Physical and Chemical Properties Properties of MatterWords to











- Slides: 19

Physical and Chemical Properties

Properties of Matter-Words to Know…

Matter Anything that has mass and takes up space!

Mass • A measure of how much matter is in an object.

Weight • A measure of the force of gravity on an object.

Volume • The amount of space that matter occupies.

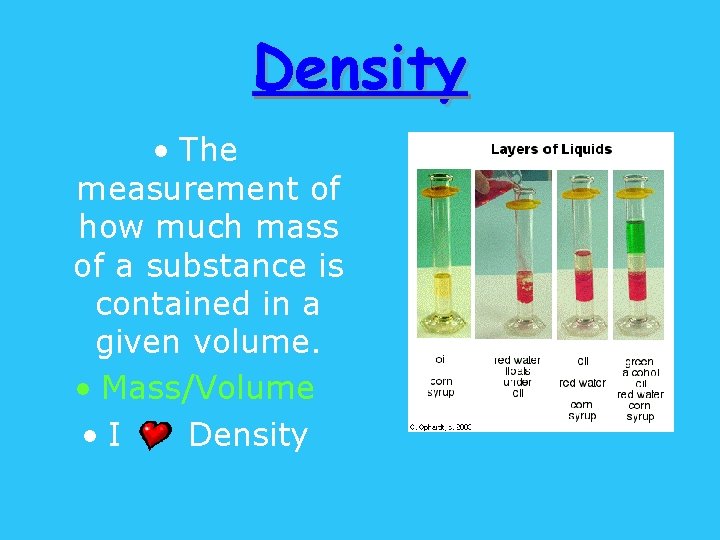
Density • The measurement of how much mass of a substance is contained in a given volume. • Mass/Volume • I Density

States of Matter • There are different “states” of matter. No, not like Texas, Oklahoma, New Mexico. States of matter are also known as phases (a physical state of matter). Elements and compounds can move from one phase to another phase without changing the chemical compound. • Solid • Liquid • Gas

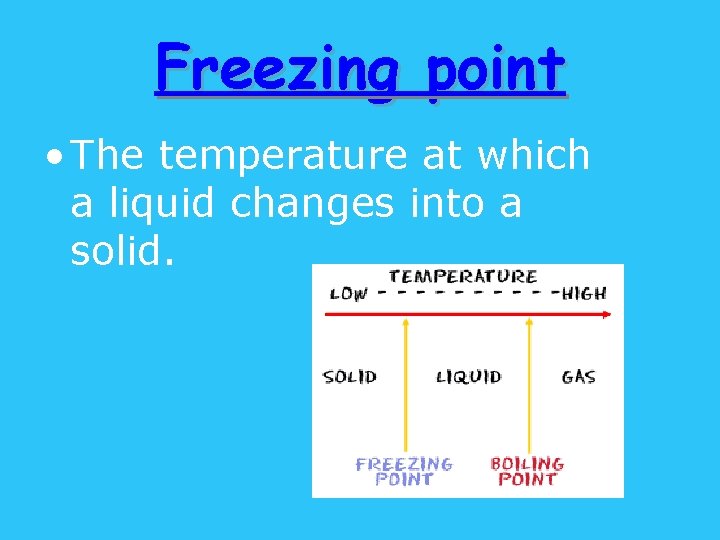
Freezing point • The temperature at which a liquid changes into a solid.

Boiling point • The boiling point of an element or compound means the temperature at which the liquid form of an element or compound is at equilibrium with the gaseous form. • the boiling point of water is 100 degrees Celsius.

Melting point • The temperatures at which the solid form of the element or compound is at equilibrium with the liquid form. • Basically the range at which the solid changes its state • The melting point of into a liquid. water is 0 degrees Celsius

Sublimation • The point where a solid goes from a solid straight to a gaseous state, skipping the liquid state.

Compound • A substance made of two or more elements chemically combined in a set ratio. – Water and salt are 2 examples of compounds.

