Outcomes of newly diagnosed multiple myeloma with deferred





- Slides: 15

Outcomes of newly diagnosed multiple myeloma with deferred ASCT after achieving at least VGPR following PAD induction chemotherapy in the Phase II PADIMAC study Wei Yee Chan, Haematology Registrar, Watford General Hospital (North Central London Rotation) On Behalf of the PADIMAC investigators 7 th November 2019

Background The current standard of care 1 in newly diagnosed transplanteligible multiple myeloma patients is induction treatment followed by ASCT. Myeloma patients are now accessing multiple lines of therapy leading to improved survival 2 with many newer agents achieving deeper responses. Patients may wish to defer their ASCT due to morbidity and mortality 3 associated with myeloablative treatment. The question remains, if it is feasible to defer ASCT, and in those who do, whether they were able to access it at relapse 4, 5.

Study Design • PADIMAC study • Phase II study • Patients were stratified to ASCT or watch and wait (W&W) according to their depth of response to treatment. • Primary end point – 2 year PFS by MRD status of patients achieving >VGPR following PAD induction who received no further treatment • Secondary endpoints • Overall response rate and toxicity of PAD • MRD negative rate following PAD and at 100 days post-PBSCH • PFS, PFS 2 and OS • Predictors of outcomes 3

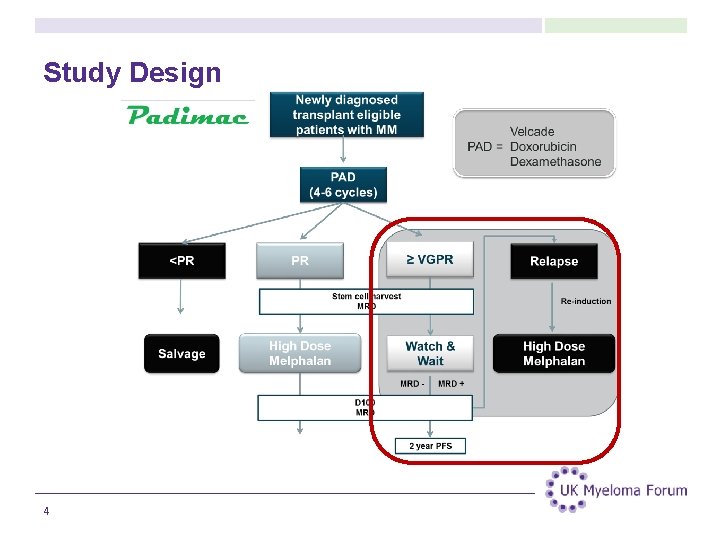
Study Design 4

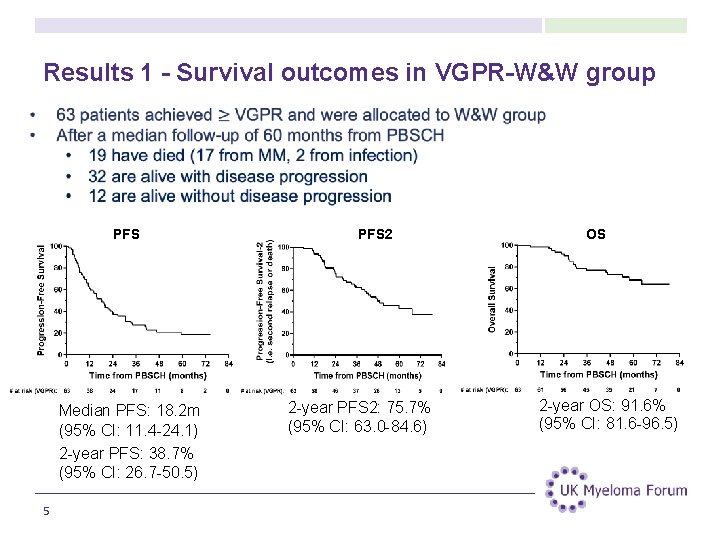
Results 1 - Survival outcomes in VGPR-W&W group PFS Median PFS: 18. 2 m (95% CI: 11. 4 -24. 1) 2 -year PFS: 38. 7% (95% CI: 26. 7 -50. 5) 5 PFS 2 2 -year PFS 2: 75. 7% (95% CI: 63. 0 -84. 6) OS 2 -year OS: 91. 6% (95% CI: 81. 6 -96. 5)

