FST 504 TECHNOLOGY OF MISCELLANEOUS FOOD COMMODITY 3


























- Slides: 41

FST 504: TECHNOLOGY OF MISCELLANEOUS FOOD COMMODITY 3 Units Section 2 Dr Mrs J. M. Babajide Department of Food Science and Technology, University of Agriculture, Abeokuta

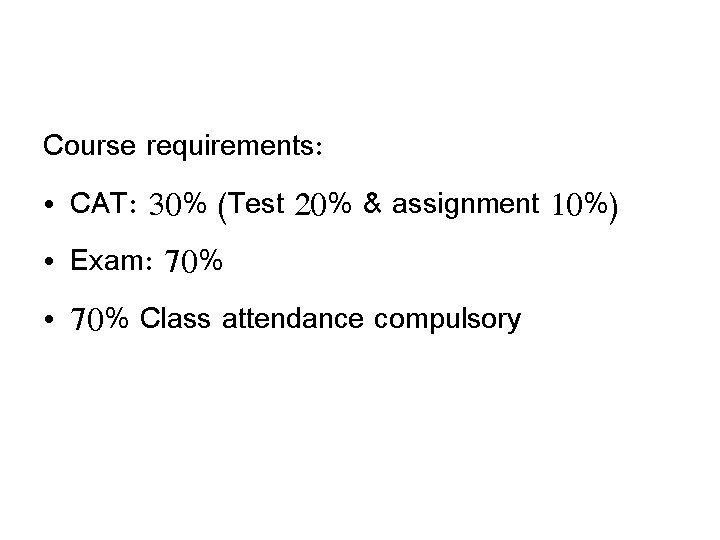
Course requirements: • CAT: 30% (Test 20% & assignment 10%) • Exam: 70% • 70% Class attendance compulsory

SUGAR AND CONFECTIONERY INTRODUCTION • Definition of sugar (sucrose) - form of carbohydrate suitable as a sweetener • Major source of sugar e. g cane and beet • World production of sugar - The world’s highest producer of sugar produce about 90 million tones/year, 60% sugar cane and 40% from sugar beet. • Sugar as an important confectionery ingredient - basic ingredient for classical sugar confectionery

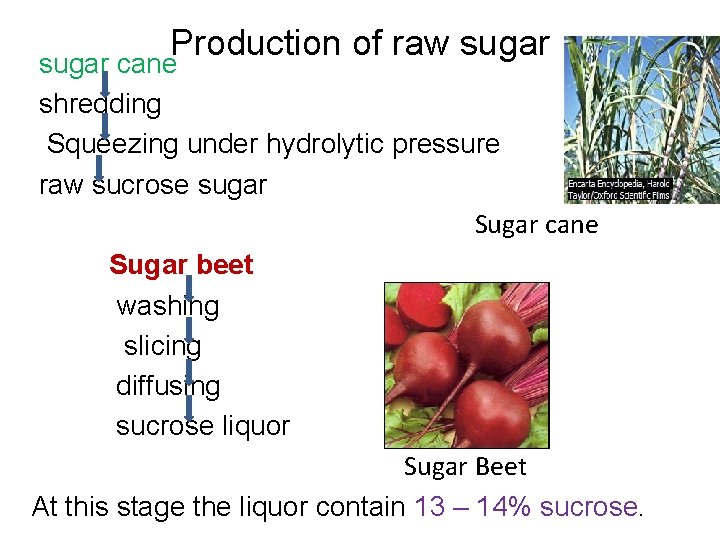
Production of raw sugar cane shredding Squeezing under hydrolytic pressure raw sucrose sugar Sugar cane Sugar beet washing slicing diffusing sucrose liquor Sugar Beet At this stage the liquor contain 13 – 14% sucrose.

