FOOD FREEZING Introduction to Food Engineering Food Freezing












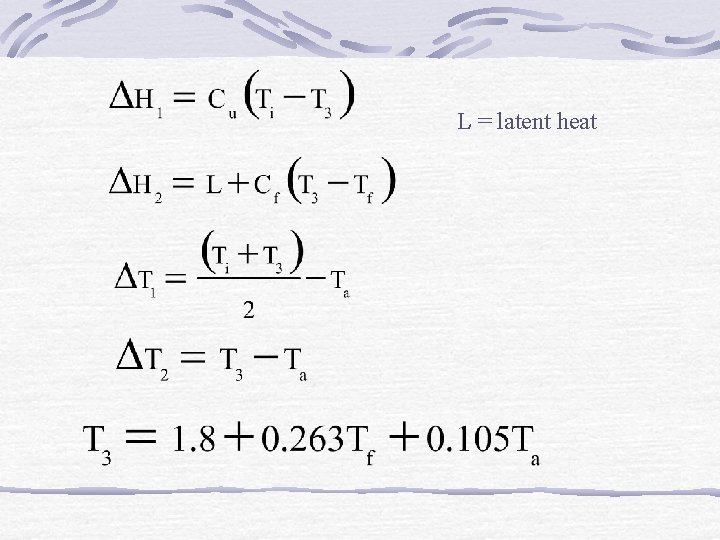

- Slides: 32

FOOD FREEZING Introduction to Food Engineering

Food Freezing The reduction of product temperature to levels below 0 C Significant reduction in growth rates of microorganisms Reduce rates of enzymatic and oxidation reactions Formation of ice crystals reduce Aw

Food Freezing Some products require rapid freezing for small ice crystal, minimum damage to product structure Other products not influence by structural changes – no need for rapid freezing Some products cannot freeze rapidly

Food Freezing Product quality = freezing time + storage condition Storage condition Fluctuations in storage temperature

Freezing Systems Aim : remove sensible heat, latent heat of fusion 20 % of water remains in liquid state To reduce temp in short time Temp of medium much lower than final product temp Large convective heat-transfer coefficients

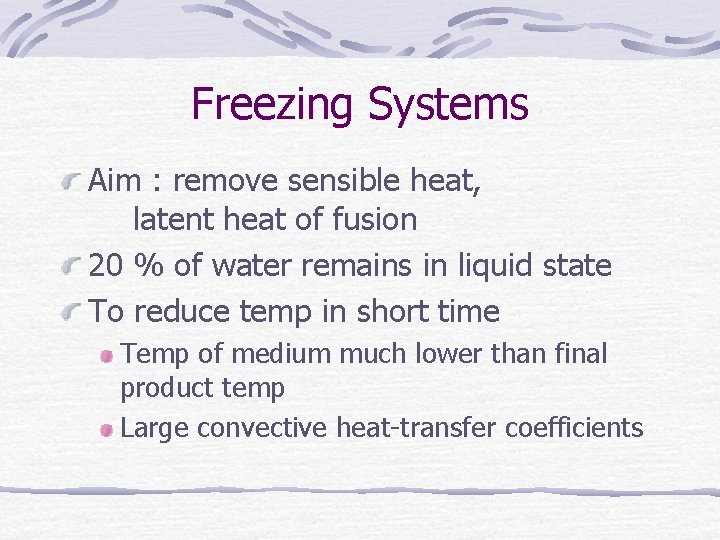
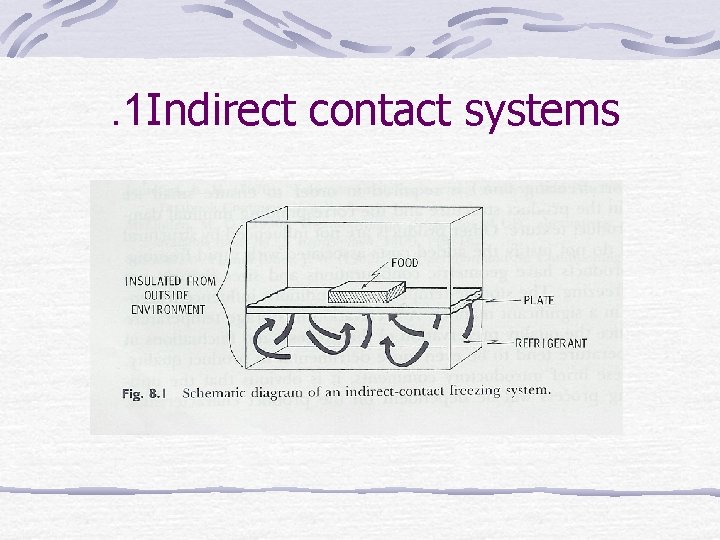
. 1 Indirect contact systems

