Jeopardy Freezing Melting Heat and Its Meas Vapor
































- Slides: 53

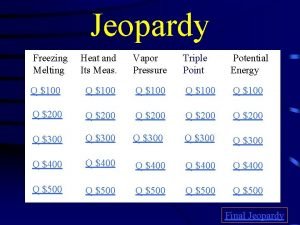
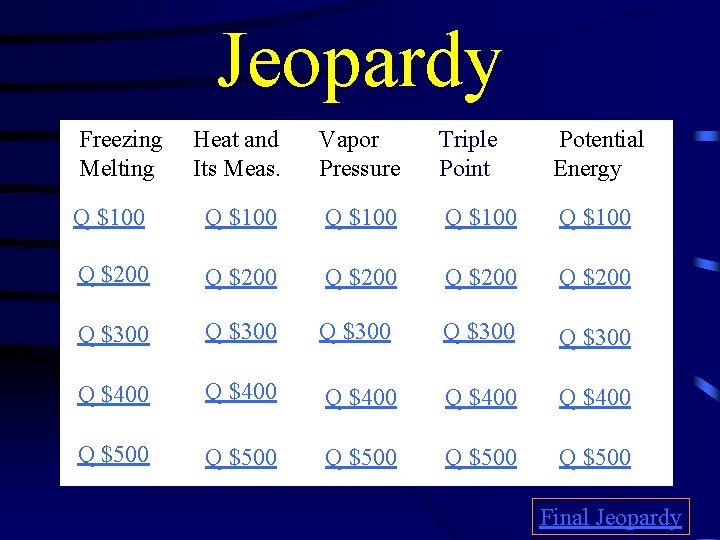
Jeopardy Freezing Melting Heat and Its Meas. Vapor Pressure Triple Point Potential Energy Q $100 Q $100 Q $200 Q $200 Q $300 Q $300 Q $400 Q $400 Q $500 Q $500 Final Jeopardy

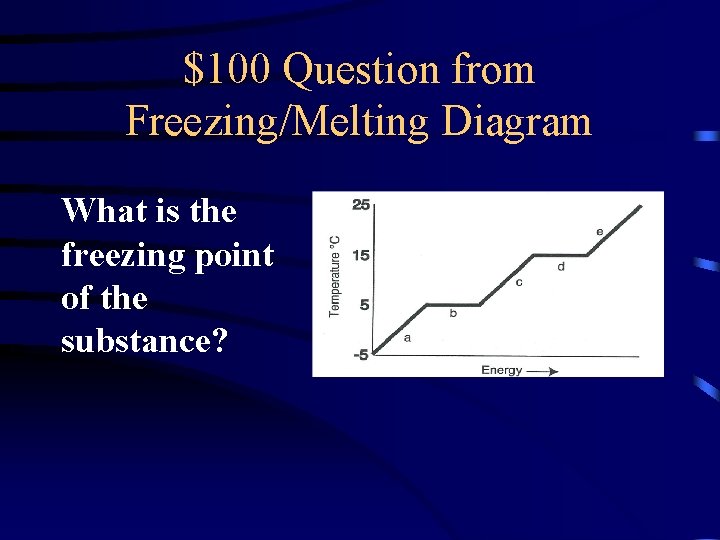
$100 Question from Freezing/Melting Diagram What is the freezing point of the substance?

$100 Answer from Freezing/Melting Diagram o 5 C

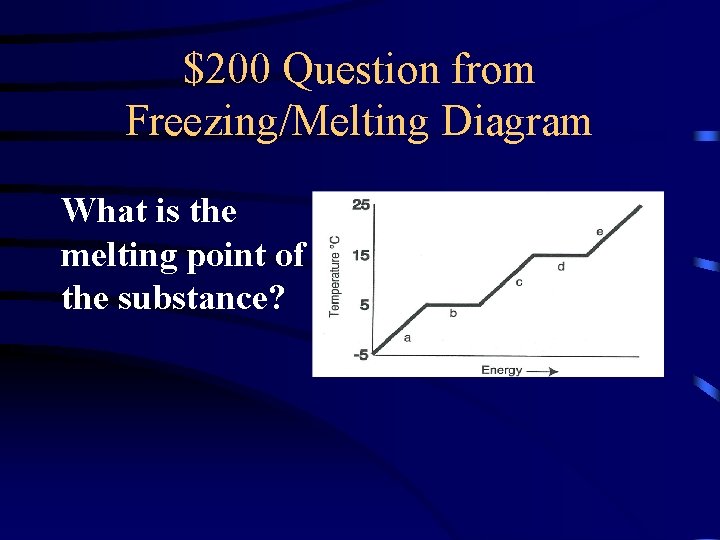
$200 Question from Freezing/Melting Diagram What is the melting point of the substance?

