FST 151 FOOD FREEZING FOOD SCIENCE AND TECHNOLOGY










































- Slides: 65

FST 151 FOOD FREEZING FOOD SCIENCE AND TECHNOLOGY 151 Food Freezing - Basic concepts (cont’d) Lecture Notes Prof. Vinod K. Jindal (Formerly Professor, Asian Institute of Technology) Visiting Professor Chemical Engineering Department Mahidol University Salaya, Nakornpathom Thailand Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 1

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 2

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 3

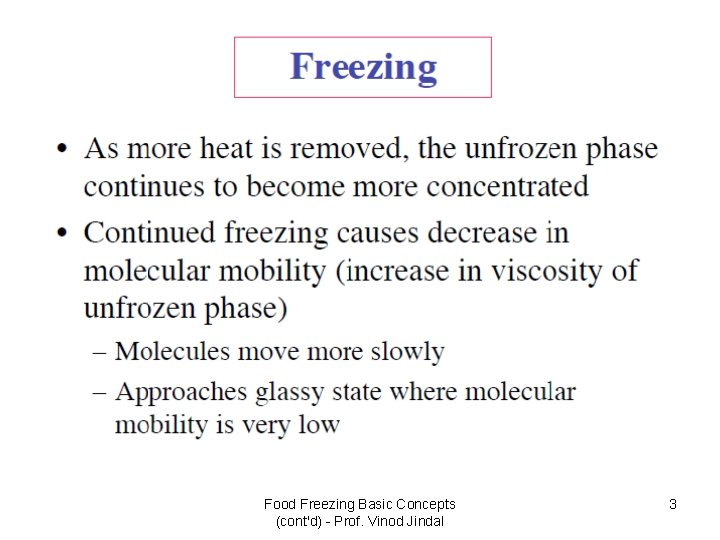
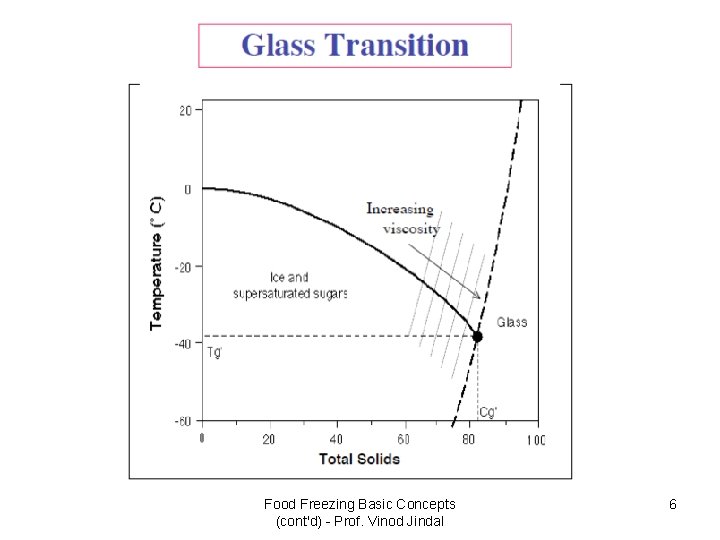
The change from amorphous to glassy state? • An amorphous phase is a condensed phase (i. e. , liquid or solid) without a regular crystalline order. Many foods crystallize only very slowly if at all so once they become supersaturated they will become increasingly viscous without crystallizing. • We can define two types of amorphous phase – rubbery and glassy. The rubbery state is more mobile (and therefore softer and less viscous) than the glassy form. • The transition from a rubbery to a glassy state is governed by molecular mobility. A reduction in temperature or the amount of water will transform rubbery state to glass and the food will become more brittle and less chemically reactive.

What is the Glassy State? • During processing and storage, the physical properties of food products change dramatically depending on water availability and temperature. • Amorphous and partially amorphous structures in foods are formed in various processes such as baking, drying and freezing. • The removal of water, by evaporation or separation as ice, produces supersaturated states of dissolved substances. • Depending on temperature and water content, these unfrozen fractions can be in either a glassy or rubbery state.

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 6

Typical Tg’ values for various food products Food Product orange juice raspberry juice strawberry apple tomato carrot egg white egg yolk beef muscle ice creams Tg’ (°C) - 30 to - 38 - 37 to - 40 - 43 - 37 - 21 - 32 - 38 - 32 - 80/ - 35 - 34

• The values shown in Table indicate that the glassy state can be achieved only for a limited number of frozen food systems at temperatures commonly employed in the distribution chain. • This leads to an important question as to what is the appropriate temperature for predicting the stability of frozen foods at temperatures above Tg’?