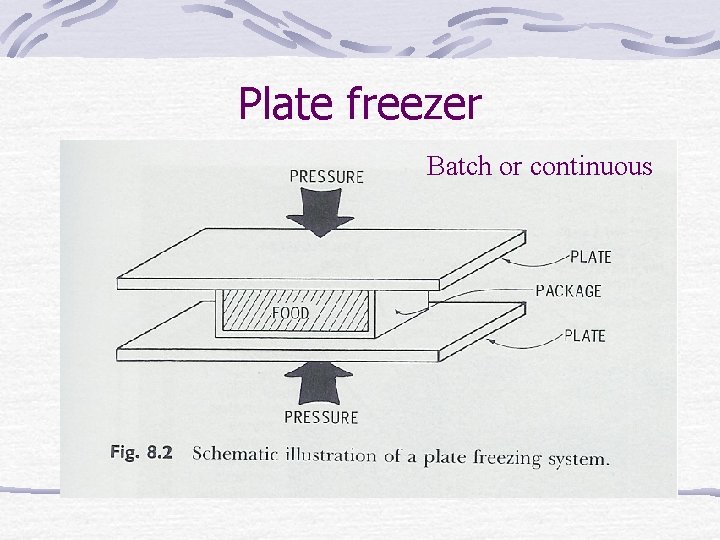
Plate freezer Batch or continuous

Air-blast freezer Product size/shape not accommodate plate freezing Package film is the barrier Source of refrigeration = cold air Batch or continuous

Freezers for liquid foods Mostly before packaging Scraped-surface system 60 -80 % latent heat removed Frozen slurry eg. ice cream Batch or continuous

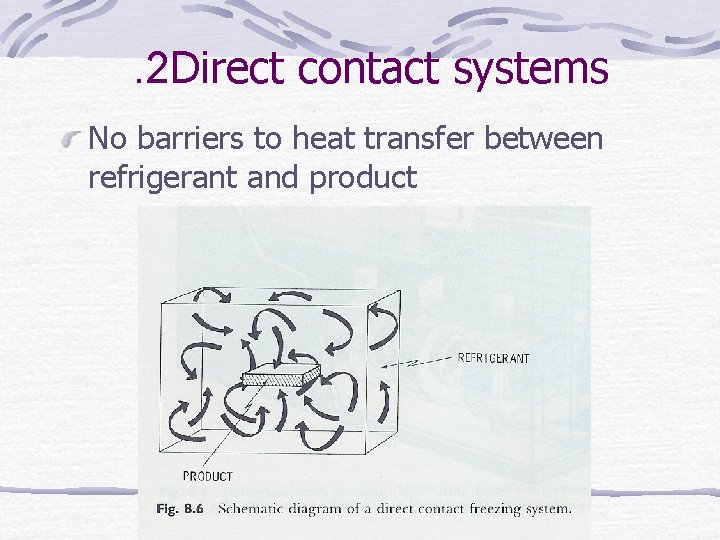
. 2 Direct contact systems No barriers to heat transfer between refrigerant and product

Air blast Low-temp air at high speeds Individual quick freezing (IQF( High convective heat-transfer coefficient Small product shape Fluidized-bed IQF