$200 Answer from Freezing/Melting Diagram o 5 C

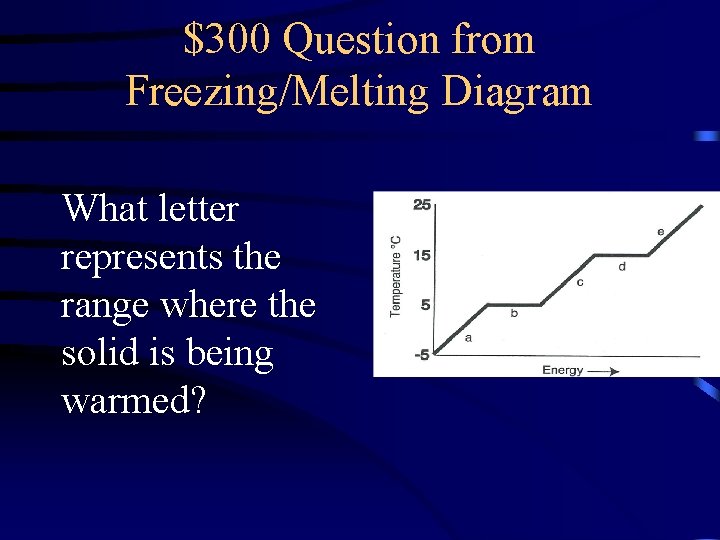
$300 Question from Freezing/Melting Diagram What letter represents the range where the solid is being warmed?

$300 Answer from Freezing/Melting Diagram A

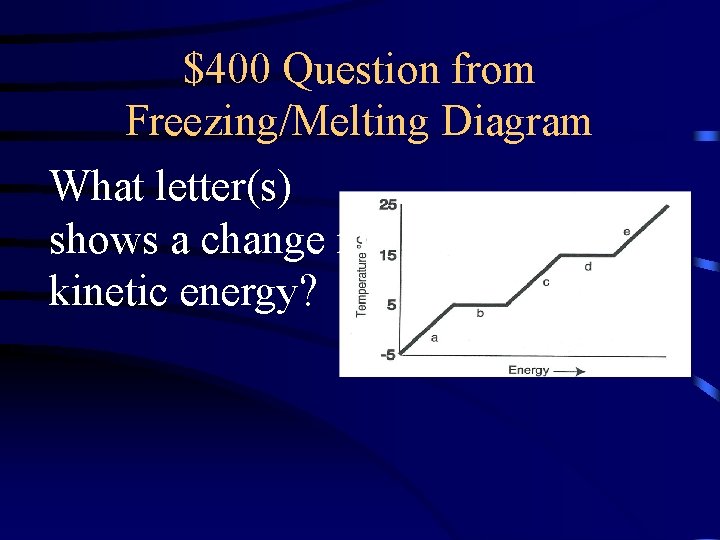
$400 Question from Freezing/Melting Diagram What letter(s) shows a change in kinetic energy?

$400 Answer from Freezing/Melting Diagram A, C, E

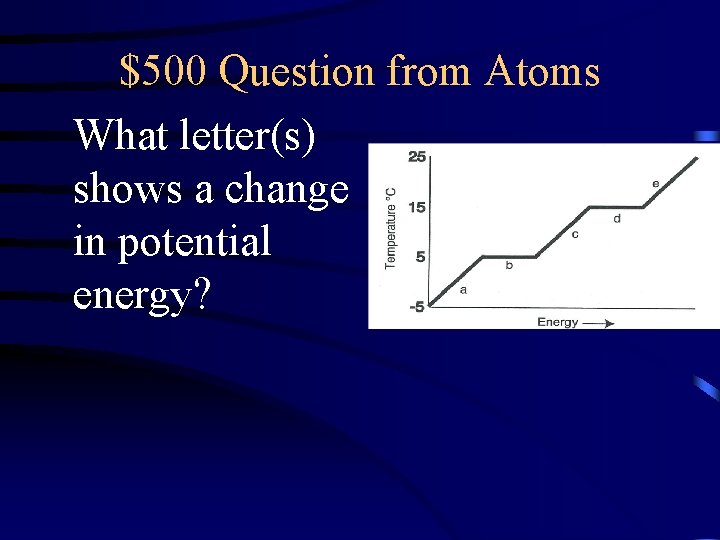
$500 Question from Atoms What letter(s) shows a change in potential energy?