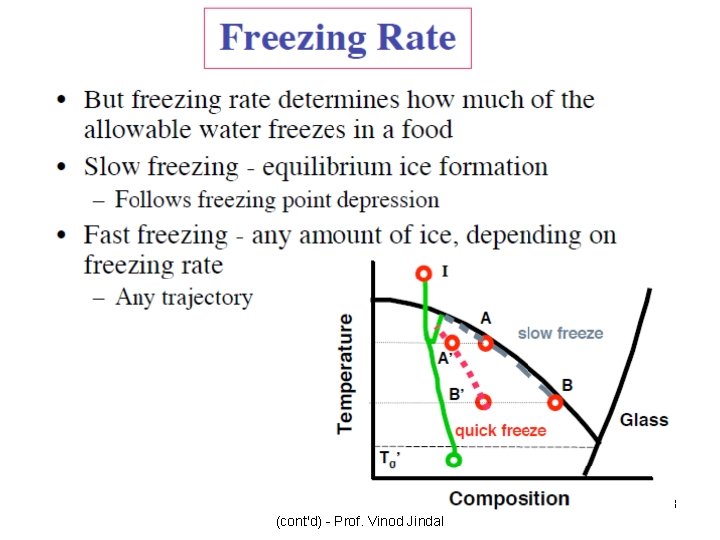
Why is the Glassy State Important in Understanding Storage Stability? • It is assumed that stability of foods, i. e. their shelf life and quality, is maintained in the glassy state. Below glass transition temperature the freeze-concentrated fraction is a glass and thus long-term stability may be expected. • It is also assumed that changes occur above the glass transition and their kinetics depends upon the difference between the storage and the glass transition temperatures. • Stability of frozen foods strongly depends upon the storage temperature. The deterioration rate may be multiplied by a factor of between 2 and 30 for an increase of the storage temperature by 10°C.

Can Glass Transitions tell us about Product Stability above Tg? • Attempts have been made to explain the drastic effect of temperature above Tg’ on rates of degradation. However, the experimental data of ice crystal growth in ice creams as a function of Tstorage -Tg’ cannot explain the influence of stabilizers which do not influence Tg’. • In practice this makes it difficult to explain the changes in the quality of frozen products by a single parameter e. g. Tg’. However, an understanding of the mobility of reactive species in frozen foods and thus their stability as a function of temperature and formulation is important.

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 11

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 12

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 13

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 14

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 15

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 16

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 17

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 18

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 19

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 20

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 21

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 22

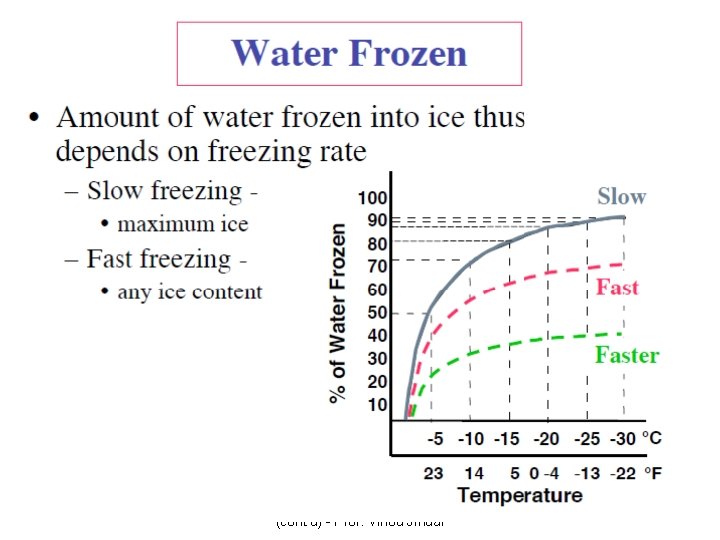
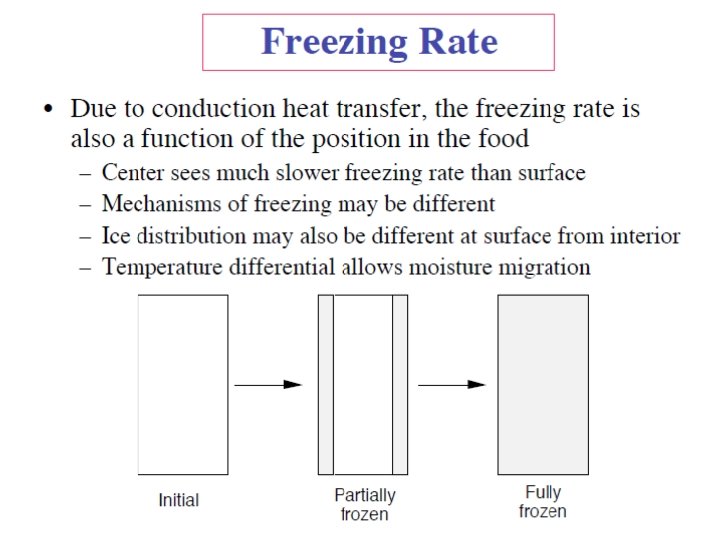
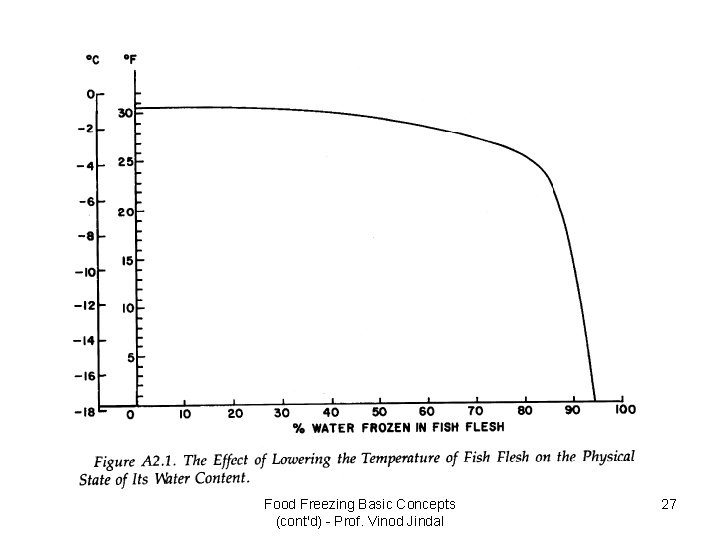
Frozen-Food Properties • Depend on thermal properties of the food product. • Phase change: Liquid (water) change to solid, the density, thermal conductivity, heat content (enthalpy), specific heat of the product change as temperature decreases below the initial freezing point for water in the food. • 1. Density – The density of solid water is less than that of liquid water – The density of a frozen food is less than the unfrozen product Intensive properties – The magnitude of change in density is proportional to the moisture content of the product • 2. Thermal conductivity – The thermal conductivity of ice is about four times larger than that of liquid water. – Same influence in thermal conductivity of a frozen food. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 23