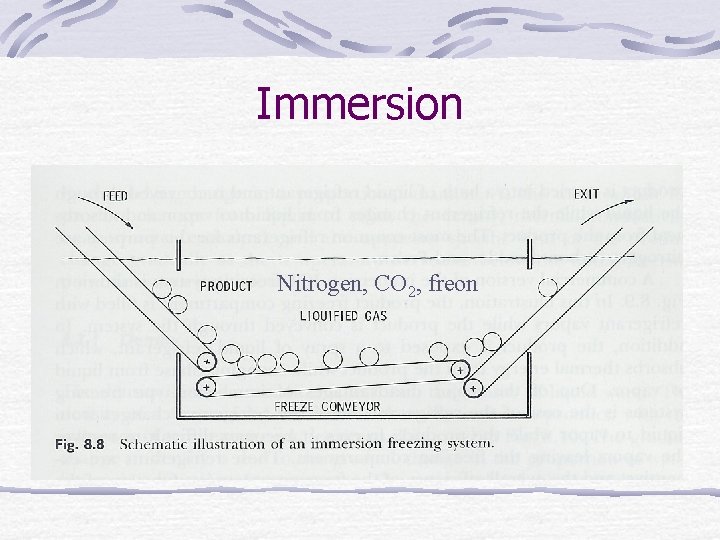
Immersion Nitrogen, CO 2, freon

Immersion Disadvantages Cost of refrigerant Difficult to recover

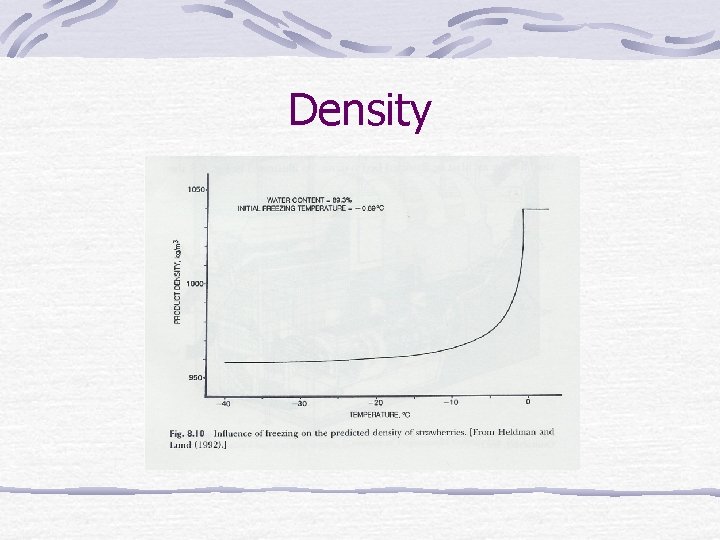
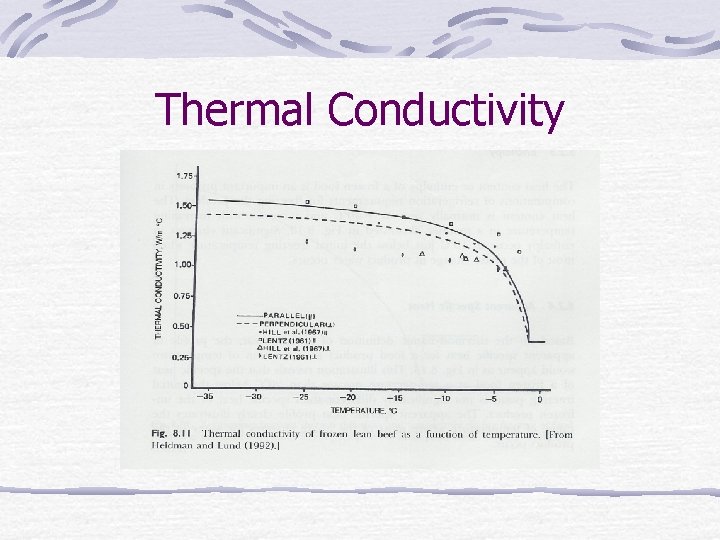
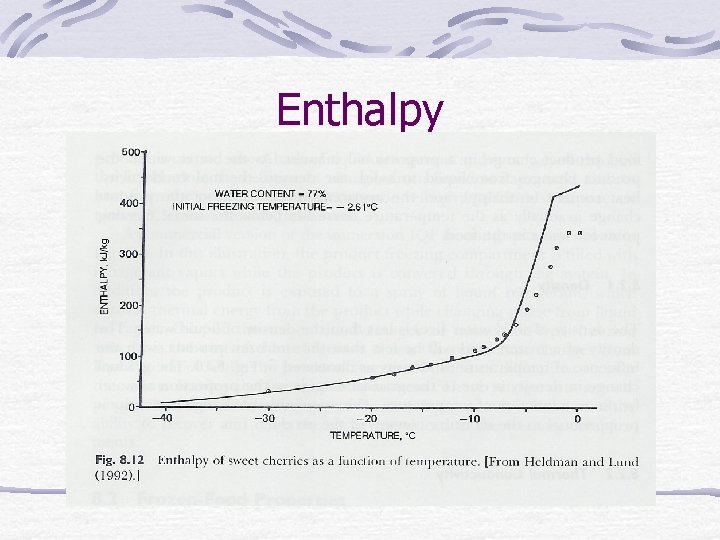
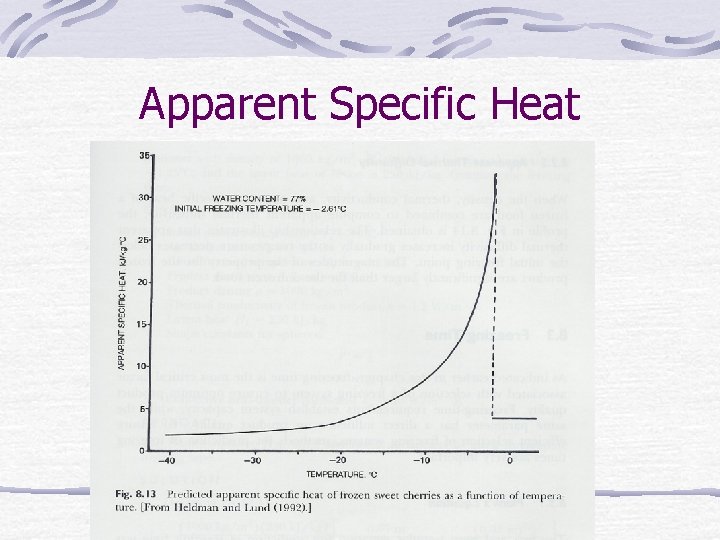
Frozen-food properties Water in food changes to solid. Density, thermal conductivity, heat content (enthalpy), apparent specific heat change.