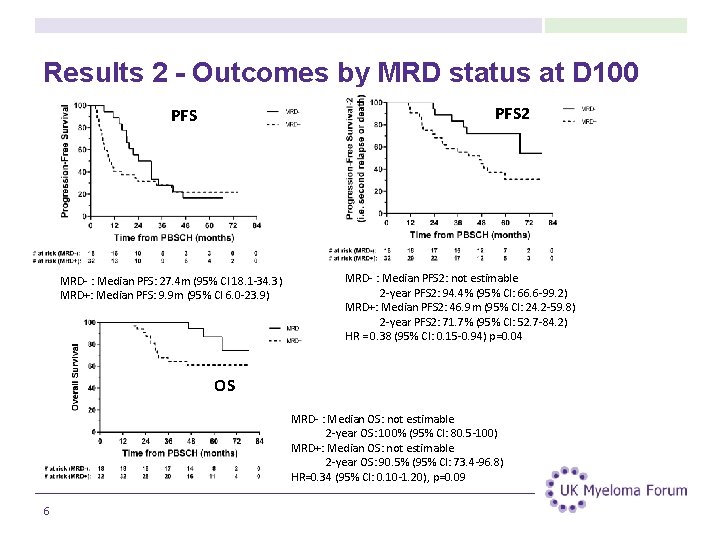
Results 2 - Outcomes by MRD status at D 100 PFS 2 PFS MRD- : Median PFS: 27. 4 m (95% CI 18. 1 -34. 3) MRD+: Median PFS: 9. 9 m (95% CI 6. 0 -23. 9) MRD- : Median PFS 2: not estimable 2 -year PFS 2: 94. 4% (95% CI: 66. 6 -99. 2) MRD+: Median PFS 2: 46. 9 m (95% CI: 24. 2 -59. 8) 2 -year PFS 2: 71. 7% (95% CI: 52. 7 -84. 2) HR = 0. 38 (95% CI: 0. 15 -0. 94) p=0. 04 OS MRD- : Median OS: not estimable 2 -year OS: 100% (95% CI: 80. 5 -100) MRD+: Median OS: not estimable 2 -year OS: 90. 5% (95% CI: 73. 4 -96. 8) HR=0. 34 (95% CI: 0. 10 -1. 20), p=0. 09 6

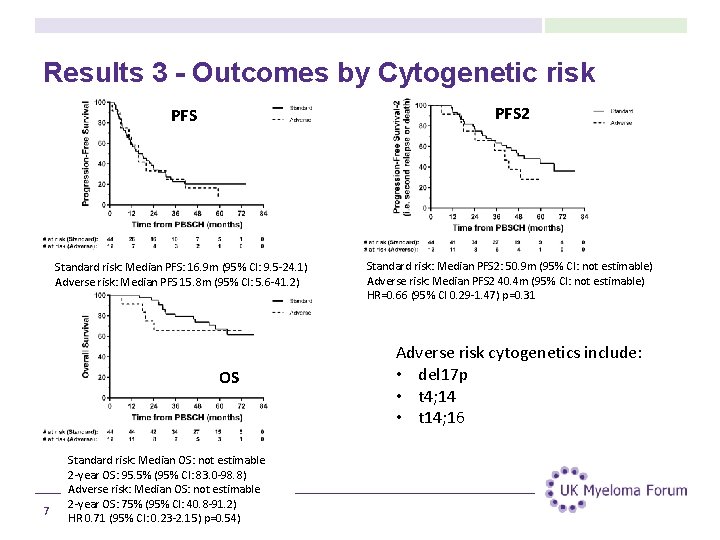
Results 3 - Outcomes by Cytogenetic risk PFS 2 Standard risk: Median PFS: 16. 9 m (95% CI: 9. 5 -24. 1) Adverse risk: Median PFS 15. 8 m (95% CI: 5. 6 -41. 2) Standard risk: Median PFS 2: 50. 9 m (95% CI: not estimable) Adverse risk: Median PFS 2 40. 4 m (95% CI: not estimable) HR=0. 66 (95% CI 0. 29 -1. 47) p=0. 31 OS 7 Standard risk: Median OS: not estimable 2 -year OS: 95. 5% (95% CI: 83. 0 -98. 8) Adverse risk: Median OS: not estimable 2 -year OS: 75% (95% CI: 40. 8 -91. 2) HR 0. 71 (95% CI: 0. 23 -2. 15) p=0. 54) Adverse risk cytogenetics include: • del 17 p • t 4; 14 • t 14; 16