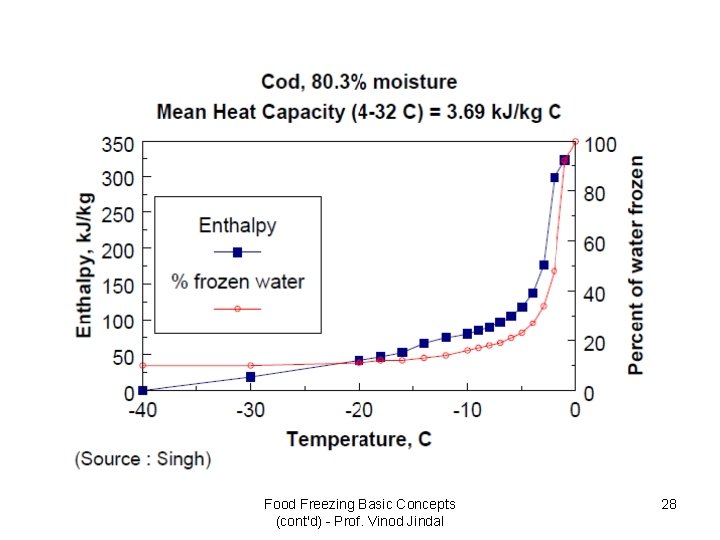
Frozen-Food Properties • 3. Enthalpy (heat content) – Important parameter for refrigeration requirement – The heat content normally zero at -40 o. C and increases with increasing temperature – Significant changes in enthalpy occur in 10 o. C below the initial freezing temperature. • 4. Apparent specific heat – Depend on function of temperature and phase changes for water in the product – The specific heat of a frozen food at a temperature greater than 20 below the initial point (-2. 61 o. C) • 5. Apparent thermal diffusivity – The apparent thermal diffusivity increases as the temperature decreases below the initial freezing point – Frozen product shows larger magnitude than unfrozen product Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 24

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 25

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 26

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 27

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 28

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 29

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 30

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 31

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 32

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 33

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 34

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 35

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 36

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 37

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 38

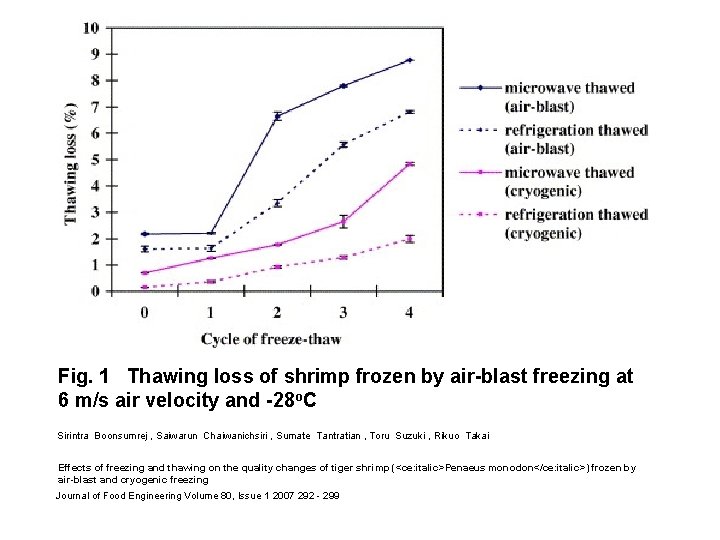
Fig. 1 Thawing loss of shrimp frozen by air-blast freezing at 6 m/s air velocity and -28 o. C Sirintra Boonsumrej , Saiwarun Chaiwanichsiri , Sumate Tantratian , Toru Suzuki , Rikuo Takai Effects of freezing and thawing on the quality changes of tiger shrimp (<ce: italic>Penaeus monodon</ce: italic>) frozen by air-blast and cryogenic freezing Journal of Food Engineering Volume 80, Issue 1 2007 292 - 299