Density

Thermal Conductivity

Enthalpy

Apparent Specific Heat

Apparent Specific Heat Below 20 C below initial freezing temp = value of unfrozen food

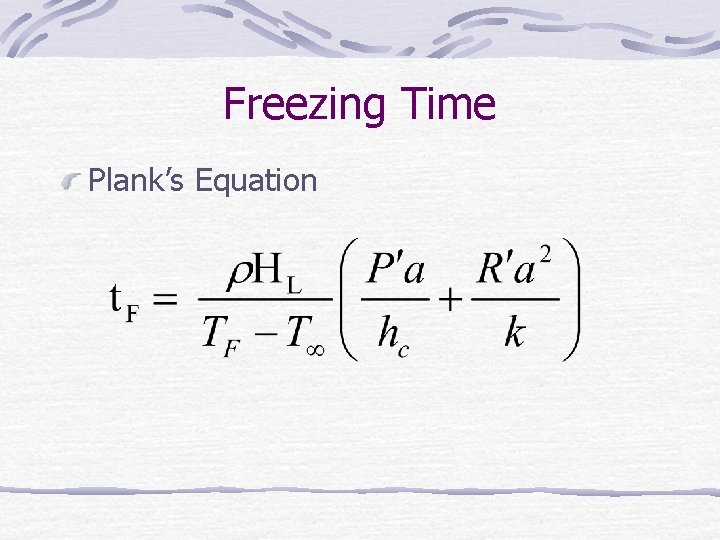
Freezing Time Plank’s Equation

t. F = freezing time = density HL = latent heat of fusion a = size (thickness, diameter) hc, k Infinite plate P =1/2 , R = 1/8 Infinite cylinder P = 1/4 , R = 1/16 Sphere P = 1/2 , R = 1/24