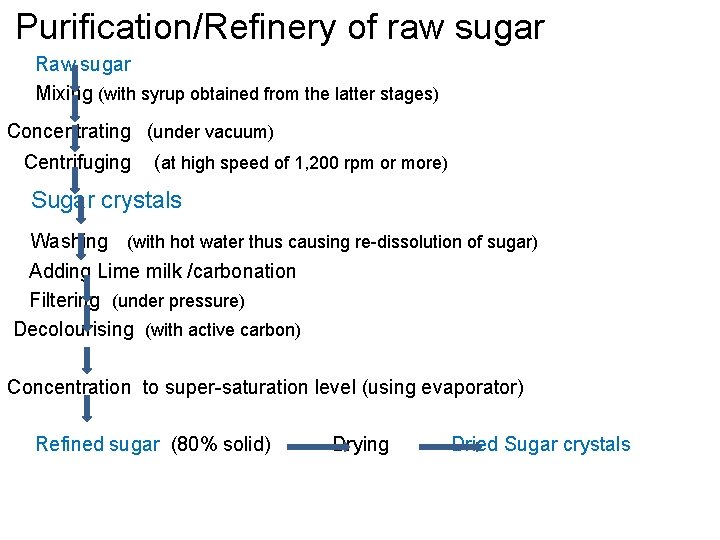
Purification/Refinery of raw sugar Raw sugar Mixing (with syrup obtained from the latter stages) concentrating (under vacuum) Centrifuging (at high speed of 1, 200 rpm or more) Sugar crystals Washing (with hot water thus causing re-dissolution of sugar) Adding Lime milk /carbonation Filtering (under pressure) Decolourising (with active carbon) Concentration to super-saturation level (using evaporator) Refined sugar (80% solid) Drying Dried Sugar crystals

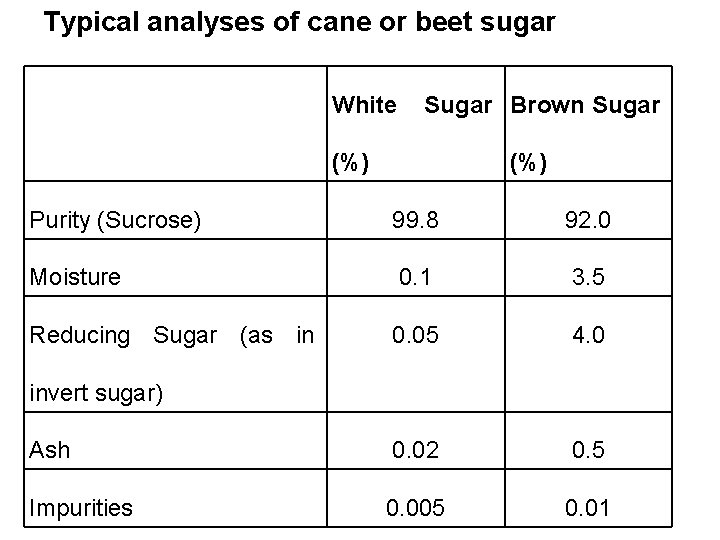
Typical analyses of cane or beet sugar White Sugar Brown Sugar (%) Purity (Sucrose) 99. 8 92. 0 Moisture 0. 1 3. 5 Reducing Sugar (as in 0. 05 4. 0 Ash 0. 02 0. 5 Impurities 0. 005 0. 01 invert sugar)

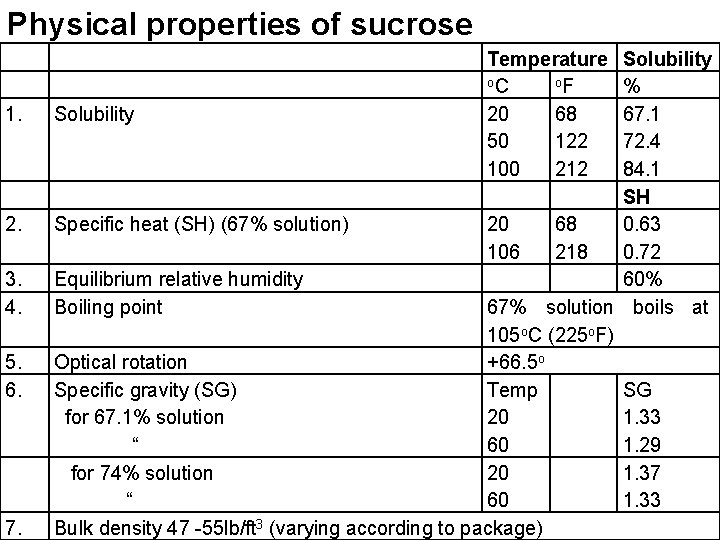
Physical properties of sucrose 1. 2. 3. 4. 5. 6. 7. Temperature o. C o. F 20 68 50 122 100 212 Solubility % Solubility 67. 1 72. 4 84. 1 SH Specific heat (SH) (67% solution) 20 68 0. 63 106 218 0. 72 Equilibrium relative humidity 60% Boiling point 67% solution boils at 105 o. C (225 o. F) Optical rotation +66. 5 o Specific gravity (SG) Temp SG for 67. 1% solution 20 1. 33 “ 60 1. 29 for 74% solution 20 1. 37 “ 60 1. 33 Bulk density 47 -55 lb/ft 3 (varying according to package)