All substances have properties… Including people! Example: People can be identified by their … Face (shape, Voice Height Finger prints Teeth DNA expressions) Eye color Hair color

What are properties? • Matter has observable and measurable qualities. • We can use general properties to identify substances. • Two basic types of properties of matter: Physical properties and Chemical properties:

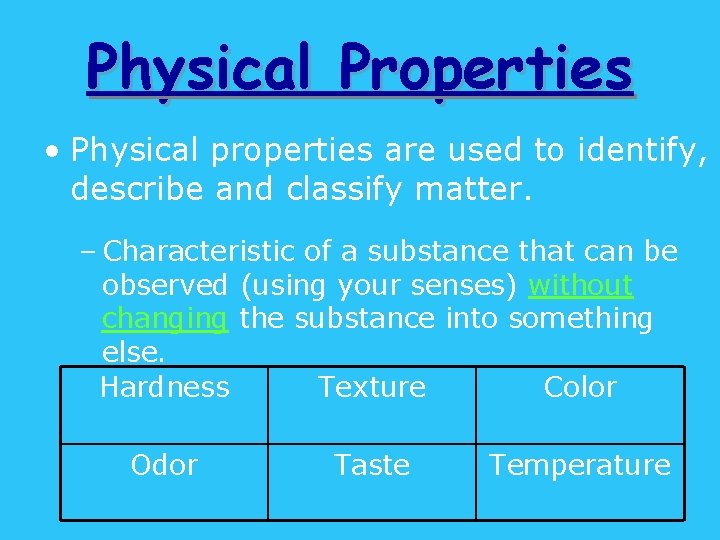
Physical Properties • Physical properties are used to identify, describe and classify matter. – Characteristic of a substance that can be observed (using your senses) without changing the substance into something else. Hardness Texture Color Odor Taste Temperature

More EXAMPLES Physical • size, shape, freezing point, boiling point, melting point, magnetism, viscosity, density, luster and many more. – Viscosity - The resistance of a liquid to flowing. – Examples: – Low viscosity-water, rubbing alcohol – High viscosity-honey

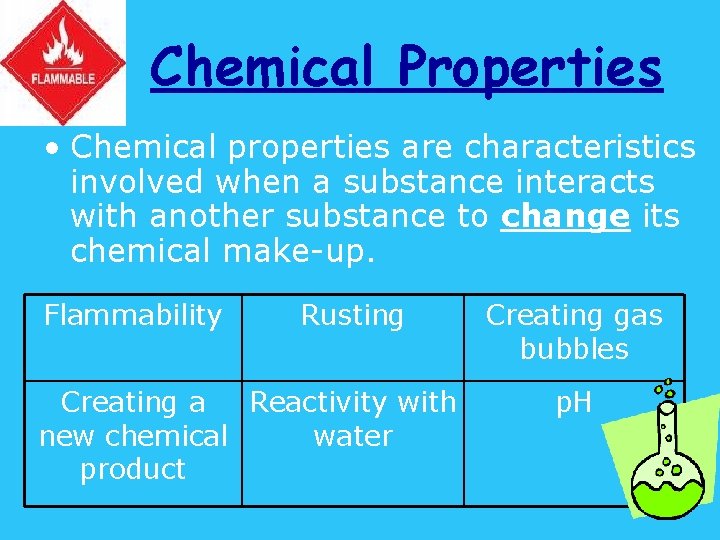
Chemical Properties • Chemical properties are characteristics involved when a substance interacts with another substance to change its chemical make-up. Flammability Rusting Creating a Reactivity with new chemical water product Creating gas bubbles p. H

Alike? Different? • Draw a double bubble map in your notes to compare and contrast physical and chemical properties.
 Chemical properties of citric acid
Chemical properties of citric acid Properties of sulphuric acid
Properties of sulphuric acid A scientist performs an experiment, and an actor performs a
A scientist performs an experiment, and an actor performs a Physical properties of dental materials pdf
Physical properties of dental materials pdf Chemical properties
Chemical properties Hardness property of matter
Hardness property of matter Physical and chemical properties of boron
Physical and chemical properties of boron Physical and chemical properties interactive
Physical and chemical properties interactive Physical and chemical properties of silver
Physical and chemical properties of silver Chemical properties of maltose
Chemical properties of maltose Physical and chemical properties
Physical and chemical properties Water chemical and physical properties
Water chemical and physical properties Physical and chemical properties sorting activity
Physical and chemical properties sorting activity Physical/chemical changes & properties color by number
Physical/chemical changes & properties color by number Compare and contrast physical and chemical changes
Compare and contrast physical and chemical changes Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Whats chemical change
Whats chemical change Physical change
Physical change Physical and chemical changes
Physical and chemical changes