MATTER Changes in State of Matter Freezing Melting




















































































- Slides: 100

MATTER Changes in State of Matter: Freezing, Melting, Evaporation, Condensation, & Sublimation DAY ONE

SPONGE Answer Questions 1 -2. Write the Letter of the Correct Answer Only 1. The measure of how much space an matter takes up is______ (a) Matter (b) Mass (c) Volume (d) Weight 2. A measure of how much matter an object contains is____. (a) matter (b) mass (c) weight (d) volume •

CONTENT STANDARD & ELEMENT Standard: S 8 P 1: Students will examine the scientific view of the nature of matter. Elements: (a). Distinguish between atoms and molecules (c). Describe the movement of particles in solid, liquids, gases and plasma states

Language of the Standards (Today's Vocabulary) • Solids • Liquids • Gases • Condensation • Sublimation • Evaporation • Freezing Point • Melting • Boiling Point

Why Is This Important ? Today I will learn about……. How to differentiate between the Changes in the Phases/States of Matter (Evaporation, Condensation, Sublimation, Melting & Freezing). This is important because it will help me to gain a better understanding of the differences between (Evaporation, Condensation, Sublimation, Melting & Freezing) and that matter changes when heat energy is added.

Essential Question 1. How do we differentiate between phases changes: Evaporation, Condensation, Melting, Freezing & Sublimation?

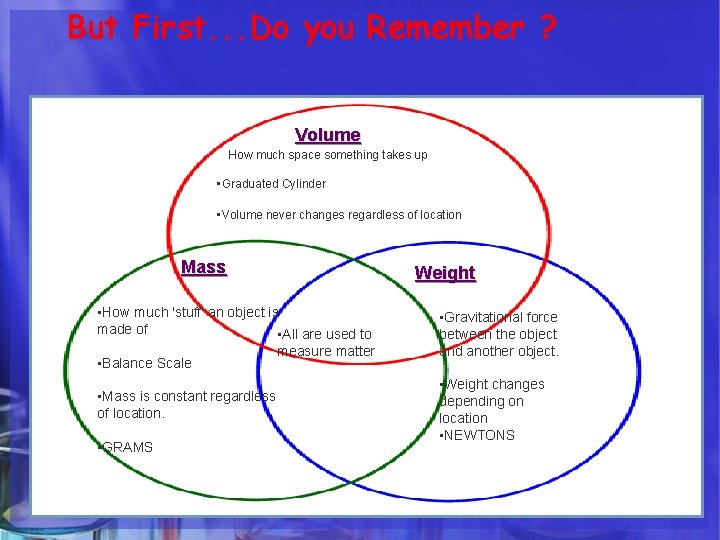
But First. . . Do you Remember ? HOW DO WE MEASURE MATTER ? Volume Mass Weight

But First. . . Do you Remember ? Volume • . How much space something takes up • Graduated Cylinder • Volume never changes regardless of location Mass Weight • How much 'stuff' an object is made of • All are used to measure matter • Balance Scale • Gravitational force between the object and another object. • Mass is constant regardless of location. • Weight changes depending on location • NEWTONS • GRAMS

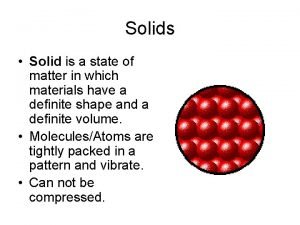
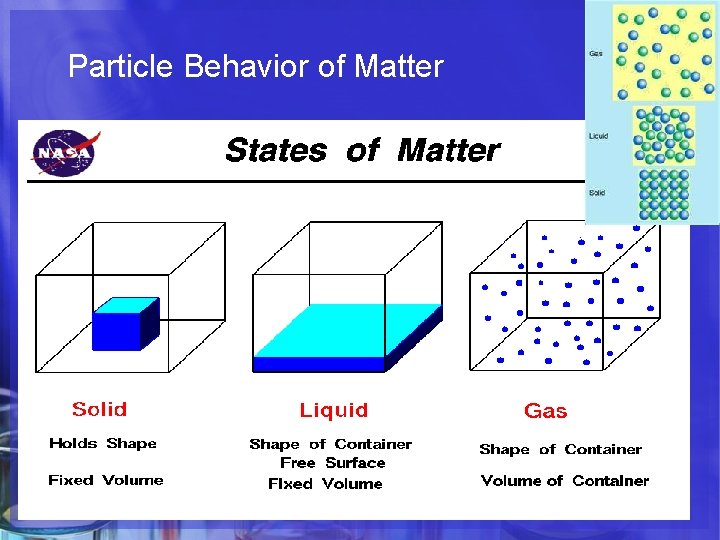
But First. . . Do you Remember ? Particle Behavior of Solid, Liquid & Gas Molecules STATE/PHASE of MATTER Does it have a fixed shape? Does it have a fixed volume? SOLID LIQUID YES NO YES GAS NO NO