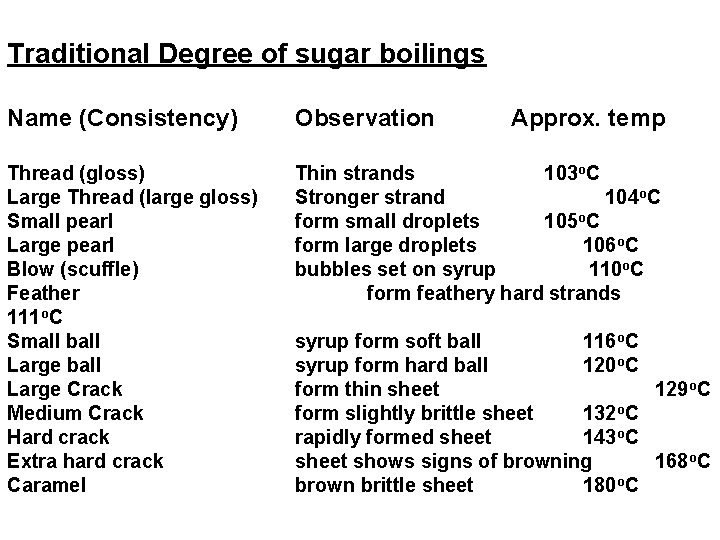
Traditional Degree of sugar boilings Name (Consistency) Observation Approx. temp Thread (gloss) Large Thread (large gloss) Small pearl Large pearl Blow (scuffle) Feather 111 o. C Small ball Large Crack Medium Crack Hard crack Extra hard crack Caramel Thin strands 103 o. C Stronger strand 104 o. C form small droplets 105 o. C form large droplets 106 o. C bubbles set on syrup 110 o. C form feathery hard strands syrup form soft ball 116 o. C syrup form hard ball 120 o. C form thin sheet 129 o. C form slightly brittle sheet 132 o. C rapidly formed sheet 143 o. C sheet shows signs of browning 168 o. C brown brittle sheet 180 o. C

PROPERTIES OF SUCROSE SUGAR 1. Solubility of Sugar • Saturation concentration of sugar: - (at room temperature a part of H 2 O will dissolve 2 parts of sugar (67%) • Factors that determine concentration of sugar: - temperature, rate of agitation, degree of under saturation and inversely to the crystal size). • Rate of dissolution of sugar: - For example , in preparing a saturated solution at room temperature, the last few % of sugar will dissolve very slowly except in the use of heat

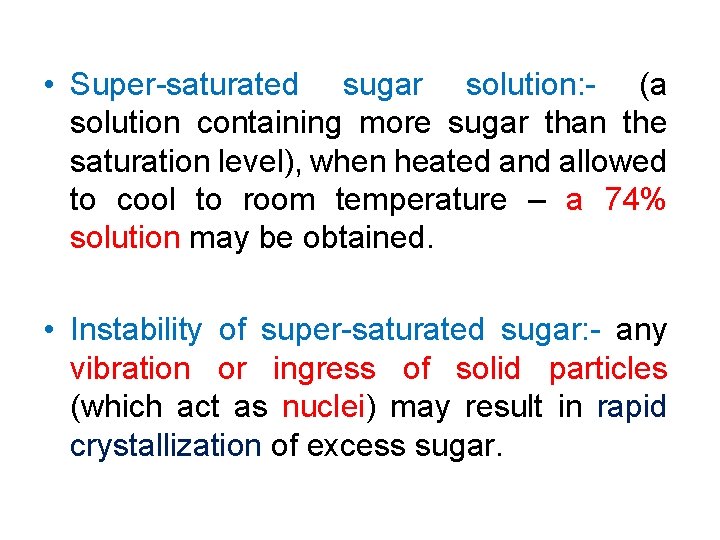
• Super-saturated sugar solution: - (a solution containing more sugar than the saturation level), when heated and allowed to cool to room temperature – a 74% solution may be obtained. • Instability of super-saturated sugar: - any vibration or ingress of solid particles (which act as nuclei) may result in rapid crystallization of excess sugar.