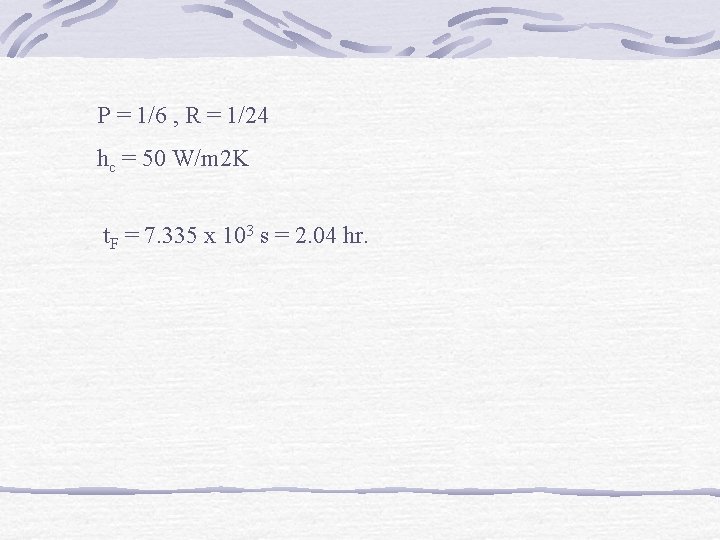
Example Spherical food product is being frozen in air-blast tunnel. Initial product temp = 10 C, cold air – 15 C. Product diameter 7 cm, density 1, 000 kg/m 3. Initial freezing temp – 1. 25 C, latent heat of fusion 250 k. J/kg. K product = 1. 2 W/m. K. Compute freezing time.

P = 1/6 , R = 1/24 hc = 50 W/m 2 K t. F = 7. 335 x 103 s = 2. 04 hr.

Limitations to Plank’s Equation Density values for frozen foods are difficult to locate/measure Latent heats of fusion – for water & water content of product Initial & final product temp not accounted for Thermal conductivity, k, for frozen product not readily available

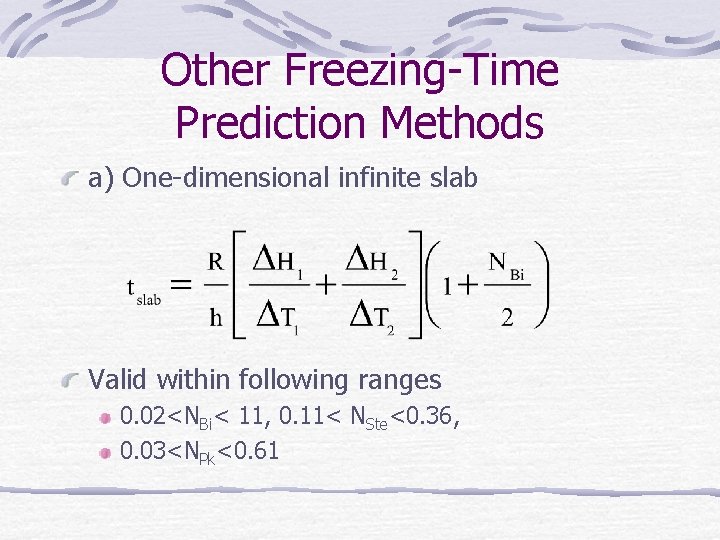
Other Freezing-Time Prediction Methods a) One-dimensional infinite slab Valid within following ranges 0. 02<NBi< 11, 0. 11< NSte<0. 36, 0. 03<NPk<0. 61