$500 Answer from Freezing/Melting Diagram B, D

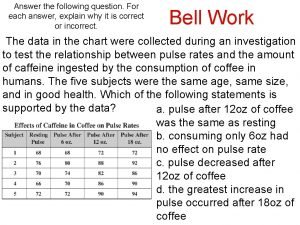
$100 Question from Heat and Its Measurement How many joules of heat are given off when 3. 5 g of water cool from 75 o. C to 25 o. C?

$100 Answer from Heat and Its Measurement -730 J

$200 Question from Heat and Its Measurement How many joules does it take to melt 25 g of ice at 0 o. C? .

$200 Answer from Heat and Its Measurement 8400 J

$300 Question from Heat and Its Measurement How many joules are given off when 120 g of steam condense to liquid water?

$300 Answer from Heat and Its Measurement ans: -270000 J

$400 Question from Heat and Its Measurement How many joules of heat are necessary to raise the temperature of 40 g of water from o o 20 C to 90 C?

$400 Answer from Heat and Its Measurement ans: 12000 J

$500 Question from Heat and Its Measurement How much heat is released when 75 g of water at 0 o. C freezes?

$500 Answer from Heat and Its Measurement -25000 J

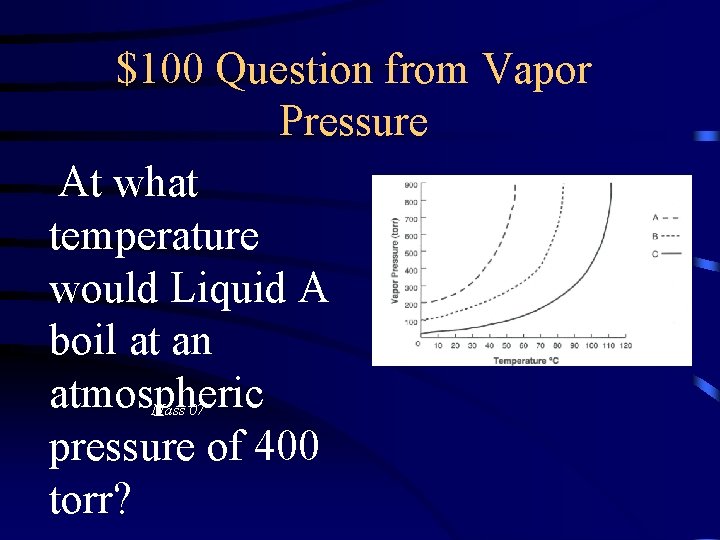
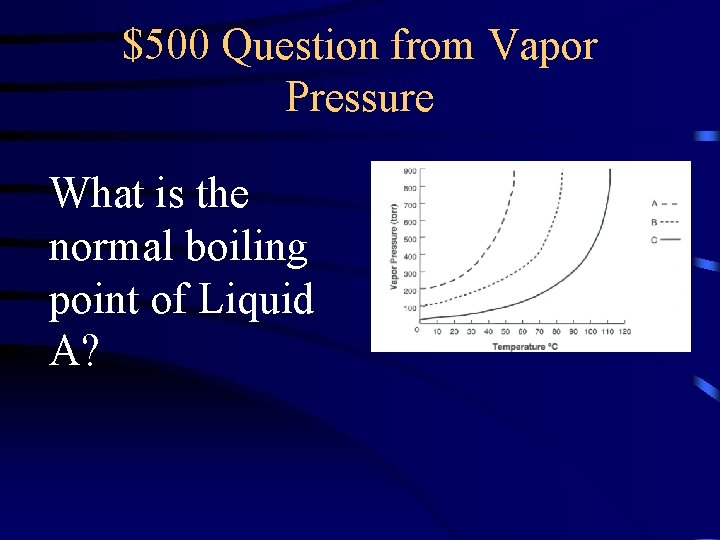
$100 Question from Vapor Pressure At what temperature would Liquid A boil at an atmospheric pressure of 400 torr? Mass 07