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 40

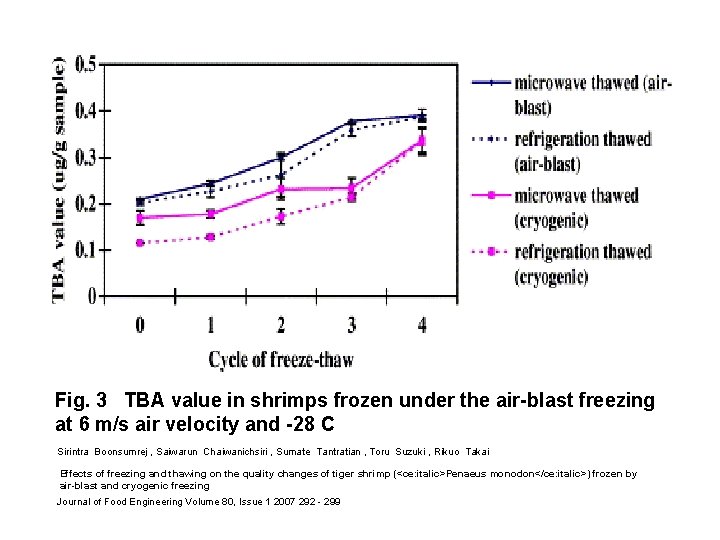
Fig. 3 TBA value in shrimps frozen under the air-blast freezing at 6 m/s air velocity and -28 C Sirintra Boonsumrej , Saiwarun Chaiwanichsiri , Sumate Tantratian , Toru Suzuki , Rikuo Takai Effects of freezing and thawing on the quality changes of tiger shrimp (<ce: italic>Penaeus monodon</ce: italic>) frozen by air-blast and cryogenic freezing Journal of Food Engineering Volume 80, Issue 1 2007 292 - 299

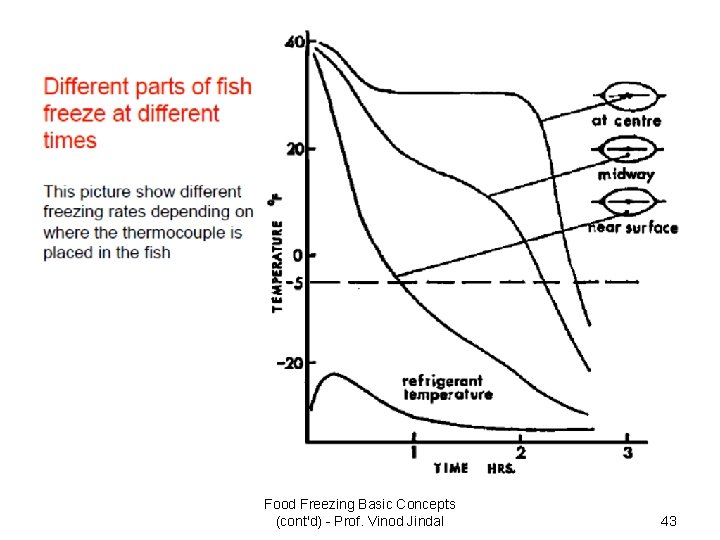
Freezing Time Calculations Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 42

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 43

Freezing Time Calculation • In freezing time calculations, the imprecise control of freezing conditions and uncertainty in thermal properties data of foods are mainly responsible for not so accurate predictions. • The overall accuracy of prediction is governed more by the uncertainty in thermal properties data rather than the calculation procedure. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 44

• There are three alternatives for obtaining thermal properties data of foods: 1) Use data from literature 2) Direct measurement 3) Using prediction equations based on the composition information Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 45

PLANK’S EQUATION • Plank’s equation is an approximate analytical solution for a simplified phase-change model. • Plank assumed that the freezing process: (a) commences with all of the food unfrozen but at its freezing temperature. (b) occurs sufficiently slowly for heat transfer in the frozen layer to take place under steady-state conditions. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 46

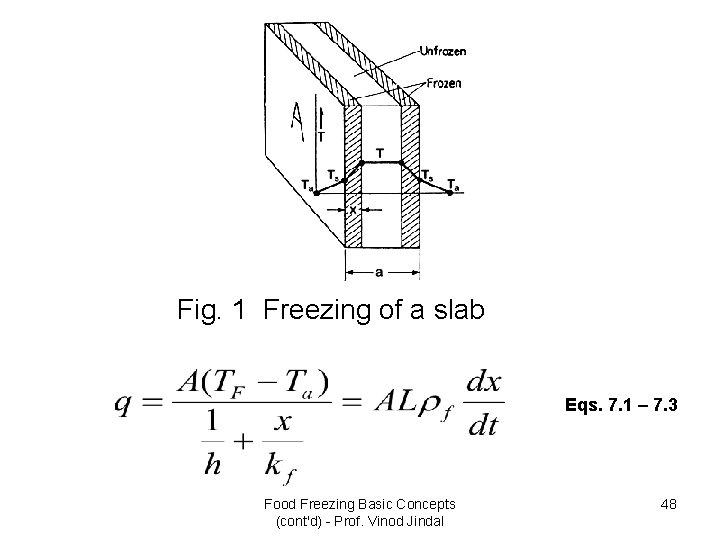
• Plank’s equation considers only phase change period during freezing process. However, Plank’s approximate solution is sufficient for many practical purposes. • This method when applied to calculate the time taken to freeze to the centre of a slab (Fig. 1) whose length and breadth are large compared with the thickness, results in the following equation: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 47

Fig. 1 Freezing of a slab Eqs. 7. 1 – 7. 3 Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 48

