Common Causes of Newly Diagnosed Anemia in Adults











- Slides: 20

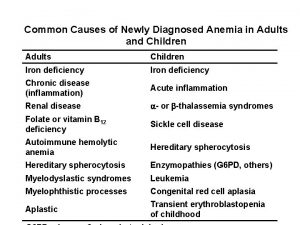
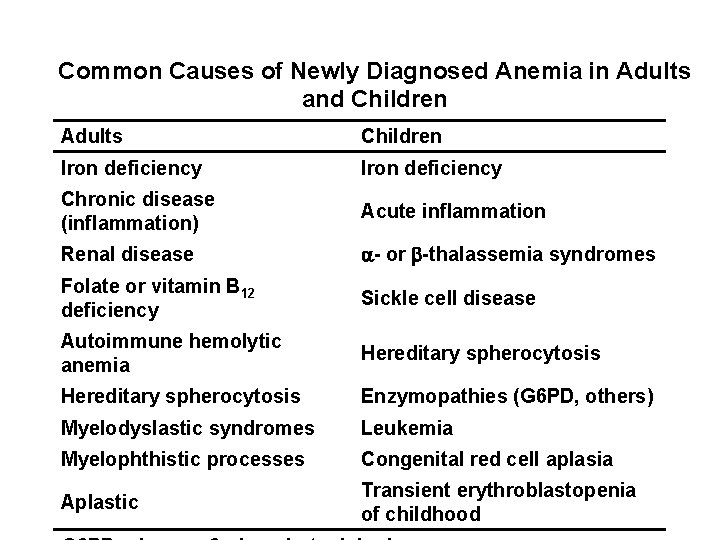
Common Causes of Newly Diagnosed Anemia in Adults and Children Adults Children Iron deficiency Chronic disease (inflammation) Acute inflammation Renal disease - or -thalassemia syndromes Folate or vitamin B 12 deficiency Sickle cell disease Autoimmune hemolytic anemia Hereditary spherocytosis Enzymopathies (G 6 PD, others) Myelodyslastic syndromes Leukemia Myelophthistic processes Congenital red cell aplasia Aplastic Transient erythroblastopenia of childhood

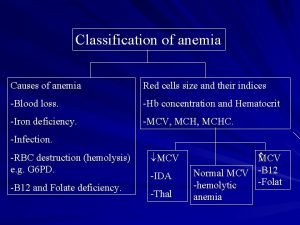
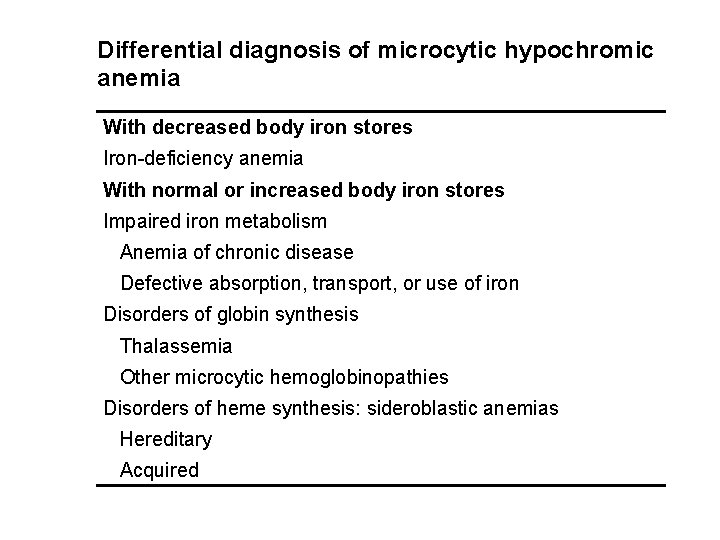
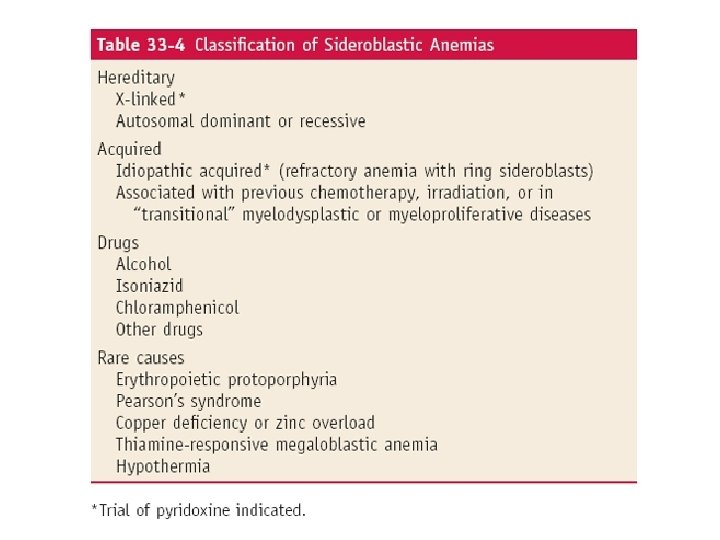
Differential diagnosis of microcytic hypochromic anemia With decreased body iron stores Iron-deficiency anemia With normal or increased body iron stores Impaired iron metabolism Anemia of chronic disease Defective absorption, transport, or use of iron Disorders of globin synthesis Thalassemia Other microcytic hemoglobinopathies Disorders of heme synthesis: sideroblastic anemias Hereditary Acquired


