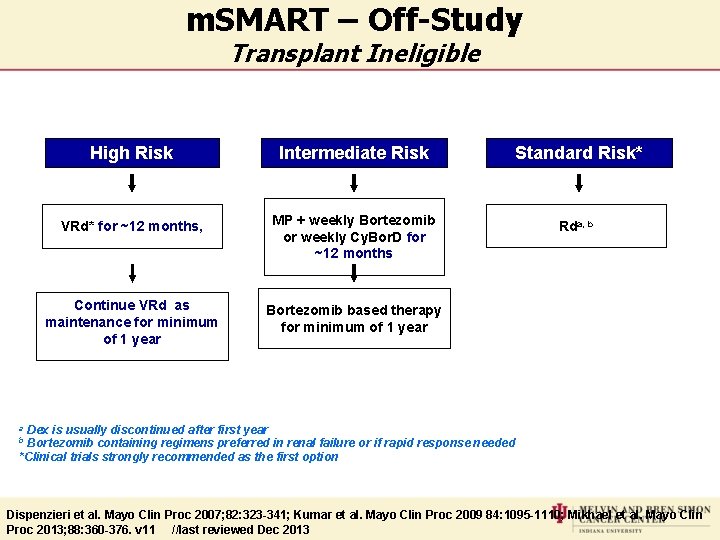
m. SMART – Off-Study Transplant Ineligible High Risk Intermediate Risk Standard Risk* VRd* for ~12 months, MP + weekly Bortezomib or weekly Cy. Bor. D for ~12 months Rda, b Continue VRd as maintenance for minimum of 1 year Bortezomib based therapy for minimum of 1 year Dex is usually discontinued after first year Bortezomib containing regimens preferred in renal failure or if rapid response needed *Clinical trials strongly recommended as the first option a b Dispenzieri et al. Mayo Clin Proc 2007; 82: 323 -341; Kumar et al. Mayo Clin Proc 2009 84: 1095 -1110; Mikhael et al. Mayo Clin Proc 2013; 88: 360 -376. v 11 //last reviewed Dec 2013

Immune Based Therapy • Monoclonal antibodies Daratumumab and Elotuzumab • Vaccine based therapy using dendritic cells • Myeloma targeted therapy using CAR-T cell. Patients own T cell engineered to attack myeloma specific antigen.

So What Are We Offering Our Patients Today? • Unlimited number of combination regimens and sequences. • Magical responses: Fast, Complete and sometimes Durable but are they the R-CHOP? • Design-stupid: Achieving CR and Maintaining it never was the goal of clinical trials. • Subset and Retrospective analysis led to speculative functional classification.

The New Paradigm • Combination therapy with novel agents provide excellent disease control. • Addition of high dose chemotherapy increase the number of patients with complete remission and very good partial response. • Consolidation therapy and maintenance therapy improve progression free survival. • Zoledronic acid appears to play important role regardless of the treatment delivered. • Five year survival is approaching 81%.
 Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Vtd protocol multiple myeloma
Vtd protocol multiple myeloma Kpd myeloma
Kpd myeloma Waldenstrom's disease
Waldenstrom's disease Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Crab criteria multiple myeloma
Crab criteria multiple myeloma Rulo formasyonu yapan hastalıklar
Rulo formasyonu yapan hastalıklar Smoldering multiple myeloma
Smoldering multiple myeloma Mgus
Mgus Treatment options
Treatment options Bespoke treatment options
Bespoke treatment options Description of waste
Description of waste Http://opn.to/a/qmyfg
Http://opn.to/a/qmyfg Treatment options
Treatment options Crohns disease meaning
Crohns disease meaning Two years ago jenny was diagnosed with schizophrenia
Two years ago jenny was diagnosed with schizophrenia How is progeria diagnosed
How is progeria diagnosed Lisa has a diagnosed learning disability
Lisa has a diagnosed learning disability How is progeria diagnosed
How is progeria diagnosed Smart multiple options
Smart multiple options Myeloma euronet
Myeloma euronet European myeloma network
European myeloma network Daratumumab macmillan
Daratumumab macmillan Myeloma
Myeloma Exercise physiology mascot
Exercise physiology mascot Alternating treatment design aba
Alternating treatment design aba Baseline
Baseline Disadvantages of mimd
Disadvantages of mimd Induction for newly qualified teachers
Induction for newly qualified teachers I newly joined the team
I newly joined the team A nurse preceptor is orienting a newly licensed nurse
A nurse preceptor is orienting a newly licensed nurse Is nigeria a newly emerging economy
Is nigeria a newly emerging economy Worker xy
Worker xy Newly industrialized population pyramid
Newly industrialized population pyramid People who work with computers while doing business
People who work with computers while doing business Brain gain definition ap human geography
Brain gain definition ap human geography Animals tend to revert from newly learned habits
Animals tend to revert from newly learned habits Newly industrialized countries
Newly industrialized countries Newly hired experienced miner training
Newly hired experienced miner training Newly industrialized country population pyramid
Newly industrialized country population pyramid A newly admitted patient was found wandering
A newly admitted patient was found wandering Cost of newly issued common stock
Cost of newly issued common stock Referatmarkeringar
Referatmarkeringar Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Luftstrupen för medicinare
Luftstrupen för medicinare Frgar
Frgar Stig karttecken
Stig karttecken Mindre än tecken
Mindre än tecken Delegerande ledarstil
Delegerande ledarstil Adressändring ideell förening
Adressändring ideell förening Toppslätskivling effekt
Toppslätskivling effekt Borra hål för knoppar
Borra hål för knoppar