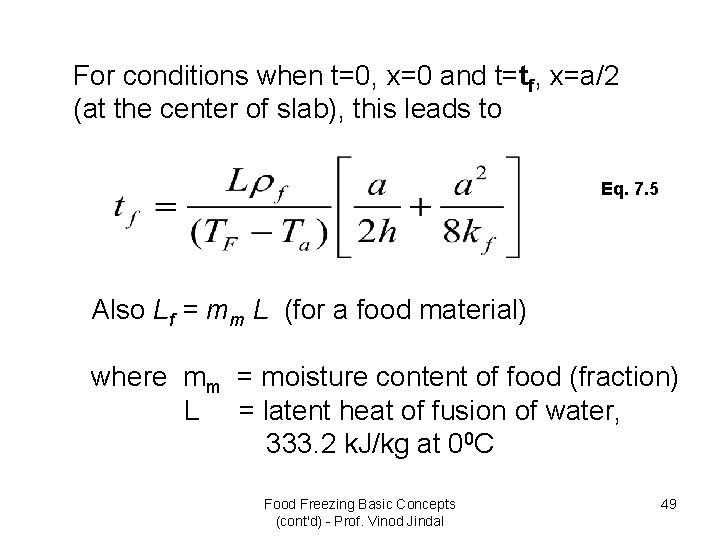
For conditions when t=0, x=0 and t=tf, x=a/2 (at the center of slab), this leads to Eq. 7. 5 Also Lf = mm L (for a food material) where mm = moisture content of food (fraction) L = latent heat of fusion of water, 333. 2 k. J/kg at 00 C Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 49

The general form of Plank’s equation is where P’ and R’ are constants accounting for the product shape with P’=1/2, R’=1/8 for infinite plate; P’=1/4, R’=1/16 for infinite cylinder; and P’=1/6 and R’=1/24 for sphere or cube. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 50

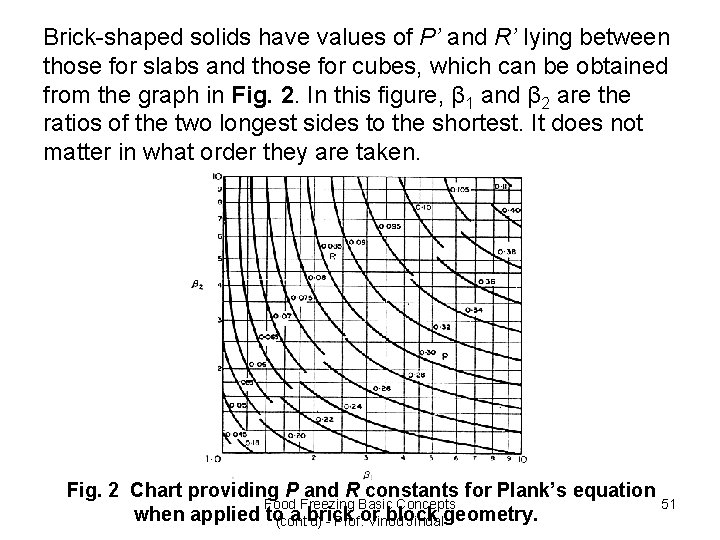
Brick-shaped solids have values of P’ and R’ lying between those for slabs and those for cubes, which can be obtained from the graph in Fig. 2. In this figure, β 1 and β 2 are the ratios of the two longest sides to the shortest. It does not matter in what order they are taken. Fig. 2 Chart providing P and R constants for Plank’s equation Food Freezing Basic Concepts 51 when applied to(cont'd) - Prof. Vinod Jindal a brick or block geometry.

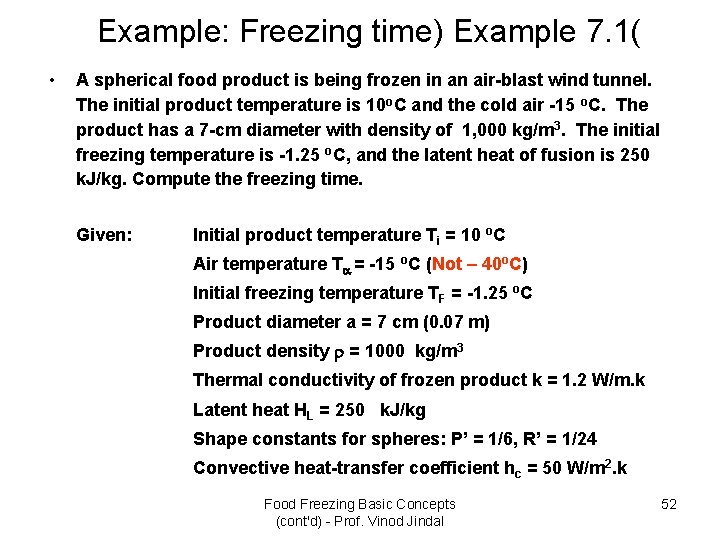
Example: Freezing time) Example 7. 1( • A spherical food product is being frozen in an air-blast wind tunnel. The initial product temperature is 10 o. C and the cold air -15 o. C. The product has a 7 -cm diameter with density of 1, 000 kg/m 3. The initial freezing temperature is -1. 25 o. C, and the latent heat of fusion is 250 k. J/kg. Compute the freezing time. Given: Initial product temperature Ti = 10 o. C Air temperature T = -15 o. C (Not – 40 o. C) Initial freezing temperature TF = -1. 25 o. C Product diameter a = 7 cm (0. 07 m) Product density = 1000 kg/m 3 Thermal conductivity of frozen product k = 1. 2 W/m. k Latent heat HL = 250 k. J/kg Shape constants for spheres: P’ = 1/6, R’ = 1/24 Convective heat-transfer coefficient hc = 50 W/m 2. k Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 52