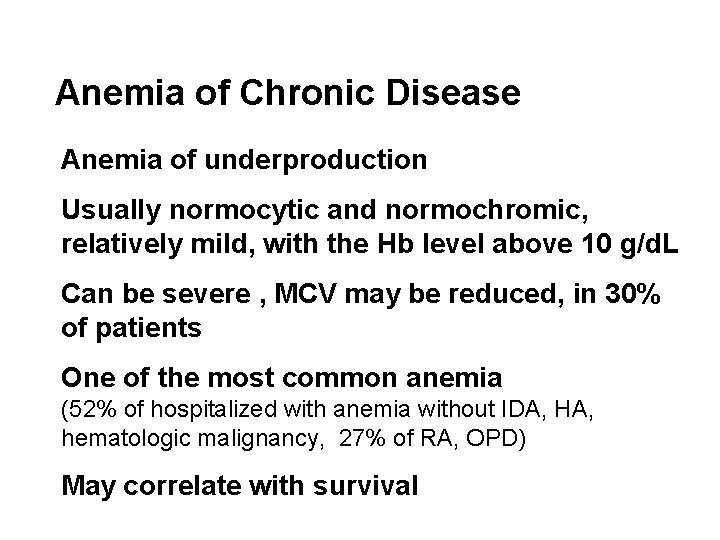
Anemia of Chronic Disease Anemia of underproduction Usually normocytic and normochromic, relatively mild, with the Hb level above 10 g/d. L Can be severe , MCV may be reduced, in 30% of patients One of the most common anemia (52% of hospitalized with anemia without IDA, HA, hematologic malignancy, 27% of RA, OPD) May correlate with survival

Causes of Anemia of Chronic Disease Block in reuse of iron by erythrocyte Shortened erythrocyte survival Direct inhibition of erythropoiesis Relative deficiency of erythropoietin




Laboratory tests to distinguish iron deficiency anemia from anemia of chronic disease (ACD) Measurement Normal Iron Units Values Deficiency ACD Low normal - ACD + Iron deficiency g/d. L 50 – 150 Serum total iron-binding g/d. L 250 – 400 capacity Transferrin saturation 20 – 50 g/L 20 – 350 Normal - Serum iron Serum ferritin Serum soluble transferrin receptor % Low normal - Normal - n. M 9 – 28 Normal Bone marrow iron stores 0 -4+ 2 – 3+ Normal 20 – 80% Iron-containing normoblasts in the bone marrow %

Diagnosis of Anemia of Chronic Disease Unequivocal diagnosis is often difficult ACD is primarily a diagnosis of exclusion (R/O infiltration by tumor, fibrosis, or infection, MDS) Dx with reticulocyte, Fe, TIBC, serum ferritin, in systemic illness R/O nutritional def, hemolysis, sequestration, BM usually not helpful DDx of IDA (serum ferritin ? , serum transferrin receptor ? ) Treatment of underlying disease. (Iron chelator has mild effect in animal ? ) If severe or symtptomatic, transfusion, r. EPO when



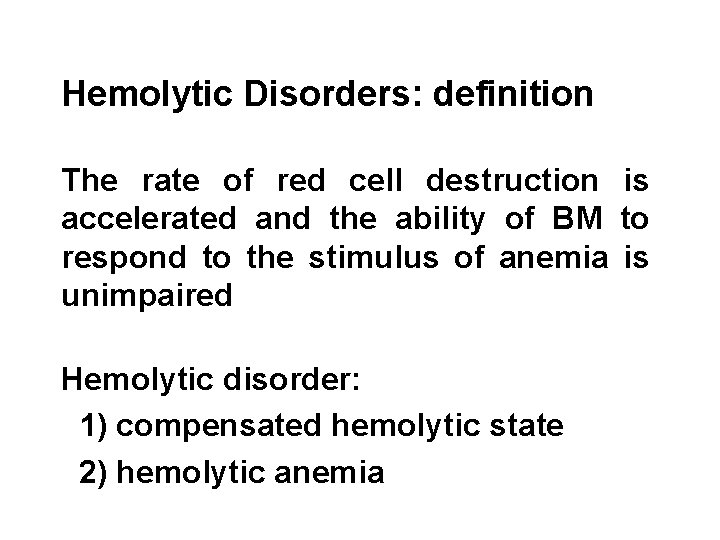
Hemolytic Disorders: definition The rate of red cell destruction is accelerated and the ability of BM to respond to the stimulus of anemia is unimpaired Hemolytic disorder: 1) compensated hemolytic state 2) hemolytic anemia