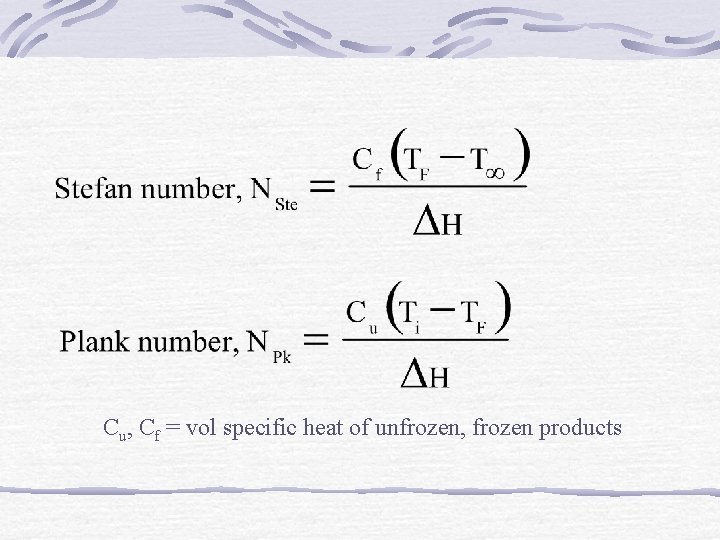
Cu, Cf = vol specific heat of unfrozen, frozen products
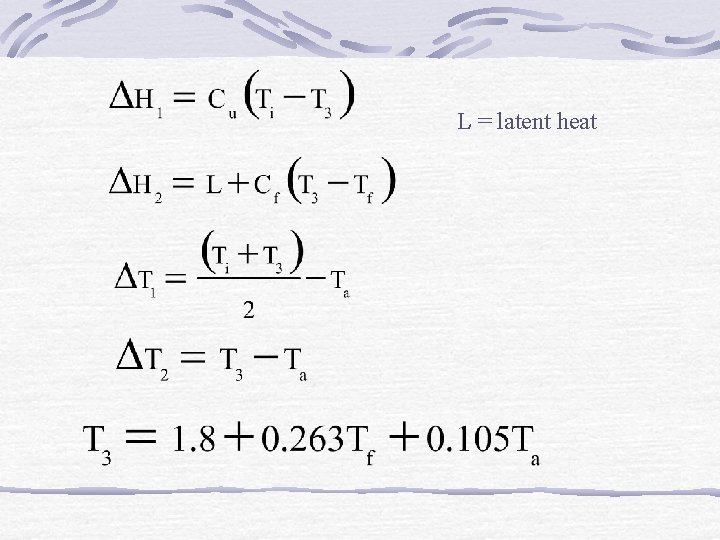
L = latent heat