Particle Behavior of Matter

ACTIVATOR Dry Ice Facts • Dry ice is what SUBLIMATION (solid to gas) is all about !!!! • Dry ice is Carbon Dioxide (CO 2) • Yes, that’s the same gas you exhale when you breathe and the same gas trees use in photosynthesis! • CO 2 is a naturally occurring molecule composed of two oxygen atoms bonded to one carbon atom. • At room temperature it is a clear odorless gas. • When warmed, goes from solid to gas; it doesn't melt… it does not evaporate…it sublimates

MORE Dry Ice FACTS !!!!

Why do they call it dry ice? ? Observe the dry ice as your teacher walks around the room. What do you notice that is unusual about the dry ice?

Sublimation • This occurs when a solid changes phase directly to a gas. There is no liquid phase. This is why it is called “dry” ice because it never gets wet like regular ice!

How do they make dry ice? ? • Every substance has a different freezing point. Companies that make dry ice simply cool air to the point where the carbon dioxide can liquefy and then become a solid. • It has all kinds of practical uses: fire extinguishers, carbonated drinks, shipping things that have to stay cold (ice cream), fog special effects, etc.

Lab Safety Procedures • Dry ice is very cold (-109 degrees Fahrenheit)…so cold its acts as if its HOT !!!! • Wear protective gloves and goggles. • Store dry ice in containers that allow the gas to escape…leave the lid slightly open

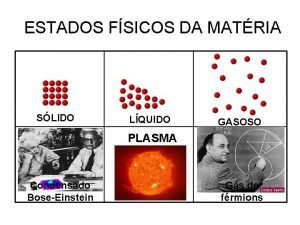
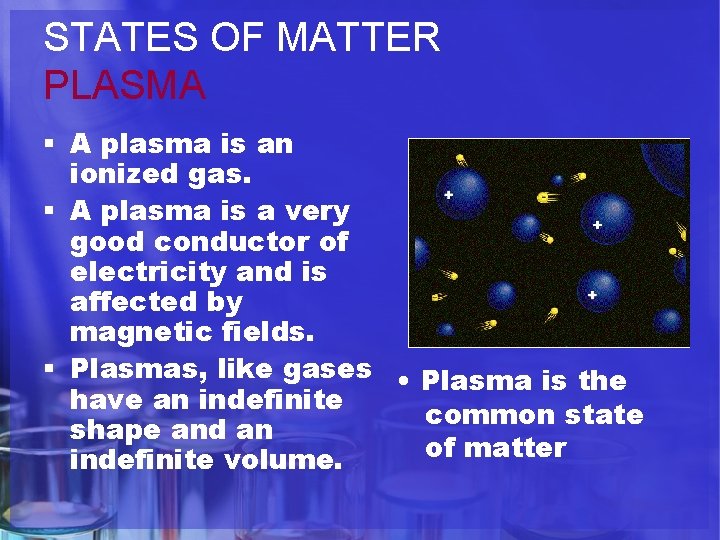
The Learning Period PHASE CHANGES SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

1. Aurora (Northern Lights)

2. Lightning

Some places where plasmas are found… 3. Flames

4. The Sun is an example of a star in its plasma state

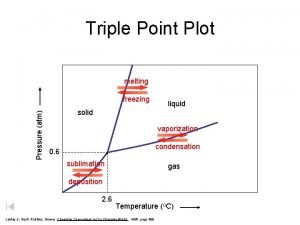
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