Results 4 – Comparing patients who received salvage ASCT at relapse versus those who did not • 51 (81. 0%) of W&W patients received treatment at relapse • 28 (54. 9%) patients had ASCT • 23 (45. 1%) patients did not have ASCT • • ASCT cohort were younger 54 (range 31 -67) vs 60 (43 -71) p=0. 05 No significant difference at baseline in: • sex, • ECOG, • ISS stage, • r-ISS stage, • Isotype, • FISH results (standard vs adverse risk), • MRD status at PBSCH, • MRD status at D 100 8

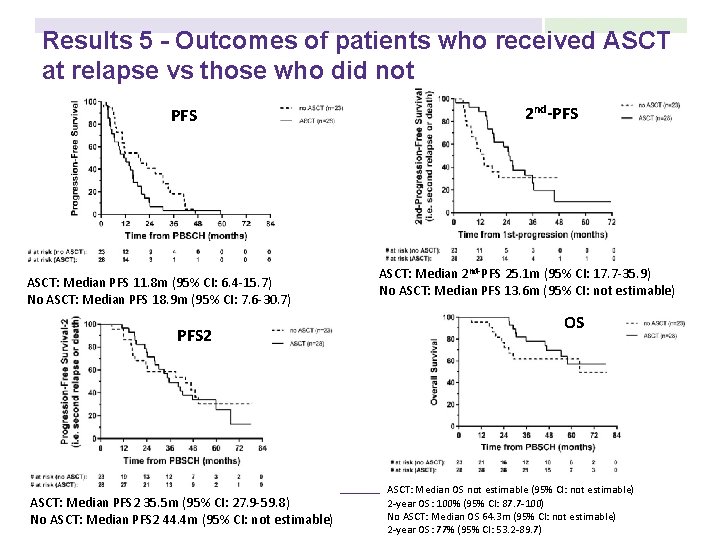
Results 5 - Outcomes of patients who received ASCT at relapse vs those who did not PFS ASCT: Median PFS 11. 8 m (95% CI: 6. 4 -15. 7) No ASCT: Median PFS 18. 9 m (95% CI: 7. 6 -30. 7) PFS 2 ASCT: Median PFS 2 35. 5 m (95% CI: 27. 9 -59. 8) No ASCT: Median PFS 2 44. 4 m (95% CI: not estimable) 2 nd-PFS ASCT: Median 2 nd-PFS 25. 1 m (95% CI: 17. 7 -35. 9) No ASCT: Median PFS 13. 6 m (95% CI: not estimable) OS ASCT: Median OS not estimable (95% CI: not estimable) 2 -year OS: 100% (95% CI: 87. 7 -100) No ASCT: Median OS 64. 3 m (95% CI: not estimable) 2 -year OS: 77% (95% CI: 53. 2 -89. 7)

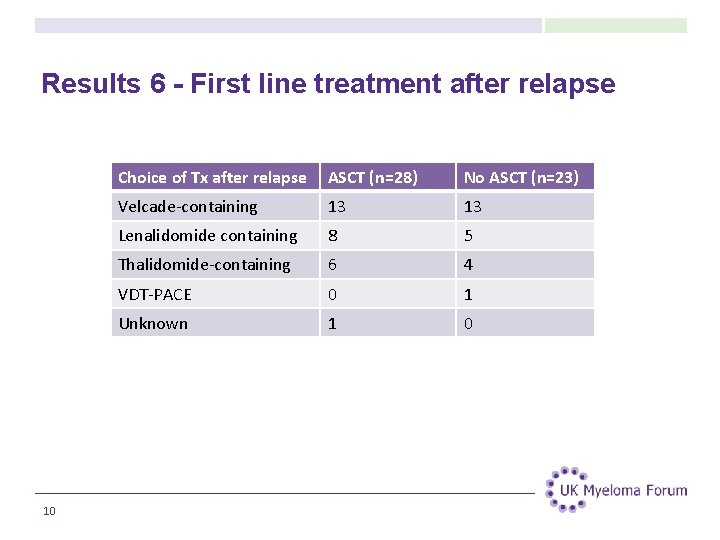
Results 6 - First line treatment after relapse 10 Choice of Tx after relapse ASCT (n=28) No ASCT (n=23) Velcade-containing 13 13 Lenalidomide containing 8 5 Thalidomide-containing 6 4 VDT-PACE 0 1 Unknown 1 0

