Matter Outline Properties of Matter States of matter



























- Slides: 39

Matter

Outline �Properties of Matter States of matter Physical properties Chemical properties �Changes in Matter Physical changes Chemical changes Law of Conservation of Mass �Mixtures, Elements and Compounds Heterogeneous, Homogeneous mixtures Separating Mixtures Elements and Compounds

What is matter? �Everything in the universe is made of matter. �Matter is made of tiny particles called atoms.

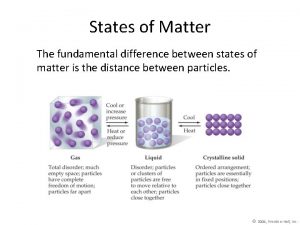
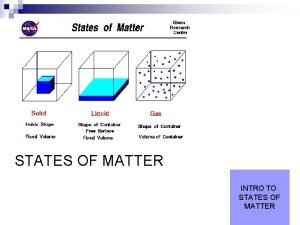
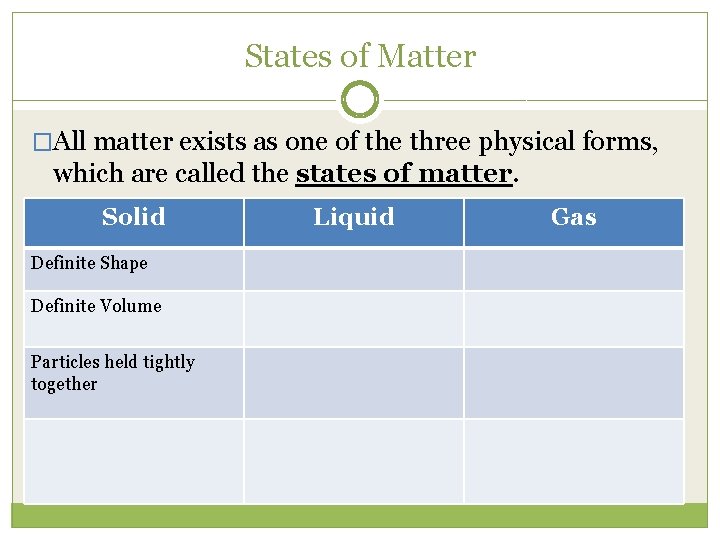
States of Matter �All matter exists as one of the three physical forms, which are called the states of matter. Solid Definite Shape Definite Volume Particles held tightly together Liquid Gas

Physical Properties �A physical property is a characteristic of matter that can be observed directly or measured without changing the substance’s composition. �Examples of Physical Properties are: �Boiling point Ductility �Melting point Elasticity �Density Brittleness �Color �Odor �Malleability

Chemical Properties �Characteristic of matter that can only be observed when one substance changes into a different substance �Examples of Chemical Properties: �Flammability �Reactivity with acids �Ability to rust

Identifying Changes �Physical Changes Physical state • Chemical Changes

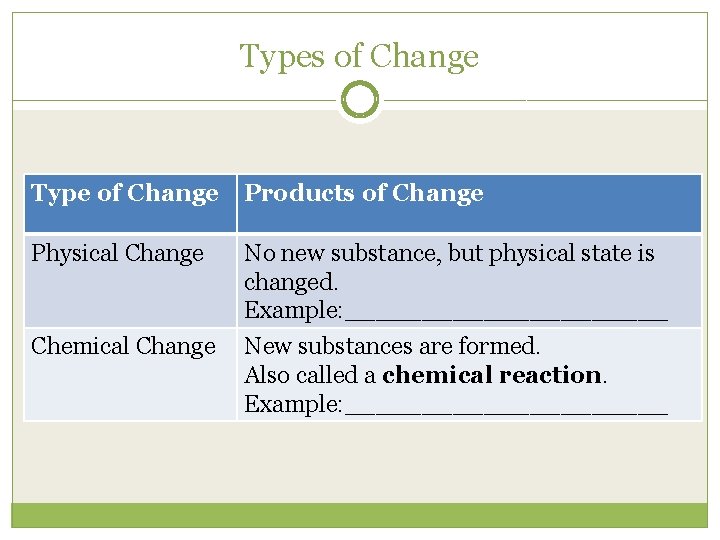
Types of Change Type of Change Products of Change Physical Change No new substance, but physical state is changed. Example: ___________ Chemical Change New substances are formed. Also called a chemical reaction. Example: ___________