$100 Answer from Vapor Pressure 32 o. C

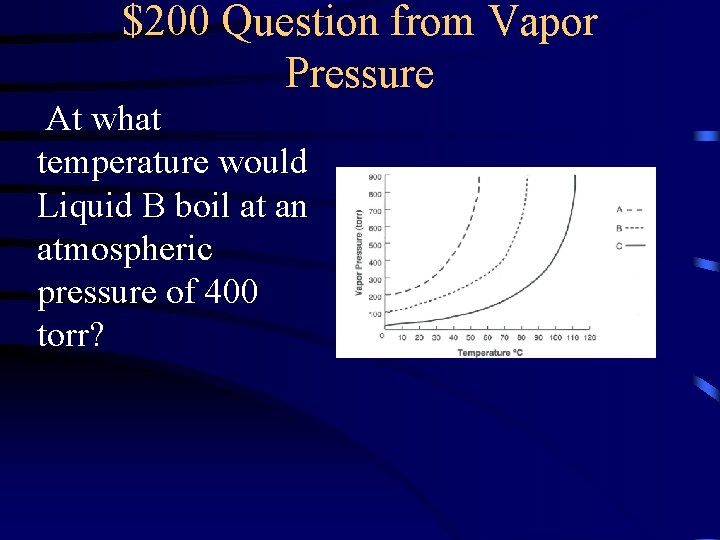
$200 Question from Vapor Pressure At what temperature would Liquid B boil at an atmospheric pressure of 400 torr?

$200 Answer from Vapor Pressure 70 o. C

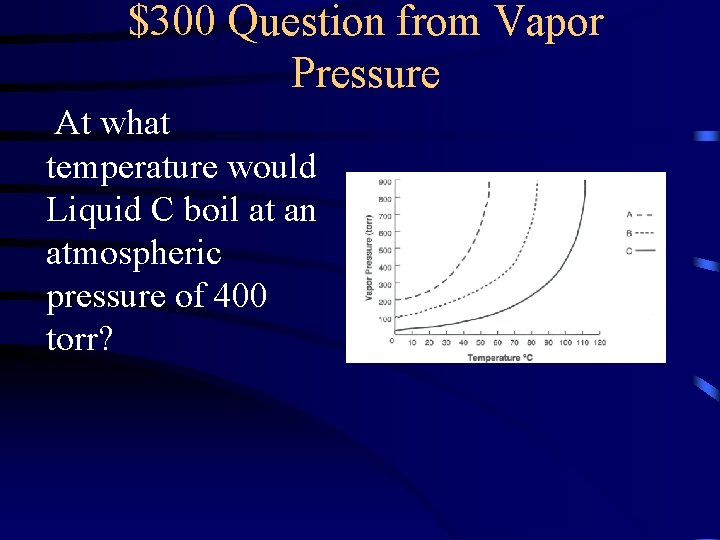
$300 Question from Vapor Pressure At what temperature would Liquid C boil at an atmospheric pressure of 400 torr?

$300 Answer from Vapor Pressure 95 o. C

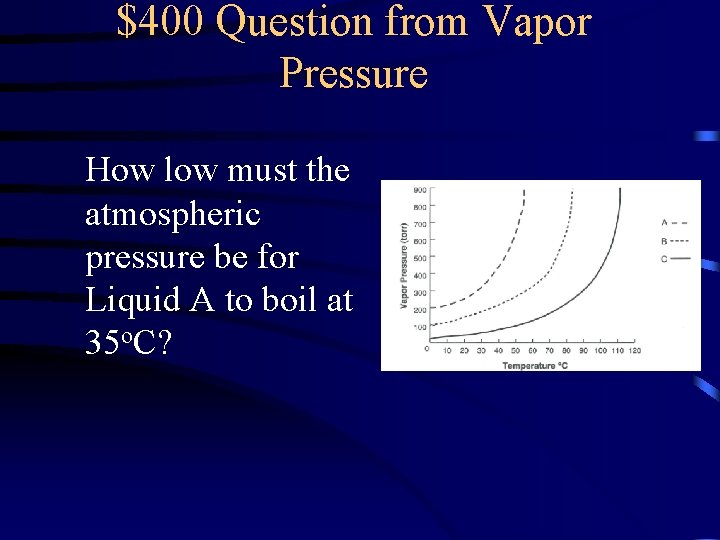
$400 Question from Vapor Pressure How low must the atmospheric pressure be for Liquid A to boil at 35 o. C?

$400 Answer from Vapor Pressure 550 torr

$500 Question from Vapor Pressure What is the normal boiling point of Liquid A?