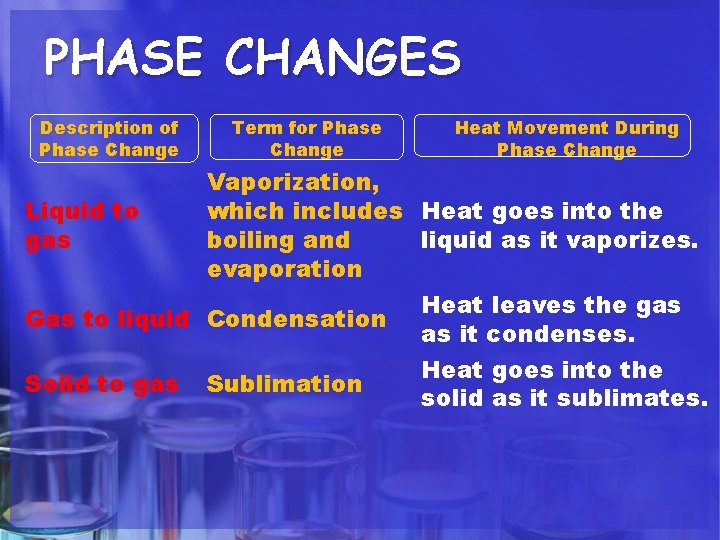
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

The Learning Period CHANGES OF PHASES OF MATTER : http: //www. bgfl. org/bgfl/custom/resources_ftp/client_ftp/ks 3/ science/changing_matter/changingmatter. swf

The Learning Period Lets See this UP CLOSE……. . PHASE CHANGE DEMOS • Sublimation • Condensation • Evaporation • Melting

Sublimation: Solid to Gas (heat goes into solid as it sublimates) • What would happen if I added some dry ice to my apple juice? • Go ahead, make a prediction. Guess!!!

Boil, Toil & Trouble!! • The heat from the apple juice is transferred to the dry ice causing it to sublimate faster (that’s the boiling effect you see & hear). • But where does the carbon dioxide gas go? ? • Can you guess what has happened to our apple juice? Try some and see!

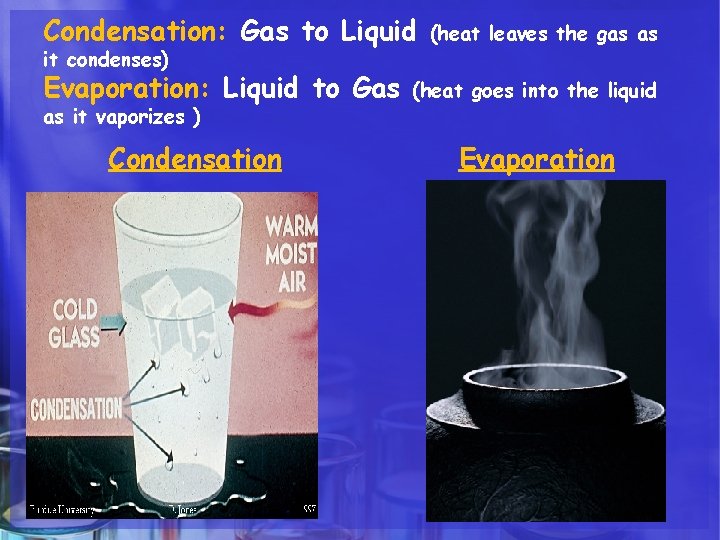
Condensation: Gas to Liquid it condenses) Evaporation: Liquid to Gas as it vaporizes ) Condensation (heat leaves the gas as (heat goes into the liquid Evaporation

Melting: Solid to Liquid melts). (Heat goes into the solid as it

Independent Work Session(CFU) QUESTIONS 1. Which describes a change from gas to a liquid? (a) evaporation (b) condensation (c) freezing (d) Sublimation 2. Why does the outside of a glass of iced tea sometimes get moist? (a) Water molecules from the tea pass through the pores in the glass. (b) Water vapor in the air turns to liquid as it condenses on the cold glass. (c) Glass attracts water droplets that are floating in the air. (d) Melting ice creates more water than the glass can hold.