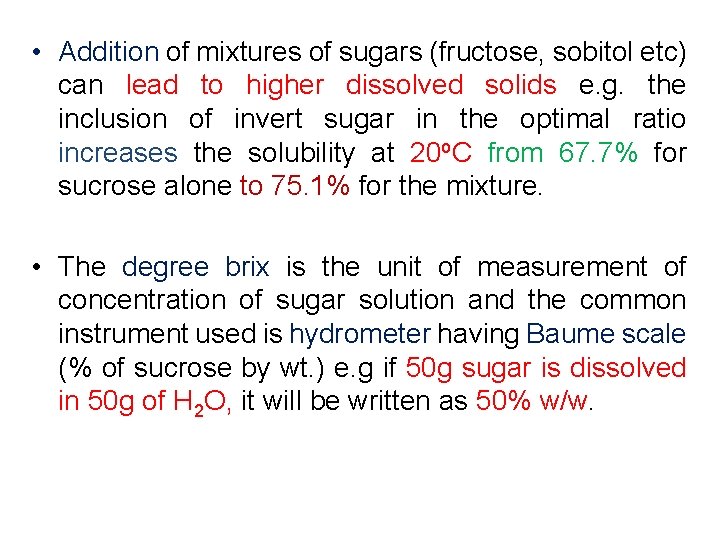
• Addition of mixtures of sugars (fructose, sobitol etc) can lead to higher dissolved solids e. g. the inclusion of invert sugar in the optimal ratio increases the solubility at 20 o. C from 67. 7% for sucrose alone to 75. 1% for the mixture. • The degree brix is the unit of measurement of concentration of sugar solution and the common instrument used is hydrometer having Baume scale (% of sucrose by wt. ) e. g if 50 g sugar is dissolved in 50 g of H 2 O, it will be written as 50% w/w.

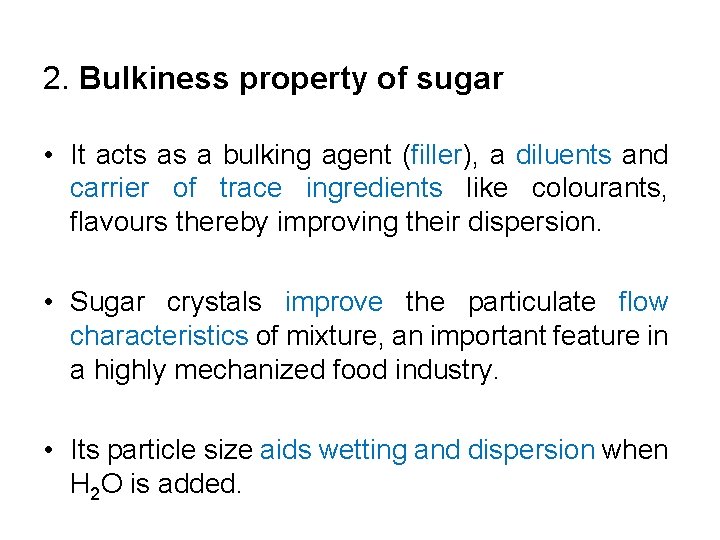
2. Bulkiness property of sugar • It acts as a bulking agent (filler), a diluents and carrier of trace ingredients like colourants, flavours thereby improving their dispersion. • Sugar crystals improve the particulate flow characteristics of mixture, an important feature in a highly mechanized food industry. • Its particle size aids wetting and dispersion when H 2 O is added.

• When mixed with fats, it enables the incorporation of air into the mixture which makes it important in generating the lightens of cake. • It provides mouth feel in soft drinks at relatively low concentration while at high concentration, it gives the characteristics e. g. in boiled sweets.

3. Relative Humidity of sugar • Sucrose sugar can tolerate to a wide range of humidity. However, it does have its limitations in its tendency to cake or solidify in it’s storage container. • Thus, sugar remains free flowing under normal European climatic conditions. When the relative humidity drops below 70%, the syrup form crystals. When the R. H is over 70%, it gives rise to conditions which encourage mould growth during storage.