$500 Answer from Vapor Pressure o 43 C

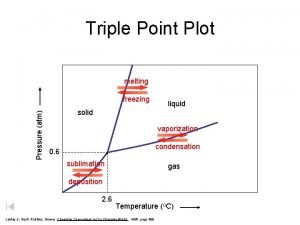
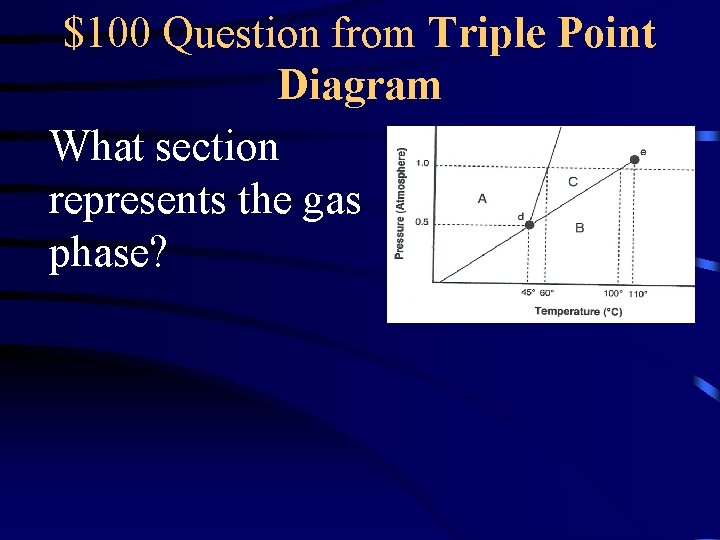
$100 Question from Triple Point Diagram What section represents the gas phase?

$100 Answer from Triple Point Diagram B

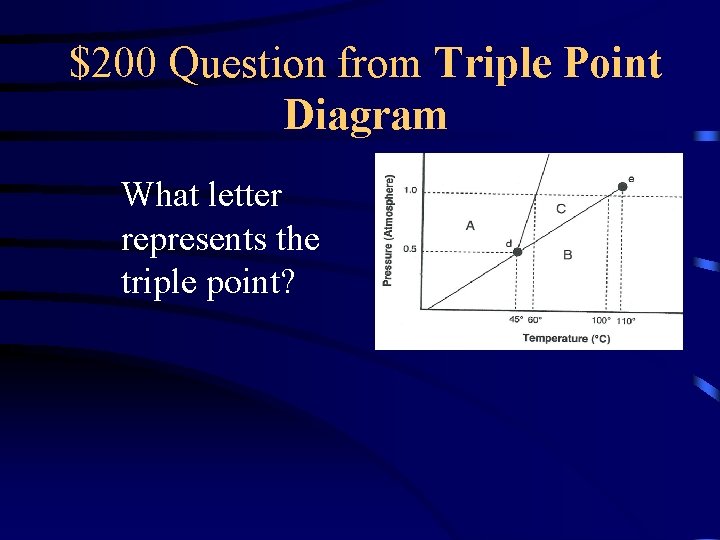
$200 Question from Triple Point Diagram What letter represents the triple point?

$200 Answer from Triple Point Diagram d

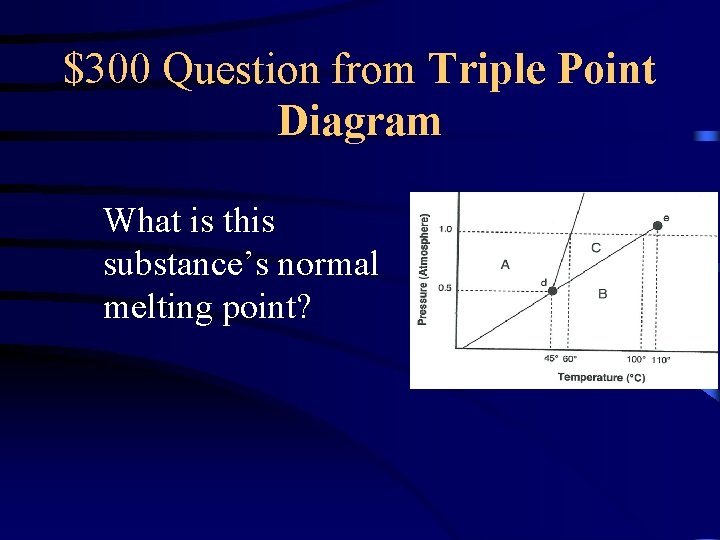
$300 Question from Triple Point Diagram What is this substance’s normal melting point?