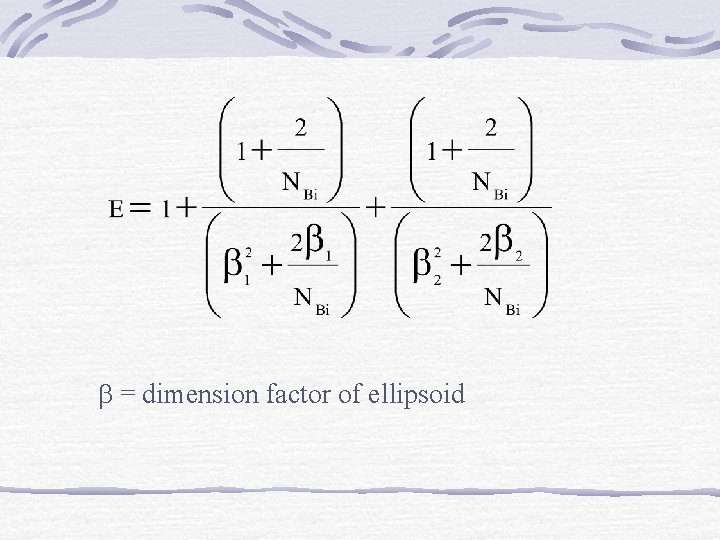
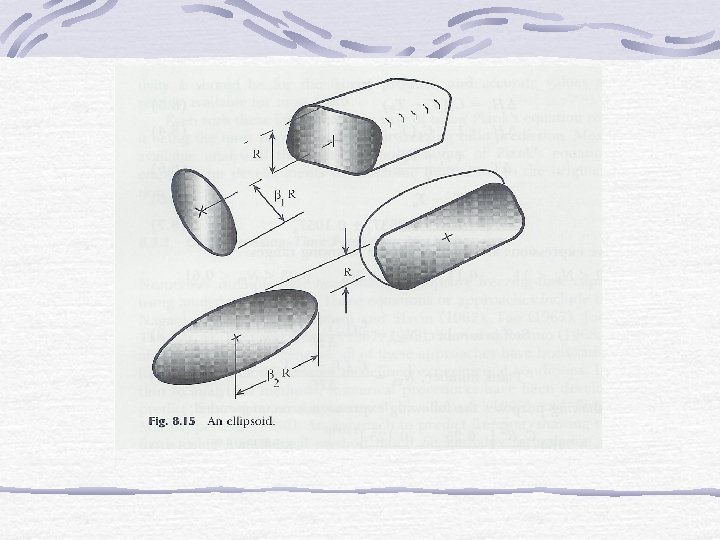
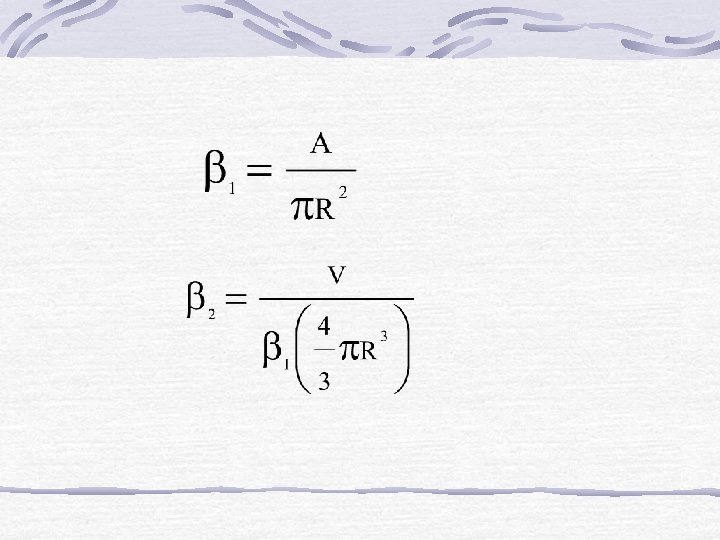
b) Ellipsoid Shapes t ellipsoid = tslab/E For infinite slab E = 1 Infinite cylinder E = 2 Sphere E = 3

= dimension factor of ellipsoid



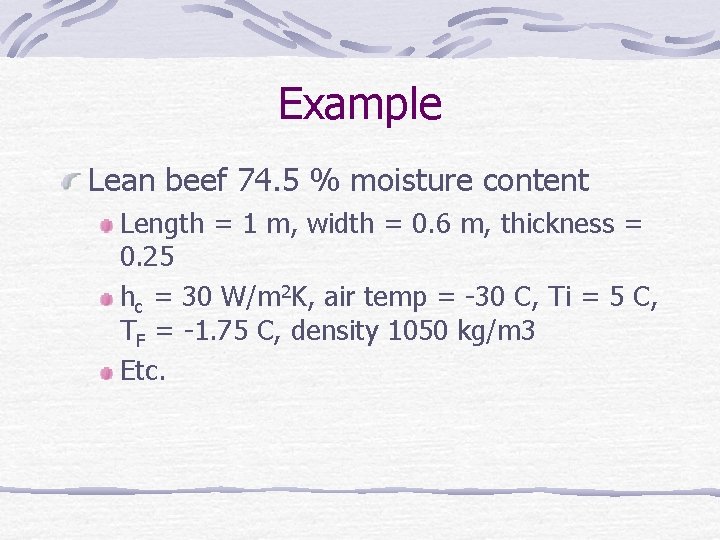
Example Lean beef 74. 5 % moisture content Length = 1 m, width = 0. 6 m, thickness = 0. 25 hc = 30 W/m 2 K, air temp = -30 C, Ti = 5 C, TF = -1. 75 C, density 1050 kg/m 3 Etc.