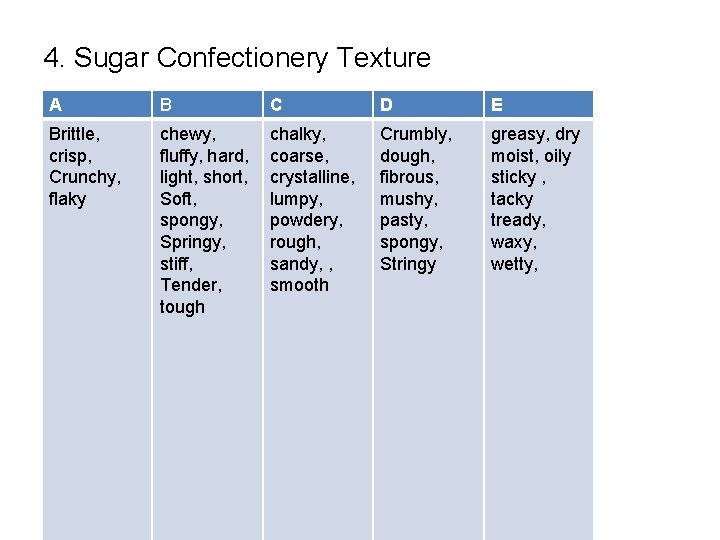
4. Sugar Confectionery Texture A B C D E Brittle, crisp, Crunchy, flaky chewy, fluffy, hard, light, short, Soft, spongy, Springy, stiff, Tender, tough chalky, coarse, crystalline, lumpy, powdery, rough, sandy, , smooth Crumbly, dough, fibrous, mushy, pasty, spongy, Stringy greasy, dry moist, oily sticky , tacky tready, waxy, wetty,

Texture variation can be achieved for confections by one or more of the following procedures: • vary the moisture content • vary the content type and strength of gelling agent • vary the sucrose-glucose syrup ratio • vary the sucrose-invert sugar solid ratio • vary the p. H • alter the process temperature conditions • vary the milk protein content • seed the batch with fondant or icing sugar • change the required level of total sugars • alter processing conditions to vary the particle size • alter the incorporated air content

FORMS/TYPES OF SUCROSE SUGAR 1. Granulated mineral water sugar 2. Granulated sugar 3. Industrial granulated 4. Cube sugar 5. Nibs 6. Caster 7. Icing sugar 8. Liquid sugar 9. Brown sugar 10. Mollases 11. Microcrystalline sugar

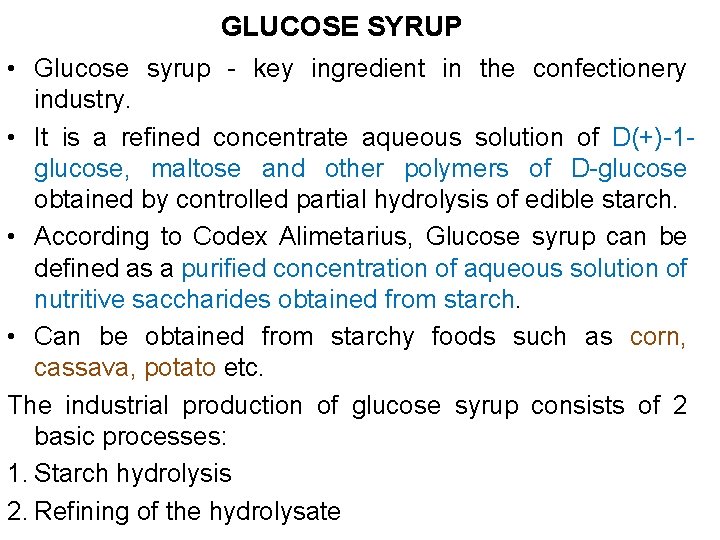
GLUCOSE SYRUP • Glucose syrup - key ingredient in the confectionery industry. • It is a refined concentrate aqueous solution of D(+)-1 glucose, maltose and other polymers of D-glucose obtained by controlled partial hydrolysis of edible starch. • According to Codex Alimetarius, Glucose syrup can be defined as a purified concentration of aqueous solution of nutritive saccharides obtained from starch. • Can be obtained from starchy foods such as corn, cassava, potato etc. The industrial production of glucose syrup consists of 2 basic processes: 1. Starch hydrolysis 2. Refining of the hydrolysate

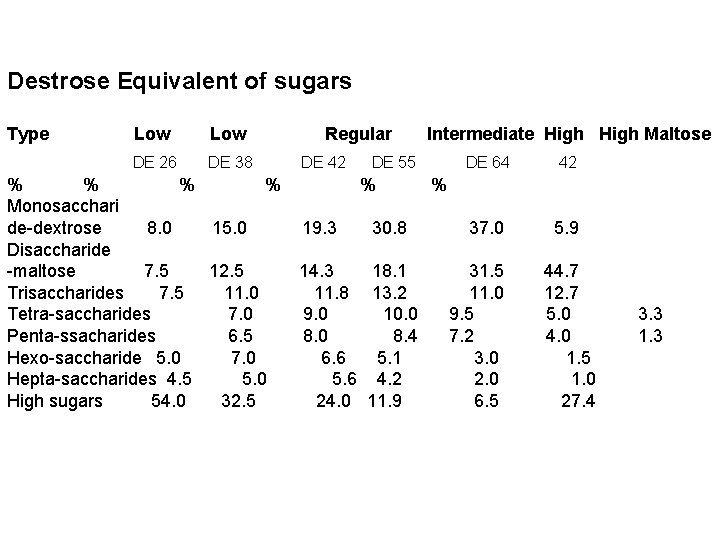
• In acid hydrolysis, dextrose equivalent (degree of hydrolysis) of 30 -35 DE could be obtained which is still of higher quality required by the food and confectionery industry. • DE is the degree of hydrolysis of starch that takes place and it is the total reducing power i. e. in the acid hydrolysis of glucose syrup, we have the composition of dextrose, maltotriose, malto-tetrose, malto-pentose, maltohexose and higher sugars in various percentages making a total of 100% for each DE, as shown below:

Destrose Equivalent of sugars Type Low Regular DE 26 DE 38 DE 42 DE 55 Intermediate High Maltose DE 64 42 % % Monosacchari de-dextrose 8. 0 15. 0 19. 3 30. 8 37. 0 5. 9 Disaccharide -maltose 7. 5 12. 5 14. 3 18. 1 31. 5 44. 7 Trisaccharides 7. 5 11. 0 11. 8 13. 2 11. 0 12. 7 Tetra-saccharides 7. 0 9. 0 10. 0 9. 5 5. 0 3. 3 Penta-ssacharides 6. 5 8. 0 8. 4 7. 2 4. 0 1. 3 Hexo-saccharide 5. 0 7. 0 6. 6 5. 1 3. 0 1. 5 Hepta-saccharides 4. 5 5. 0 5. 6 4. 2 2. 0 1. 0 High sugars 54. 0 32. 5 24. 0 11. 9 6. 5 27. 4

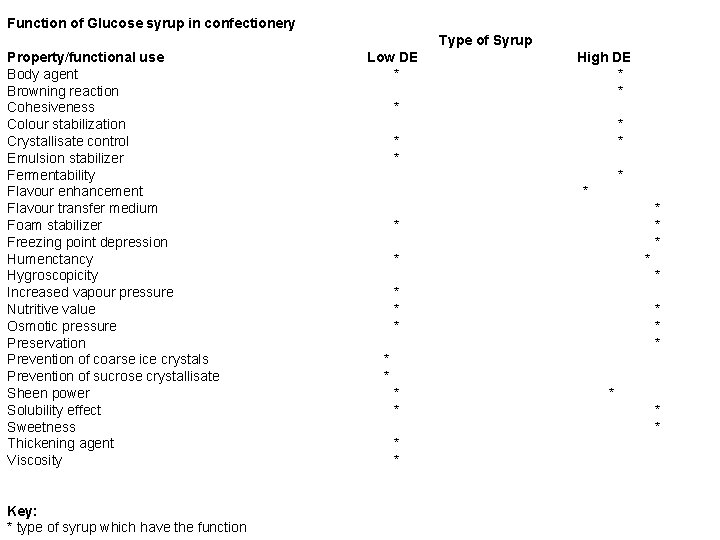
Function of Glucose syrup in confectionery Type of Syrup Property/functional use Body agent Browning reaction Cohesiveness Colour stabilization Crystallisate control Emulsion stabilizer Fermentability Flavour enhancement Flavour transfer medium Foam stabilizer Freezing point depression Humenctancy Hygroscopicity Increased vapour pressure Nutritive value Osmotic pressure Preservation Prevention of coarse ice crystals Prevention of sucrose crystallisate Sheen power Solubility effect Sweetness Thickening agent Viscosity Key: * type of syrup which have the function Low DE * High DE * * * * * * * * * * * *

BOILED SWEETS • High boiled sweets are sugar products which are glossy in appearance. They can be considered as sugar liquids with very high viscosities. • The finished product of boiled sweet is a super cooled liquid at ambient temperature with a solid content of 97 – 98%.

• Although there is super saturation at the solid state with respect to sucrose, but because of the addition of glucose syrup, the formulation cannot crystallize. • Other ingredients that can be added to boiled sweets are flavours, milk, fruits, chocolate, colours etc.