$300 Answer from Triple Point Diagram 60 o. C

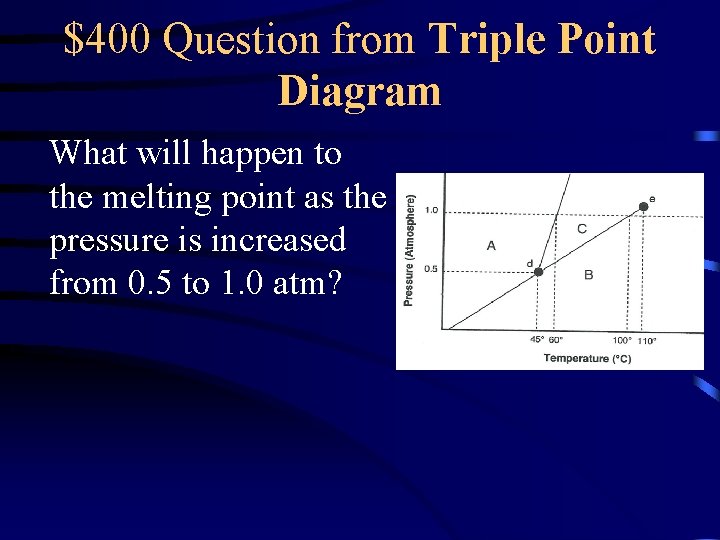
$400 Question from Triple Point Diagram What will happen to the melting point as the pressure is increased from 0. 5 to 1. 0 atm?

$400 Answer from Triple Point Diagram It increases from o 45 to 60 C

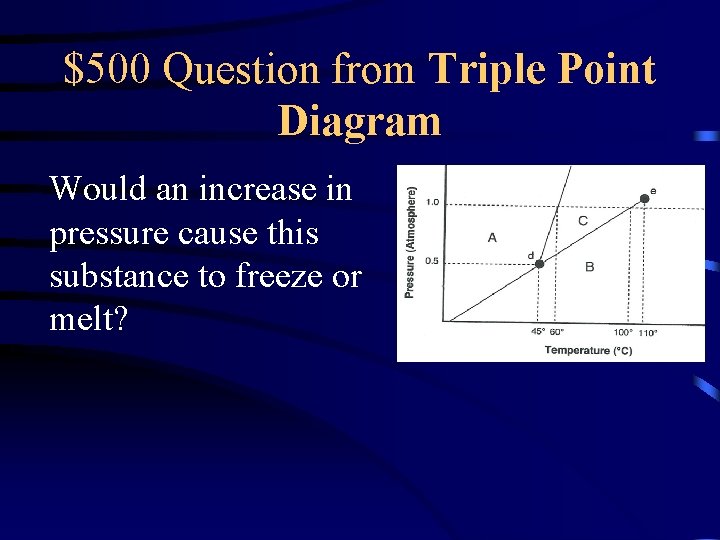
$500 Question from Triple Point Diagram Would an increase in pressure cause this substance to freeze or melt?

$500 Answer from Triple Point Diagram freeze

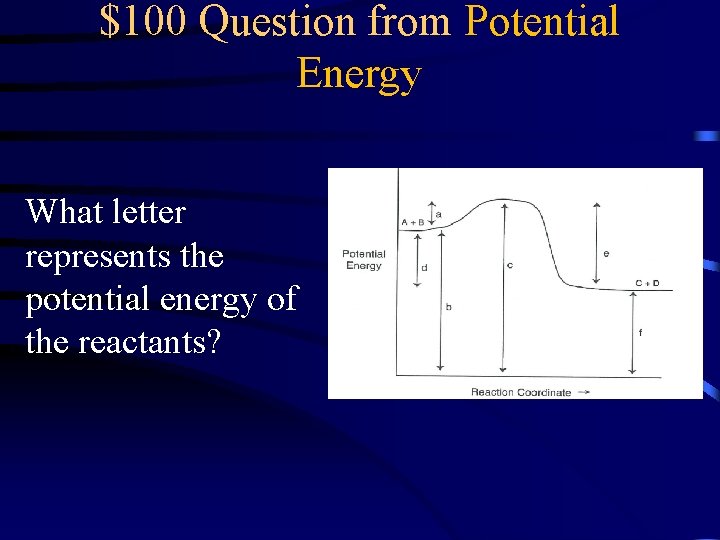
$100 Question from Potential Energy What letter represents the potential energy of the reactants?