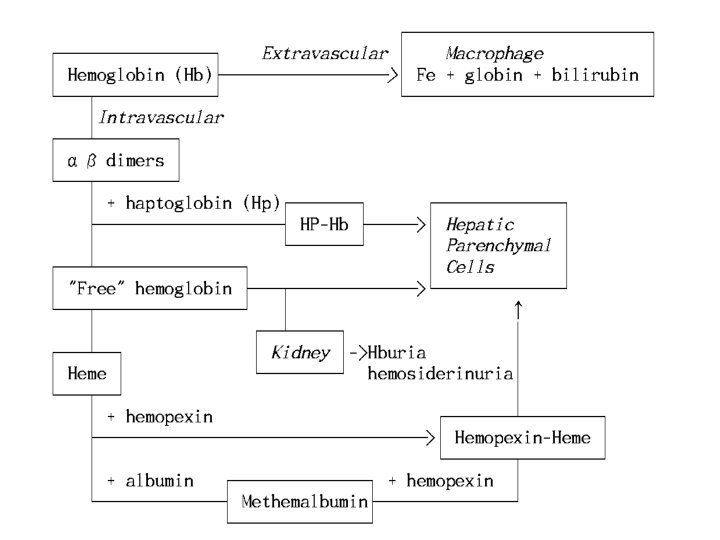
Pathophysiology of Hemolysis Site of Red Cell Destruction 1) Intravascular lysis: release of RBC content directly into PB trauma, C' fixation, toxin 2) Extravascular lysis: RBC's are taken up by macrophage in the spleen and liver, where they are destroyed surface abnormality deformability: membrane, surface/volume ratio, intracellular component 3) Both sites

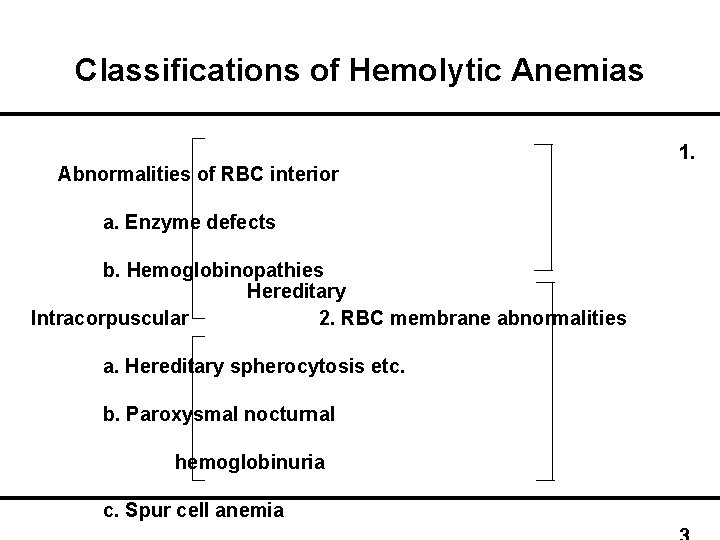
Classifications of Hemolytic Anemias Abnormalities of RBC interior a. Enzyme defects b. Hemoglobinopathies Hereditary Intracorpuscular 2. RBC membrane abnormalities a. Hereditary spherocytosis etc. b. Paroxysmal nocturnal hemoglobinuria c. Spur cell anemia 1.

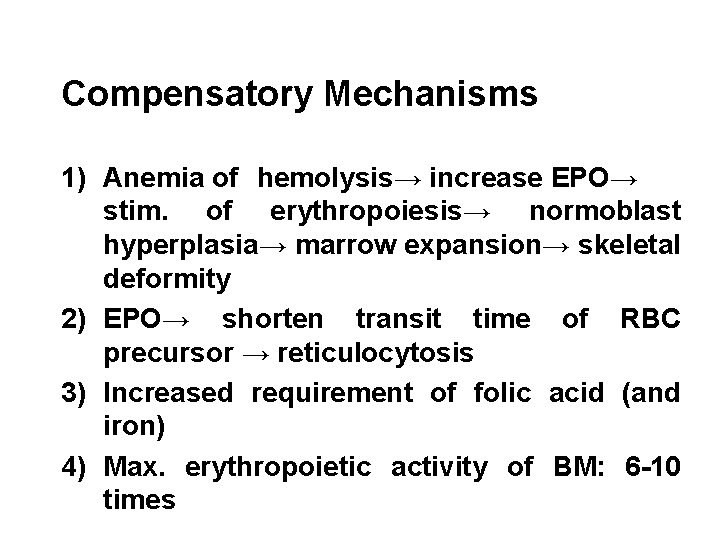
Compensatory Mechanisms 1) Anemia of hemolysis→ increase EPO→ stim. of erythropoiesis→ normoblast hyperplasia→ marrow expansion→ skeletal deformity 2) EPO→ shorten transit time of RBC precursor → reticulocytosis 3) Increased requirement of folic acid (and iron) 4) Max. erythropoietic activity of BM: 6 -10 times