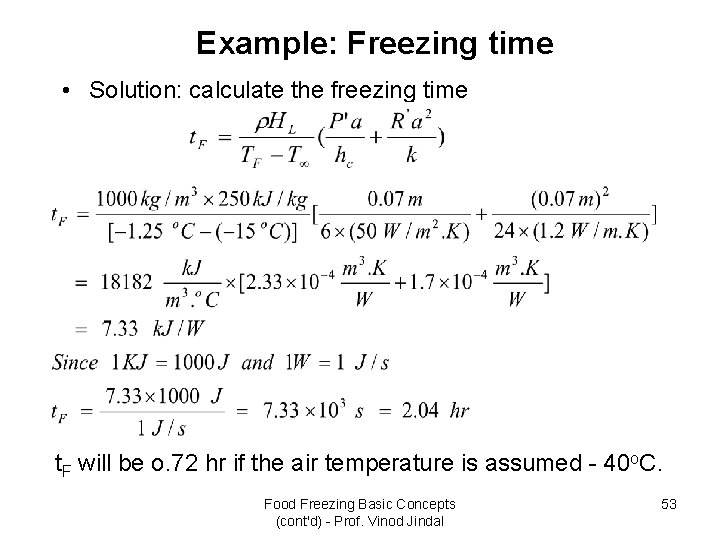
Example: Freezing time • Solution: calculate the freezing time t. F will be o. 72 hr if the air temperature is assumed - 40 o. C. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 53

• Plank's equation results in the underestimation of freezing times because of the assumptions made in its derivation. • The initial freezing temperature (TF) for most foods is not reliably known. Although the initial freezing temperature is tabulated for many foods, the initial and final product temperatures are not accounted for in the computation of freezing times. • Also we often do not know for sure what values of ρf and kf to select. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 54

• Despite the limitations, Plank’s equation is the most popular method for predicting freezing time. • Most other available methods are based on the modification of Plank’s equation. • Because of data uncertainty alone, freezing time estimates should be treated as being accurate to within ± 20% at best. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 55

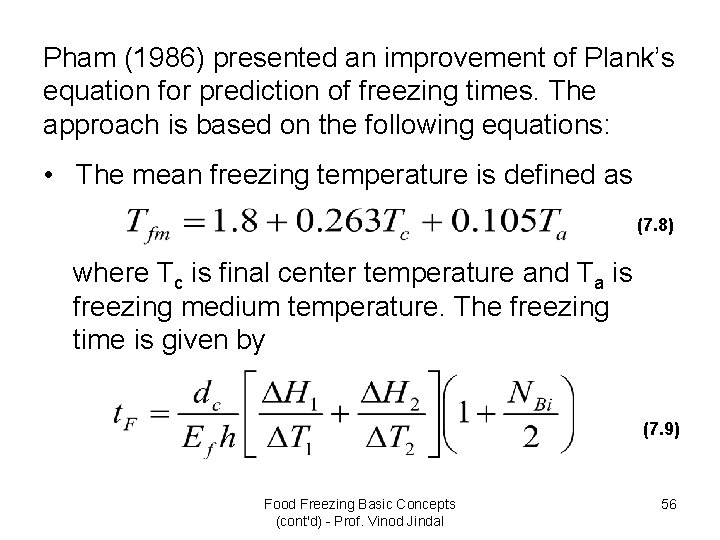
Pham (1986) presented an improvement of Plank’s equation for prediction of freezing times. The approach is based on the following equations: • The mean freezing temperature is defined as (7. 8) where Tc is final center temperature and Ta is freezing medium temperature. The freezing time is given by (7. 9) Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 56

where dc = characteristic dimension ‘r’ or shortest distance Ef = a shape factor (‘ 1’ for slab, ‘ 2’ for cylinder and ‘ 3’ for sphere) (7. 10) (7. 11) (7. 12) (7. 13) ΔH 1 = Enthalpy change during pre-cooling, J/m 3 ΔH 2 = Enthalpy change during phase change and postcooling period, J/m 3 Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 57

Freezing Time of Finite Shaped Objects In Pham’s method, the value of Ef is adjusted (Eq. 7. 16): Ef = G 1 + G 2 E 1 + G 3 E 2 where the values of G 1, G 2 and G 3 are given in Table 7. 1 and E 2 are calculated from Eqs. 7. 17 & 7. 19 and Eqs. 7. 18 & 7. 20, respectively. We can now follow Example 7. 2 (Singh and Heldman) and compare the freezing time calculations based on Pham’s approach and Plank’s equation. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 58