$100 Answer from Potential Energy B

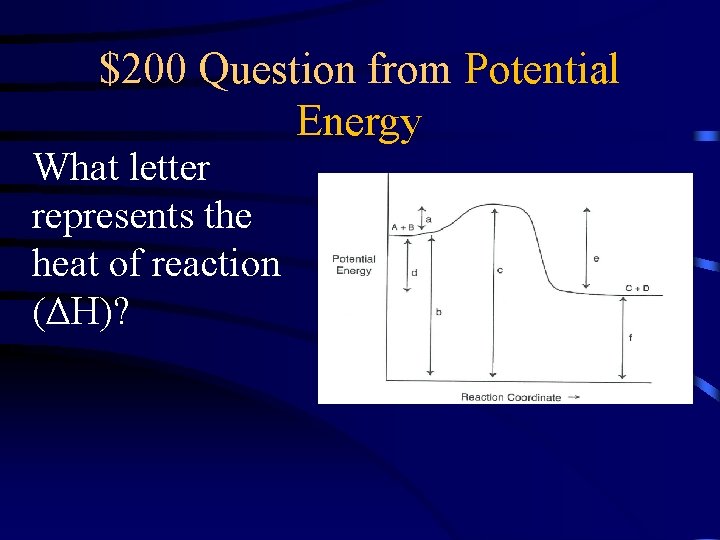
$200 Question from Potential Energy What letter represents the heat of reaction (ΔH)?

$200 Answer from Potential Energy D

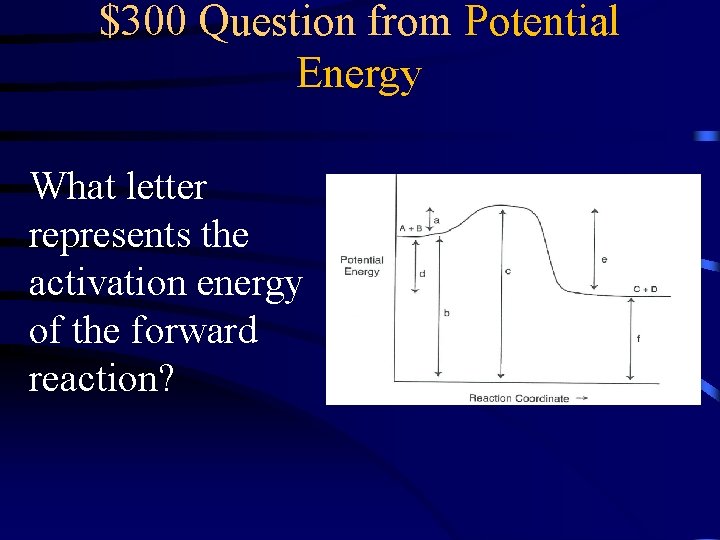
$300 Question from Potential Energy What letter represents the activation energy of the forward reaction?

$300 Answer from Potential Energy A

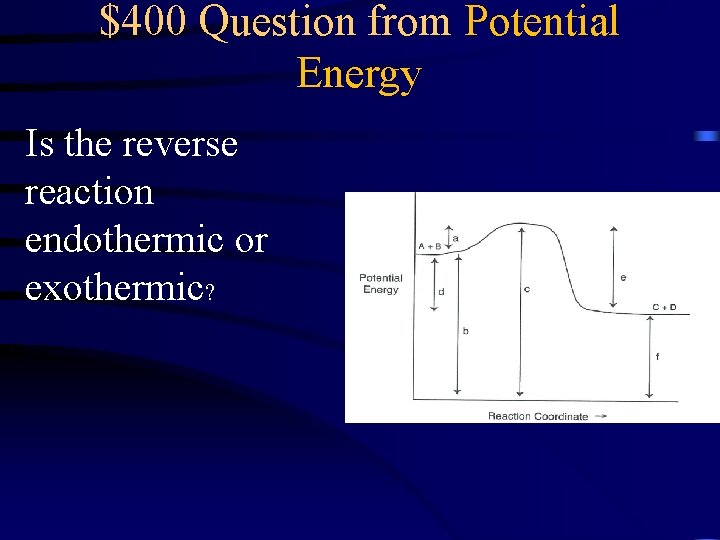
$400 Question from Potential Energy Is the reverse reaction endothermic or exothermic?