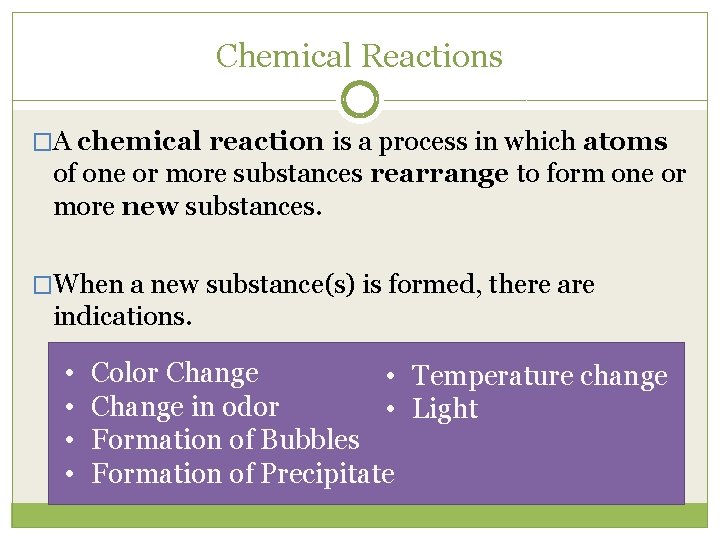
Chemical Reactions �A chemical reaction is a process in which atoms of one or more substances rearrange to form one or more new substances. �When a new substance(s) is formed, there are indications. • • Color Change • Temperature change Change in odor • Light Formation of Bubbles Formation of Precipitate

Conservation of Mass �The Law of Conservation of Mass states that mass is neither created nor destroyed during a chemical reaction. Mass is always conserved in a chemical reaction.

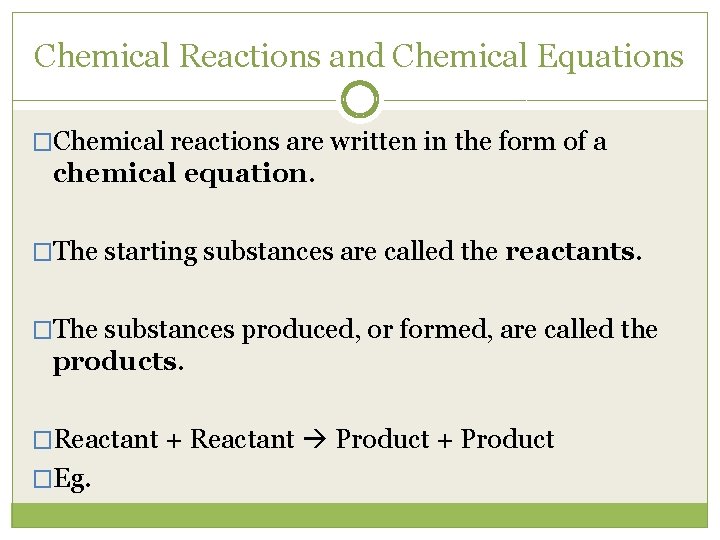
Chemical Reactions and Chemical Equations �Chemical reactions are written in the form of a chemical equation. �The starting substances are called the reactants. �The substances produced, or formed, are called the products. �Reactant + Reactant Product + Product �Eg.