Results 7 - Reasons for not having ASCT 11 Reasons No ASCT (n=23) Other trial or treatment options preferable 6 Age and frailty 5 Refractory disease 4 Lost to follow up / Unknown 8

Conclusion • MRD Status at D 100 post-PBSCH is prognostic of longer term outcomes in a response adapted treatment pathway • Only around half of patients allocated to ASCT deferral actually received ASCT at time of relapse. • The reasons for patients not proceeding to ASCT at relapse are multifactorial and complex. This likely includes age at relapse, accumulated comorbidities and the availability of other treatment options at the time. • Patients should be counselled on deferral of ASCT that at the time of relapse, they may not be suitable for ASCT, and therefore may never use their stored stem cells. Despite this, there will likely be other treatment options available. 12

References 1) Gay F. , Engelhardt M. , Terpos E. , Wäsch R. , Giaccone L. , Auner H. W et al. From Transplant To Novel Cellular Therapies in Multiple Myeloma: European Myeloma Network Guidelines and Future Perspectives. Haematologica February 2018 103: 197 -211 2) Raab M. , Fink L, Schoen P, Gonzalez-Mc. Quire S. , Flinois A. , Cavo M et al. Evolution of multiple myeloma treatment practices in Europe from 2014 to 2016. Br J Haematol. 2019 Jun; 185(5): 981 -984 3) Braunstein M. , Niesvizky R. Deferring autologous stem cell transplantation for consolidation of minimal residual disease in multiple myeloma. Seminars in Oncology 43 (2016) 709– 711 4) Mina R. , Lonial S. Is there a role of stem cell transplant in multiple myeloma. Cancer 2019; 125: 2534 -2543 5) 6) Kumar S. K. , Buadi F. K. , Rajkumar S. V. Pros and cons of frontline autologous transplant in multiple myeloma: the debate over timing. Blood (2019) 133 (7): 652 -659. 13

Acknowledgements Authors N Counsell 2, R Popat 1, R de Tute 3, D De-Silva 1, EH Phillips 2, J Cavenagh 4, T Adedayo 2, N Braganca 2, C Roddie 1, M Streetly 5, S Schey 6, M Koh 7, J Crowe 8, M Quinn 9, TC Morris 9, S D’Sa 10, A Virchis 11, G Cook 12, C Crawley 13, G Pratt 14, M Cook 15, L Clifton-Hadley 2, N Rabin 16, R Owen 3, K Yong 1 Affiliations 1 UCL Cancer Institute, London, 2 Cancer Research UK & UCL Cancer Trials Centre, London, 3 Leeds Teaching Hospitals NHS Trust , 4 St. Bartholomew’s Hospital, London, 5 Guy’s Hospital, London, 6 Kings Hospital, London, 7 St George’s Hospital, London, 8 Royal United Hospital, Bath, 9 Belfast Trust, Belfast 10 Mount Vernon Cancer Centre, Herts, 11 Royal Free Hospital Foundation NHS Trust, London 12 St James’s University Hospital, Leeds, 13 Addenbrookes Hospital, Cambridge, 14 Heart of England NHS Foundation Trust, Birmingham 15 Queen Elizabeth Hospital, Birmingham, 16 University College London Hospitals NHS Foundation Trust, London This work was part carried out at the UCL Cancer Institute which is an NIHR Biomedical Research Centre. This study received support from Janssen UK. 14

Acknowledgements Thank you to the UK Myeloma Forum for awarding me the bursary to attend the International Myeloma Workshop Conference 2019. 15
 Kpd multiple myeloma
Kpd multiple myeloma Waldenstrom's
Waldenstrom's Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Smoldering lymphoma
Smoldering lymphoma Multiple myelom tanı kriterleri
Multiple myelom tanı kriterleri Smoldering multiple myeloma
Smoldering multiple myeloma Crab mm
Crab mm Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Vtd protocol multiple myeloma
Vtd protocol multiple myeloma Kelly criterion example
Kelly criterion example Is crohns autoimmune
Is crohns autoimmune Lisa has a diagnosed learning disability
Lisa has a diagnosed learning disability Two years ago jenny was diagnosed with schizophrenia
Two years ago jenny was diagnosed with schizophrenia Progeria life expectancy
Progeria life expectancy How is progeria diagnosed
How is progeria diagnosed Myeloma
Myeloma