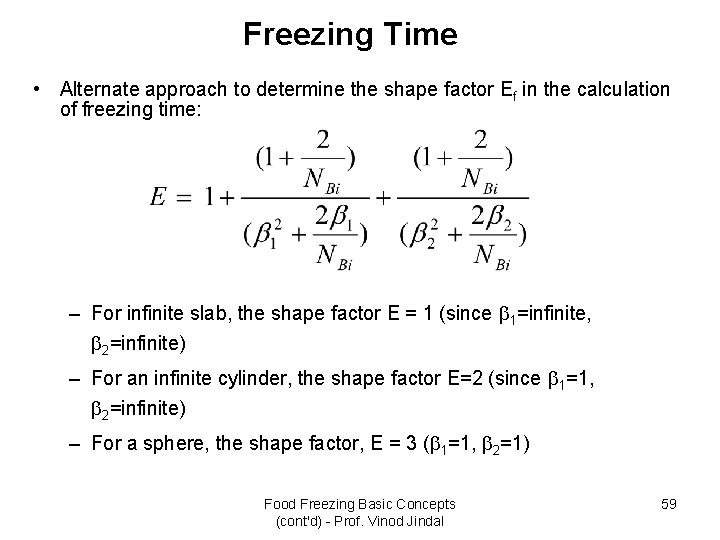
Freezing Time • Alternate approach to determine the shape factor Ef in the calculation of freezing time: – For infinite slab, the shape factor E = 1 (since 1=infinite, 2=infinite) – For an infinite cylinder, the shape factor E=2 (since 1=1, 2=infinite) – For a sphere, the shape factor, E = 3 ( 1=1, 2=1) Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 59

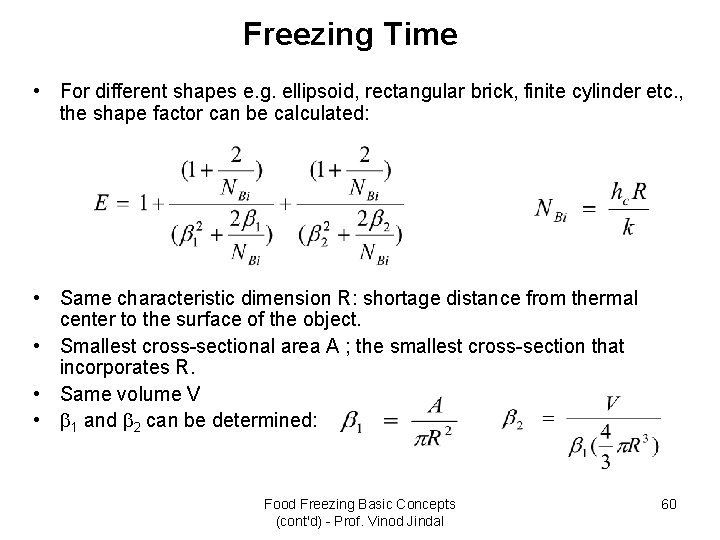
Freezing Time • For different shapes e. g. ellipsoid, rectangular brick, finite cylinder etc. , the shape factor can be calculated: • Same characteristic dimension R: shortage distance from thermal center to the surface of the object. • Smallest cross-sectional area A ; the smallest cross-section that incorporates R. • Same volume V • 1 and 2 can be determined: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 60

Example: Freezing Time • Lean beef with 74. 5% moisture content and 1 m length, 0. 6 m width, and 0. 25 m thickness is being frozen in an air-blast freezer with h c = 30 W/m 2. K and air temperature of -30 o. C. If the initial product temperature is 5 o. C. Estimate the time required to reduce the product temperature to -10 o. C. An initial freezing temperature of -1. 75 o. C has been measured for the product. The thermal conductivity of frozen beef is 1. 5 W/m. K, and the specific heat of unfrozen beef is 3. 5 k. J/kg. K. A product density of 1050 kg/m 3 can be assumed, and a specific heat of 1. 8 k. J/kg. K for frozen beef can be estimated from properties of ice. – – – Product length d 2 = 1 m Product width d 1 = 0. 6 m Product thickness a = 0. 25 m Convective heat-transfer coefficient hc = 30 W/m 2. k Air temperature T = -30 o. C Initial product temperature Ti = 5 o. C Initial freezing temperature TF = -1. 75 o. C Product density = 1050 kg/m 3 Enthalpy change ( H) = 0. 745 333. 22 k. J/kg = 248. 25 k. J/kg (estimate) Thermal conductivity k of frozen product = 1. 5 W/m. K Specific heat of product (Cpu) = 3. 5 k. J/kg. K Specific heat of frozen product (Cpf) = 1. 8 k. J/kg. K Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 61

Solution: Freezing Time (1) Determine shape factor: (2) The Biot number is : (3) Shape factor E: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 62

Solution: Freezing Time (4) T 3 : (5) H 1: Cu(Ti-T 3) (6) T 1 and T 2 : Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 63

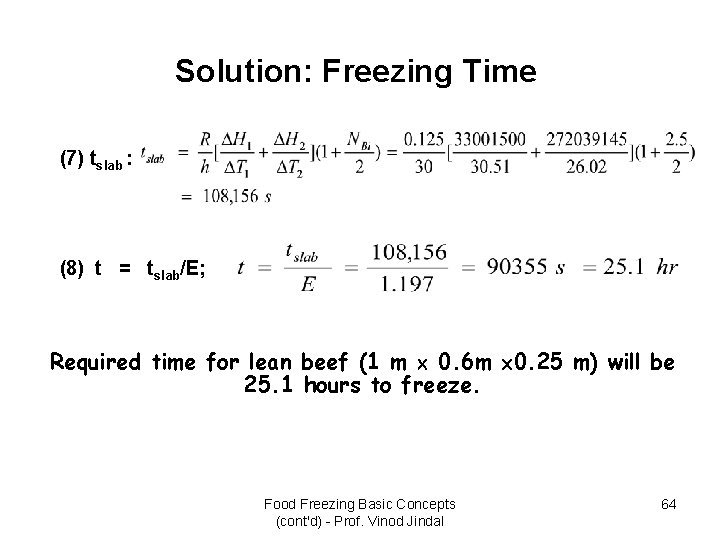
Solution: Freezing Time (7) tslab : (8) t = tslab/E; Required time for lean beef (1 m 0. 6 m 0. 25 m) will be 25. 1 hours to freeze. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 64