3. Temperature measures_____. (a) how slow particles move (b) how hot something is (c) the temperature of something (d) how fast particles move 4.

CLOSING “I WONDER…. . ” Directions: Based on what you learned today paper write down……. What did I notice… What did I expect… I wonder what would happen….

HOMEWORK 1. Review the Test 1 STUDY GUIDE for the first unit test on NEXT WEEK ……. •

MATTER Changes in State of Matter: Freezing, Melting, Evaporation, Condensation, & Sublimation DAY TWO

SPONGE • Clear your desk • Study graphic organizers & all notes for QUIZ

CONTENT STANDARD & ELEMENT Standard: S 8 P 1: Students will examine the scientific view of the nature of matter. Elements: (a). Distinguish between atoms and molecules (c). Describe the movement of particles in solid, liquids, gases and plasma states

Language of the Standards (Today's Vocabulary) • Solids • Liquids • Gases • Condensation • Sublimation • Evaporation • Freezing Point • Melting • Boiling Point

Why Is This Important ? Today I will……. assess what I have mastered about states of matter and changes in those states. This is important because it is important for me understand what standards I have mastered.

Essential Questions 1. How do we differentiate between Solid, Liquids & Gas ? 2. How do we differentiate b/w Evaporation, Condensation, Melting, Freezing & Sublimation?

QUIZ TODAY AGENDA 1. QUIZ REVIEW (Staircase Review & Sorting Activity) 2. QUIZ TIME 3. GRADES & Feedback

The Learning Period: STAIR CASE REVIEW SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

The Learning Period Sorting Activity • Place the steps in order • CFU • QUIZ TIME !!!!!!

CLOSING • Question: What skills do you need to master based on how you think you performed? & The Quiz RESULTS are IN !!!!!!!

MATTER Classifying Matter: ELEMENTS & THE PERIODIC TABLE DAY THREE

EQ: How is the periodic table arranged? TIWL: how the periodic table is arranged, because it is arranged by its physical and chemical properties. • SPONGE: • How many elements are listed on the periodic table of elements? • A. 28 • B. 60 • C. 72 • D. more than 100 • *Place Homework in the center of table

CONTENT STANDARD & ELEMENT Standard: S 8 P 1: Students will examine the scientific view of the nature of matter. Element: (f) Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements.

Language of the Standards • • • (Today's Vocabulary) Periodic Table Elements Atomic number Atomic mass Family Period Groups Metalloids Nonmetals

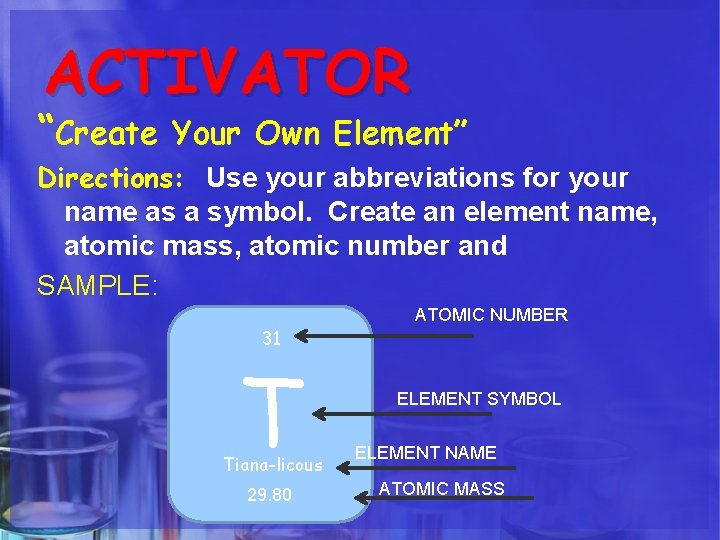
ACTIVATOR “Create Your Own Element” Directions: Use your abbreviations for your name as a symbol. Create an element name, atomic mass, atomic number and SAMPLE: 31 T Tiana-licous 29. 80 ATOMIC NUMBER ELEMENT SYMBOL ELEMENT NAME ATOMIC MASS