Production of High Boiled Sweets (HBS) There are 3 main production methods for HBS. They are • Open pans • Vacuum cookers • Continuous cookers Each of this require different ratio of sugar to glucose syrup to give the best result. Sucrose: glucose • Open pan 70: 30 to 66. 5: 33. 5 • Vacuum cookers 65: 35 to 50: 50 • Continuous cookers 60: 40 to 45: 55

• Approximate temperature of 156 OC is used during open pans. • Vacuum cooking can be as low as 110 – 129 OC Precautions during HBS production : • During cooling, prevent seeding (introduction of nuclei), this is because a grain of sugar drop into the mass will induce crystallisation • Ensure good doctoring • Stop stirring after attaining desired temperature

Product types of boiled sweets • High boiled sweets manufacturing technology ranges from lollipops, candies, cones, medicated confectioneries, lettered rock, soft centred sweets, butter boilings, laminated (crackened or honey combed sweets to grained Edinburgh rock; marshmallow, Nougat, butterscotch, candy etc. • Description of some Boiled sweets E. G Laminated or Honey comb sweet: • This is a multilayered sweets with a crunchy texture made from many layers of cooked sugar having its centre filled with honey, nut paste, peanuts or other suitable fillings and finally wrapped in a thin envelope of high boiled sweets or sugar.

Sweets Candy cane lollipop Rockets Marshmallow

Gums, Jellies and Pastilles: • Gums, Jellies and Pastilles constitute a large class of confectionery which can be manufactured with many variations. • They are comparatively low boiled and contain about 20% moisture. • Obtained by the use of various types of water binding gelling agents such as gum Arabic, starch, gelatin, agar and pectin.

Tablets and Lozenges: • Tablets are made by compressing powdered or granulated ingredients in a confined space (die) until the particles bond together. • They have very smooth surface and very little amount of moisture. • Ingredients: Base material (sucrose), binders (gum) lubricants, starch (which swells upon contact with water and breaks up the tablet).

• Lozenges are made from icing sugar, mixed with a binder, sheeted, but into shape and allowed to dry. • When menthols/mints, vitamin C or other sore throat medicines are added, they are called medicated lozenges. • In effervescent tablets, citric acid and sodium bicarbonate are included. Colours and flavours can also be added. • Lozenges tend to have hard rough finishing while compressed tablets have smooth shiny surfaces.

Chewing and Bubble gum: • Chewing gums are sticky candy to be chewed but not swallowed. It is composed of mixed natural (chiclemilky juice of the tropical sapodilla tree Archras zapota of Central America) and synthetic gums, resins together with various sugars and flavouring materials (such as mints). • The difference between chewing gum and bubble gum is the ability of Bubble gum to make bubbles and stretch when blown. Bubble gum contains higher levels of polymers or rubbers. • In sugar free or sugar less chewing gum, sorbitol, mannitol, xylitol are used.

SOFT DRINK BEVERAGES What is Soft Drink Beverage? • Soft drinks are non-alcoholic carbonated or non-carbonated beverages usually containing a sweetening agent, edible acids and natural or artificial flavours. Examples of Soft Drink • Soft drinks include, cola beverages, fruit flavoured drinks, ginger ale, and root beer, also include soda water, seltzer water and tonic water.

History of Soft drink • The first attempt to manufacture carbonated soft drinks were the result of a desire to duplicate the naturally effervescent, mineral-rich waters that flowed from the springs at the well-known European spas. • John Pemberton invented caramel coloured syrup in 1886, when diluted and carbonated, this syrup is called coca-cola because it originally contained cocaine from coco leaf and rich in caffeine from the kolanut. This premiere flavoured soft drink was first patented in 1893.

• In 1984, in response to the public demand for more healthful and less fattening foods as follows - 1. Soft drink manufactures began formulating with natural juices. 2. Vitamin enriched soft drinks 3. Sugar, caffeine, sodium -free soft drinks also became popular in the late twentieth century.

PRODUCTION OF SOFT DRINK • Water treatment using sand filter /activated carbon / superchlorination and coagulation. • Carbonation of treated water to give the characteristic effervescence sparkle) soft drinks. (fizz and • During carbonation, Chilling is carried out

• Finished soft drinks can be produced by diluting a mixture of non-carbonated water and flavoured sugar syrup with highly carbonated water then bottled • or syrup is measured directly with bottles then filled with carbonated water injected under high pressure. • The bottles are capped by another machine on the assembly line, packed inspected, then in cartons or cases ready for

Soft Drink Packaging • Carbonated soft drinks are packaged for sale in variety of containers such as glass bottles, tin or aluminum cans and plastic bottles. • Non-carbonated soft drinks can be packaged not only in bottles and cans but also in treated card board carton (tetrapak) since they are not under pressure.