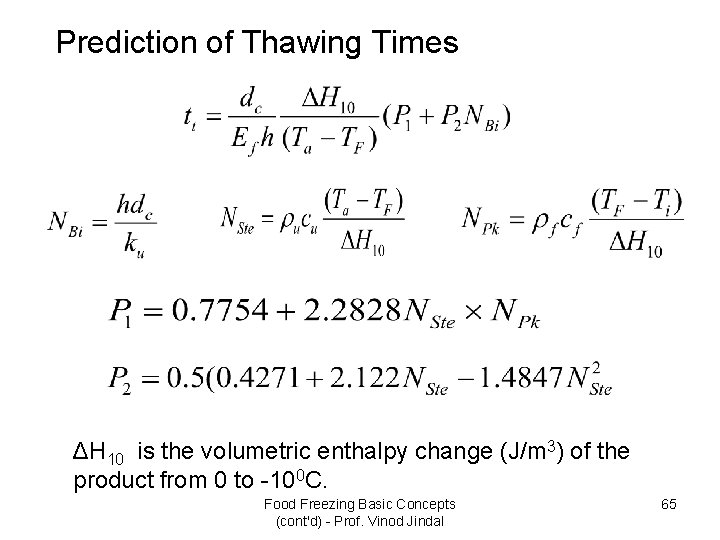
Prediction of Thawing Times ΔH 10 is the volumetric enthalpy change (J/m 3) of the product from 0 to -100 C. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 65
 Fluidized bed freezer
Fluidized bed freezer Fst freezing
Fst freezing Sucrose solubility curve
Sucrose solubility curve K5 think central
K5 think central Fst 201
Fst 201 Fst flame spray technologies
Fst flame spray technologies Fst ssv vva
Fst ssv vva Enotes fst
Enotes fst Fst programming
Fst programming Linkage disequilibrium definition
Linkage disequilibrium definition Fst model
Fst model Fst
Fst Principles of food preservation by freezing
Principles of food preservation by freezing What is her favourite subject?
What is her favourite subject? What is 82 rounded to the nearest ten
What is 82 rounded to the nearest ten Chm 151 final exam
Chm 151 final exam Model netics
Model netics Conforme a tua infinita graça numero
Conforme a tua infinita graça numero Econ 151
Econ 151 Software consists of:
Software consists of: 118/151
118/151 Cs 151 uci
Cs 151 uci Tổng kết vốn từ 151
Tổng kết vốn từ 151 Immurel
Immurel Alan ableson queens
Alan ableson queens 1,151,725 bytes
1,151,725 bytes Mcb 151
Mcb 151 Econ 151
Econ 151 Mozzanica antincendio
Mozzanica antincendio Econ 151
Econ 151 Sjsu cs 151
Sjsu cs 151 Cs 151 sjsu
Cs 151 sjsu Hardness texture color and freezing point are examples of
Hardness texture color and freezing point are examples of Usth
Usth Spike unist internship
Spike unist internship State council of science and technology
State council of science and technology Mail @ stcu.int
Mail @ stcu.int Science technology university yemen
Science technology university yemen Masdar institute of science and technology
Masdar institute of science and technology Madhav institute of technology and science
Madhav institute of technology and science Jordan university of science and technology
Jordan university of science and technology Jordan university of science and technology
Jordan university of science and technology Grade 4 term 3 natural science
Grade 4 term 3 natural science Sts chapter 6
Sts chapter 6 Technology adverb form
Technology adverb form Stone age science and technology
Stone age science and technology Tufts science and technology center
Tufts science and technology center Natural sciences and technology grade 6 term 4
Natural sciences and technology grade 6 term 4 Grade 6 natural science and technology term 2
Grade 6 natural science and technology term 2 Types of natural science
Types of natural science Schorarly
Schorarly Journal of semiconductor technology and science
Journal of semiconductor technology and science Jordan university of science and technology
Jordan university of science and technology Indo-us dollar matching grant project
Indo-us dollar matching grant project Advanced science and technology letters
Advanced science and technology letters The university of shanghai for science and technology
The university of shanghai for science and technology Unit 2 relationship
Unit 2 relationship Natural science grade 6 term 4
Natural science grade 6 term 4 Indian institute of space science and technology
Indian institute of space science and technology Dhs science and technology directorate org chart
Dhs science and technology directorate org chart Fnu engineering courses
Fnu engineering courses Columbia science and technology law review
Columbia science and technology law review Hamzeh khanpour
Hamzeh khanpour Norwegian museum of science and technology
Norwegian museum of science and technology Shri dadaji institute of technology and science
Shri dadaji institute of technology and science Npust scholarship
Npust scholarship