The Learning Period: Elements • A pure substance that cannot be broken down into simpler substances by chemical means • There are 115 elements are known, of which 92 are known to occur in nature

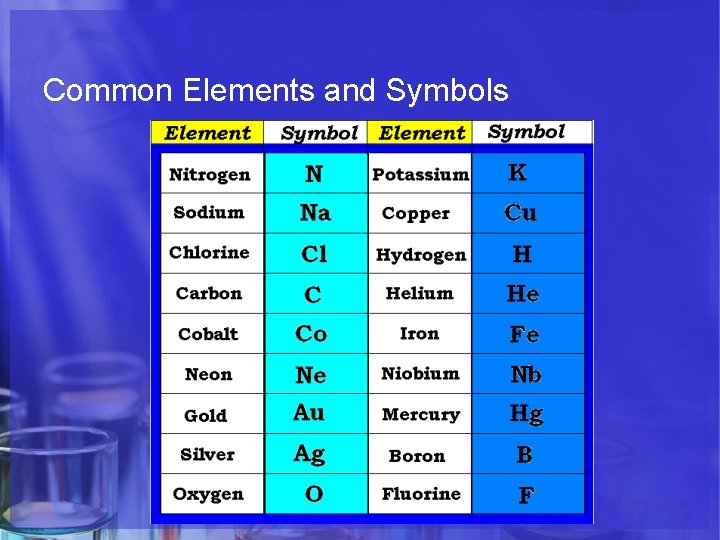
Common Elements and Symbols

Symbols C Cu Carbon Copper • All elements have their own unique symbol. • It can consist of a single capital letter, or a capital letter and one or two lower case letters.

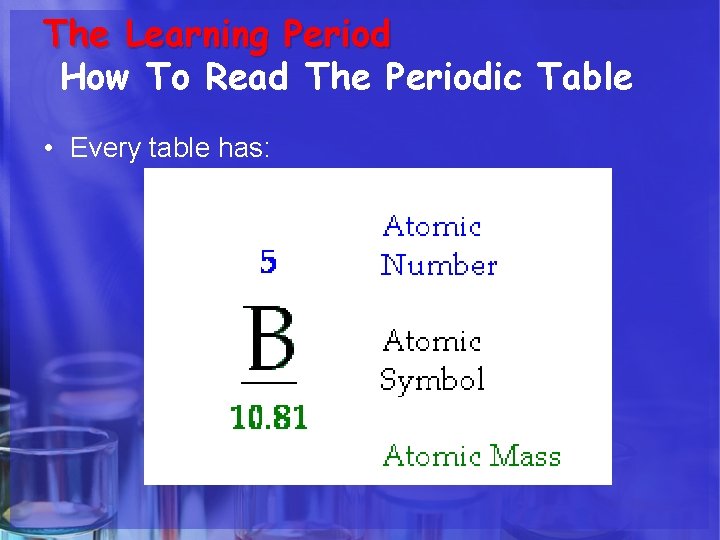
The Learning Period How To Read The Periodic Table • Every table has:

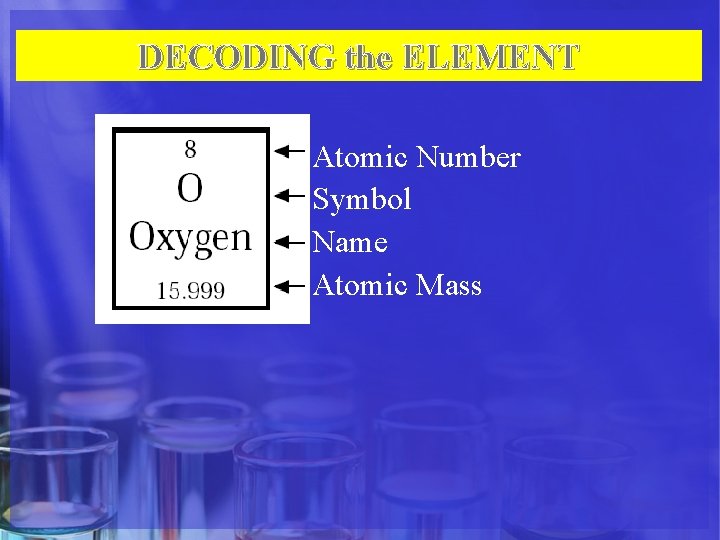
Atomic Number • The number of protons in an atom identifies the element. • The number of protons in an atom is referred to as the atomic number of that element.