$400 Answer from Potential Energy Endothermic

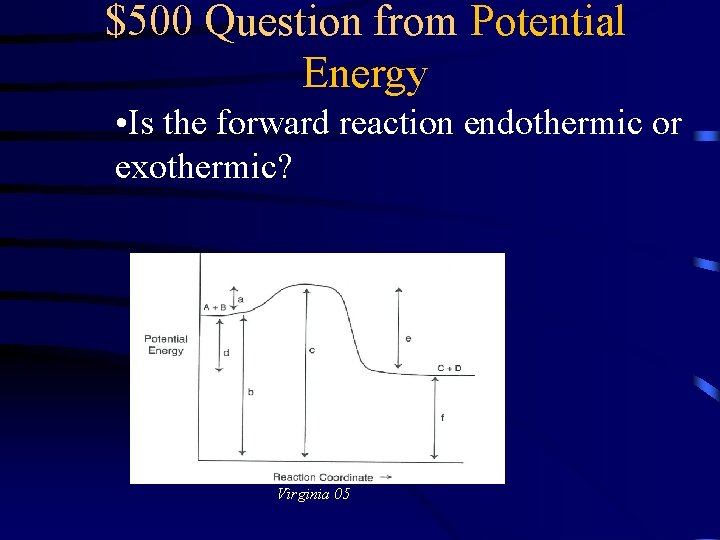
$500 Question from Potential Energy • Is the forward reaction endothermic or exothermic? Virginia 05

$500 Answer from Potential Energy Exothermic

Final Jeopardy Determine the heat required to convert 62. 0 grams of ice at -10. 3 °C to water at 100. 0 °C. The specific heat capacity of ice is 2. 05 J/g °C.

Final Jeopardy Answer Ans. 4. 79 E 4 J 47 900 J
 Heat meas
Heat meas Superaquecimento
Superaquecimento Figurative language fahrenheit 451
Figurative language fahrenheit 451 6 common phase changes
6 common phase changes Melting evaporation condensation freezing
Melting evaporation condensation freezing Freezing melting evaporation
Freezing melting evaporation What is incongruent melting
What is incongruent melting What is meas
What is meas Fisheriris
Fisheriris Por que meas abandonado
Por que meas abandonado Unit of latent heat
Unit of latent heat The emigree poem
The emigree poem Its halloween its halloween the moon is full and bright
Its halloween its halloween the moon is full and bright Hardness texture color and freezing point are examples of
Hardness texture color and freezing point are examples of Why is earth called the blue planet
Why is earth called the blue planet Vapor pressure worksheet
Vapor pressure worksheet Example of ion dipole
Example of ion dipole Specific heat capacity table pdf
Specific heat capacity table pdf Dry heat cooking examples
Dry heat cooking examples Heat transfer jeopardy
Heat transfer jeopardy Thermal energy jeopardy
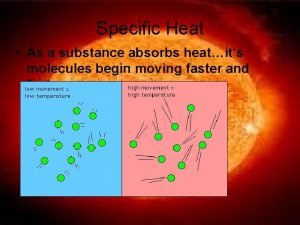
Thermal energy jeopardy When a substance absorbs heat its molecules will
When a substance absorbs heat its molecules will When a train increases its velocity its momentum
When a train increases its velocity its momentum Rainy sunny cloudy windy
Rainy sunny cloudy windy If its square its a sonnet
If its square its a sonnet Its not easy but its worth it
Its not easy but its worth it Cool chilly cold freezing
Cool chilly cold freezing Nashville egg donation
Nashville egg donation Expansion upon freezing
Expansion upon freezing Expansion upon freezing
Expansion upon freezing Mobile tcp in mobile computing
Mobile tcp in mobile computing Market form of
Market form of Air freezing point
Air freezing point Pham method freezing
Pham method freezing Fluidised bed freezer
Fluidised bed freezer Hpt makes frozen food products
Hpt makes frozen food products Freezing method of food preservation
Freezing method of food preservation Introduction to food engineering
Introduction to food engineering Pure solvent
Pure solvent Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Pure solvent
Pure solvent Expansion upon freezing
Expansion upon freezing A biome that is cold, dry and treeless.
A biome that is cold, dry and treeless. Expansion upon freezing
Expansion upon freezing Expansion upon freezing
Expansion upon freezing Upper air soundings
Upper air soundings Freezing point of brine fahrenheit
Freezing point of brine fahrenheit Dehydrofreezing
Dehydrofreezing Transmission/timeout freezing
Transmission/timeout freezing Freezing point of xylene
Freezing point of xylene Molar mass from freezing point depression calculator
Molar mass from freezing point depression calculator Aim do now
Aim do now Personification of a lake during winter
Personification of a lake during winter Fst freezing
Fst freezing