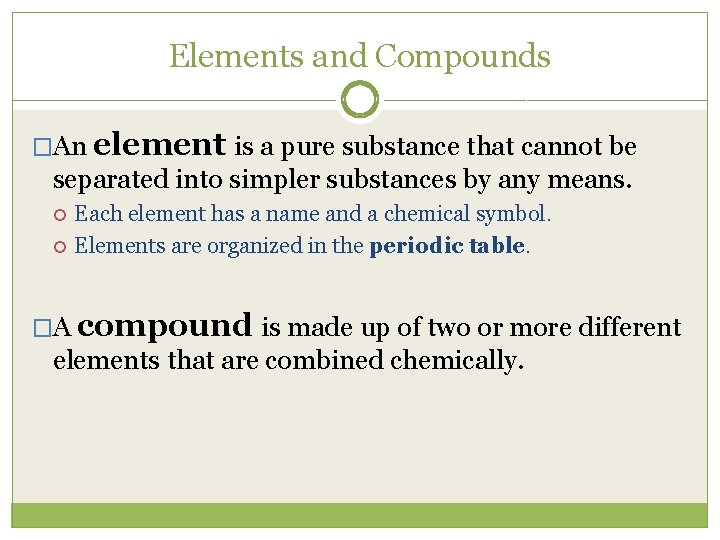
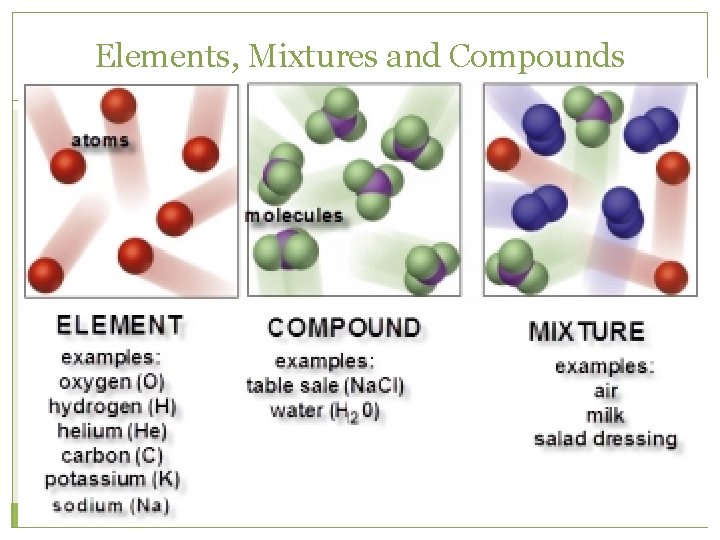
Elements and Compounds �An element is a pure substance that cannot be separated into simpler substances by any means. Each element has a name and a chemical symbol. Elements are organized in the periodic table. �A compound is made up of two or more different elements that are combined chemically.

Mixtures �A mixture is a combination of two or more pure substances. Each substance in a mixture keeps its chemical properties. �A pure substance is a substance that is the same throughout. Example:

Compounds: Law of Definite Proportions �A compound is always composed of the same elements in the same proportion by mass. �Mass of a compound = sum of the masses of the elements that make up the compound

Compounds: Law of Multiple Proportions �When different compounds are formed by a combination of the same elements, �If two elements form more than one compound between them, then the ratios of the masses of the second element (which combine with a fixed mass of the first element) will be ratios of small whole numbers �Example:

Elements, Mixtures and Compounds













Mixtures �Types of Mixtures: Homogeneous: “the same” A mixture that has constant composition throughout. Example: Heterogeneous: “different” A mixture that does not blend smoothly and has a non-uniform composition Example:



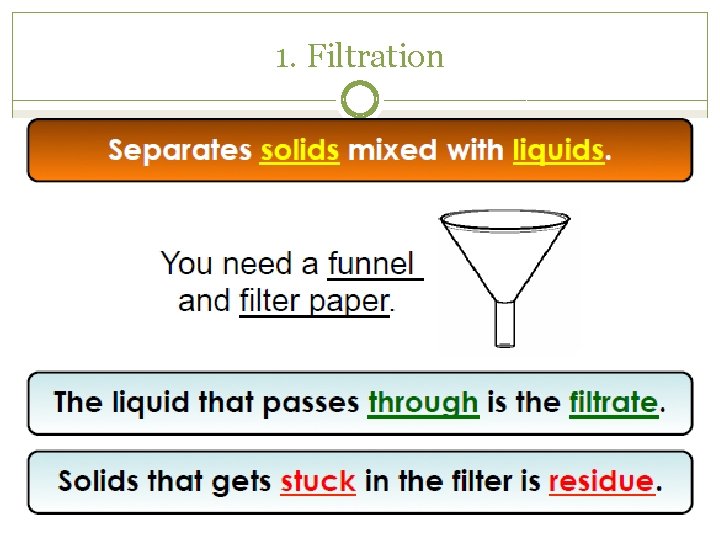
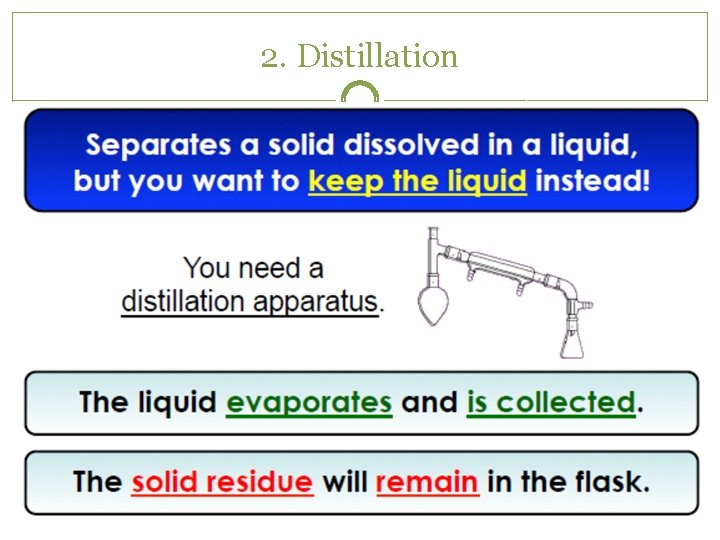
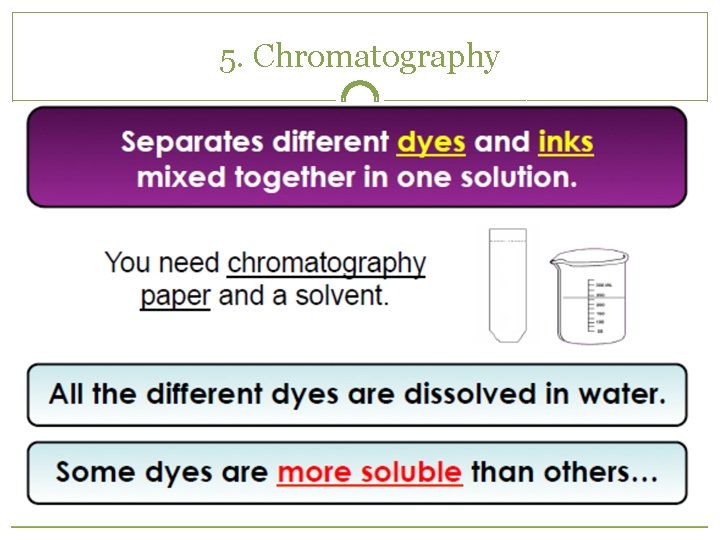
Separating Mixtures �Because substances in a mixture are physically combined, the processes used to separate a mixture are physical (no need for chemical reactions) �Filtration �Distillation �Crystallization �Sublimation �Chromatography

1. Filtration

2. Distillation


3. Crystallization �Formation of pure solid particles of a substance from a solution containing the dissolved substance.

4. Sublimation or Evaporation �Sublimation: Solid changes to vapor without melting �Eg. �Evaporation: Liquid changes to gas �Eg.


5. Chromatography
 Slave states free states
Slave states free states Southern states vs northern states
Southern states vs northern states How does the constitution guard against tyranny
How does the constitution guard against tyranny What is a quote sandwich examples
What is a quote sandwich examples Lesson 2 physical properties answer key
Lesson 2 physical properties answer key Lesson outline lesson 2 wave properties answer key
Lesson outline lesson 2 wave properties answer key Whats the study of matter and energy
Whats the study of matter and energy Four states of matter
Four states of matter Four states of matter
Four states of matter 5 states of matter
5 states of matter Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Changing states of matter
Changing states of matter Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Solid liquid gas venn diagram
Solid liquid gas venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that Chapter 12 states of matter
Chapter 12 states of matter Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter basics
States of matter basics States of matter foldable
States of matter foldable Heat thermal energy and temperature
Heat thermal energy and temperature Uses of heat
Uses of heat The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Stayes of matter
Stayes of matter Solid liquid gas plasma
Solid liquid gas plasma Mind map on states of matter
Mind map on states of matter Phase change concept map solid liquid gas
Phase change concept map solid liquid gas Whats states of matter
Whats states of matter States of matter
States of matter Properties of matter jeopardy
Properties of matter jeopardy What is the particle theory of matter
What is the particle theory of matter States of matter
States of matter Four states of matter
Four states of matter States of matter solid liquid gas
States of matter solid liquid gas Interconversion of states of matter
Interconversion of states of matter Classifying matter flowchart
Classifying matter flowchart Five states of matter
Five states of matter Examples of gas
Examples of gas States of matter
States of matter 4 fe
4 fe