Clinical Manifestation: Depend on the duration of process and its severity 1. Chronic congenital anemia • Variable from no anemia to severe enough to require transfusion • Jaundice; acholuric, bilirubin seldom rise above 4 -5 mg/d. L with normal liver, kernicterus in newborn • Crisis; usually precipitated by an intercurrent infection • aplastic crisis, hemolytic crisis, megaloblastic crisis • Splenomegaly, mild to moderate, occasionally huge • Cholelithiasis; pigmented stone due to excessive bilirubin load • Leg ulcer (bilateral and areas over malleoli or above these prominence) • Skeletal abnormality 2. Acquired hemolytic anemia • If developed acutely, Sx of acute febrile illness, abdominal or back pain, myalgia, headache, malaise, vomiting, shaking chill, fever • If developed insidiously, similar to that of congenital anemia

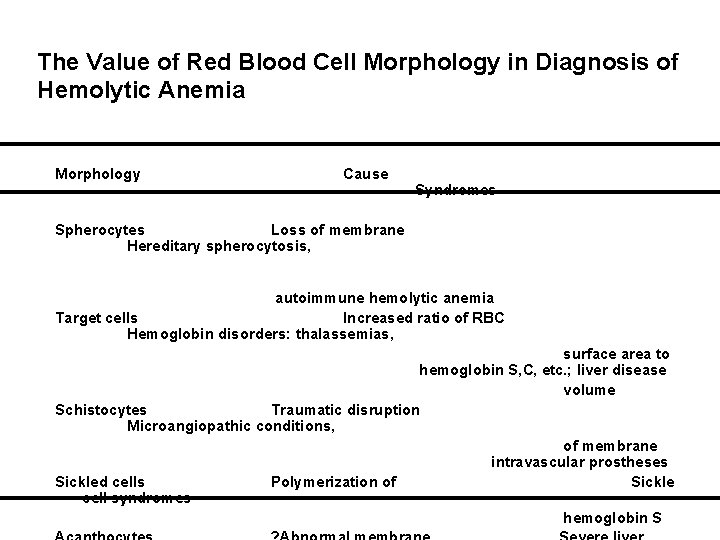
The Value of Red Blood Cell Morphology in Diagnosis of Hemolytic Anemia Morphology Cause Syndromes Spherocytes Loss of membrane Hereditary spherocytosis, autoimmune hemolytic anemia Target cells Increased ratio of RBC Hemoglobin disorders: thalassemias, surface area to hemoglobin S, C, etc. ; liver disease volume Schistocytes Traumatic disruption Microangiopathic conditions, of membrane intravascular prostheses Sickled cells Polymerization of Sickle cell syndromes hemoglobin S

 Folate deficiency symptoms
Folate deficiency symptoms Megaloblastic anemia causes
Megaloblastic anemia causes Megaloblastic anemia vs pernicious anemia
Megaloblastic anemia vs pernicious anemia Capital cf
Capital cf Hyperammonemia symptoms
Hyperammonemia symptoms Hemolysis symptoms
Hemolysis symptoms Pernicious anemia vs megaloblastic
Pernicious anemia vs megaloblastic Anemia structure
Anemia structure Microcytic anemia tails
Microcytic anemia tails Types of hemolytic anemia
Types of hemolytic anemia Acute and chronic
Acute and chronic Dj monica cruz
Dj monica cruz How is progeria diagnosed
How is progeria diagnosed Two years ago jenny was diagnosed with schizophrenia
Two years ago jenny was diagnosed with schizophrenia Crohns disease meaning
Crohns disease meaning Lisa has a diagnosed learning disability
Lisa has a diagnosed learning disability Proximate causation vs ultimate causation
Proximate causation vs ultimate causation Social behavior examples in animals
Social behavior examples in animals Faulty radiographs
Faulty radiographs Ap human geography unit 3 vocab
Ap human geography unit 3 vocab Newly industrialized population pyramid
Newly industrialized population pyramid