Some special beverage categories are: 1. Non-carbonated soft drinks which are produced with some ingredients except CO 2 and techniques of carbonated soft drink but not protected from spoilage. They are usually pasteurized in bulk or continuous flash pasteurized either prior to filling or in the bottle. 2. Powdered soft drinks are made by blending flavouring materials such as dry acids, gums, sweeteners and artificial colour.

3. Nutraceutical beverages are drinks formulated with special functional ingredients that promote some aspect of health or reduce the risk of certain diseases.

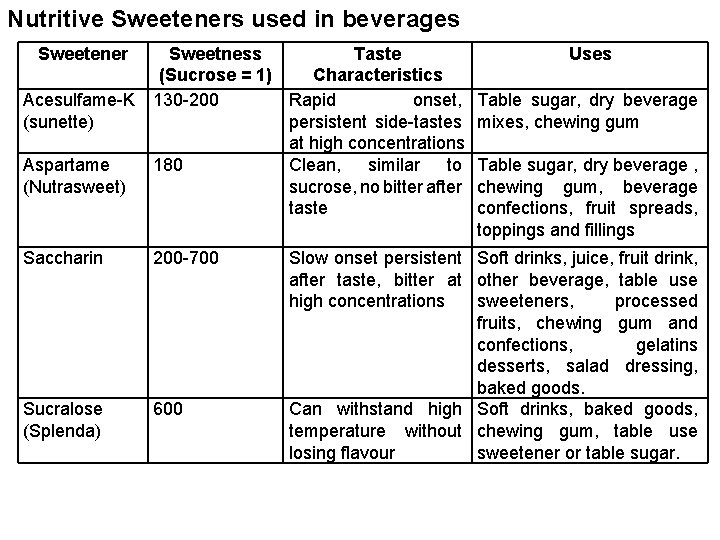
Nutritive Sweeteners used in beverages Sweetener Sweetness Taste (Sucrose = 1) Characteristics Acesulfame-K 130 -200 Rapid onset, (sunette) persistent side-tastes at high concentrations Aspartame 180 Clean, similar to (Nutrasweet) sucrose, no bitter after taste Saccharin 200 -700 Sucralose (Splenda) 600 Uses Table sugar, dry beverage mixes, chewing gum Table sugar, dry beverage , chewing gum, beverage confections, fruit spreads, toppings and fillings Slow onset persistent Soft drinks, juice, fruit drink, after taste, bitter at other beverage, table use high concentrations sweeteners, processed fruits, chewing gum and confections, gelatins desserts, salad dressing, baked goods. Can withstand high Soft drinks, baked goods, temperature without chewing gum, table use losing flavour sweetener or table sugar.

PROJECT Student will produce: • Sugar syrup with various consistency • High boiled sweets of various types
 Fst 201
Fst 201 Aws400
Aws400 Vereins und verbandsadministration
Vereins und verbandsadministration Enotes fst
Enotes fst Fst freezing
Fst freezing Freezing of fish
Freezing of fish Fst programming
Fst programming What is fst
What is fst Fst model
Fst model Fst
Fst Trgs 504
Trgs 504 Idea vs 504
Idea vs 504 Factorizacion de 504
Factorizacion de 504 504 plan oklahoma
504 plan oklahoma Celiac 504 plan
Celiac 504 plan 504 plan catholic schools
504 plan catholic schools Single family housing section 504 repair pilot program
Single family housing section 504 repair pilot program 504 home repair program
504 home repair program Blink htlm
Blink htlm Manifestation determination meeting agenda
Manifestation determination meeting agenda Section 504
Section 504 Bridge loan
Bridge loan Section 504
Section 504 Norma 504
Norma 504 Www.iecex.com
Www.iecex.com Sec 504
Sec 504 Unit 2 food food food
Unit 2 food food food Sequence of food chain
Sequence of food chain Suppository solid dosage form
Suppository solid dosage form Miscellaneous detail
Miscellaneous detail Miscellaneous fasteners
Miscellaneous fasteners Carpentry miscellaneous tools
Carpentry miscellaneous tools Miscellaneous operators in javascript
Miscellaneous operators in javascript Suppository is a semi solid dosage form
Suppository is a semi solid dosage form Vera claythorne quotes
Vera claythorne quotes Electronic cookware miscellaneous
Electronic cookware miscellaneous Miscellaneous base for suppositories
Miscellaneous base for suppositories Miscellaneous commercial insurance
Miscellaneous commercial insurance Miscellaneous commercial insurance
Miscellaneous commercial insurance Letter writing from and to address
Letter writing from and to address Fatty acid composed of
Fatty acid composed of Four parts of a stock
Four parts of a stock