Atomic Symbol • The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen, " etc. ). • These symbols are used every where in the world • Usually, a symbol is the abbreviation of the element or the abbreviated Latin name of the element.

Atomic Mass • The atomic mass is the average mass of an element in atomic mass units ("amu"). • Though individual atoms always have a whole number of amus, the atomic mass on the periodic table is shown as a decimal number because it is an average of all the isotopes of an element.

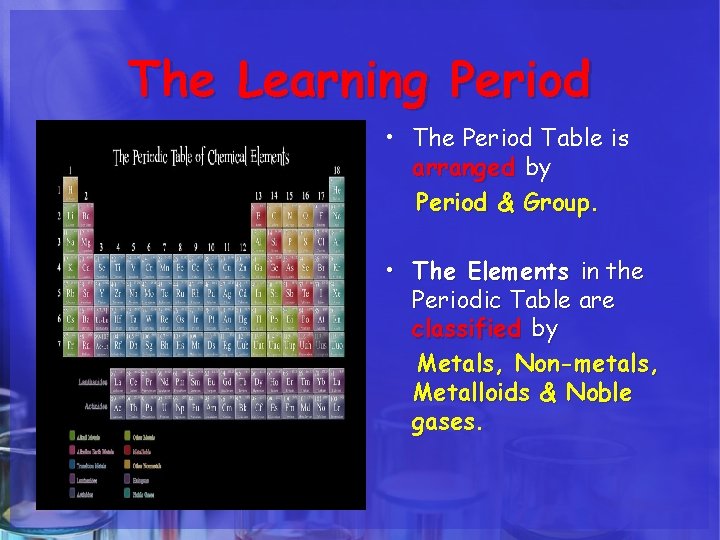
The Learning Period • The Period Table is arranged by Period & Group. • The Elements in the Periodic Table are classified by Metals, Non-metals, Metalloids & Noble gases.

ARRANGEMENT The Period Table is arranged by Period & Group. • The periodic table is made up of rows of elements and columns. • A row is called a period • A column is called a group/family



PERIODS (row) • The elements in a period are not alike in properties. • In fact, the properties change greatly across even given row. • The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas. GROUP/FAMILY (column) • Elements in each family have similar but not identical properties. • For example, lithium (Li), sodium (Na), potassium (K), and other members of family IA are all soft, white, shiny metals. • All elements in a family have the same number of valence electrons

CLASSIFICATION The Elements in the Periodic Table are classified by Metals, Non-metals, Metalloids & Noble gases. • Metals: Elements that are usually solids at room temperature. Most elements are metals. Non-Metals: Elements in the upper right corner of the periodic Table. Their chemical and physical properties are different from metals. • Metalloids: Elements that lie on a diagonal line between the Metals and non-metals. Their chemical and physical properties are intermediate between the two. • Nobel Gases: colorless gases that are extremely un-reactive.


Noble Gases

Properties of Metals • Metals are good conductors of heat and electricity. • Metals are shiny. • Metals are ductile (can be stretched into thin wires). • Metals are malleable (can be pounded into thin sheets). • A chemical property of metal is its reaction with water which results in corrosion.

Properties of Non-Metals • Non-metals are poor conductors of heat and electricity. • Non-metals are not ductile or malleable. • Solid non-metals are brittle and break easily. • They are dull. • Many non-metals are gases. Sulfur

Properties of Metalloids • Metalloids (metal-like) have properties of both metals and non-metals. • They are solids that can be shiny or dull. • They conduct heat and electricity better than nonmetals but not as well as metals. • They are ductile and malleable. Silicon

Noble Gases • Noble Gases are colorless gases that are extremely un-reactive. • One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. • Because they do not readily combine with other elements to form compounds, the noble gases are called inert. • The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. • All the noble gases are found in small amounts in the earth's atmosphere.

DECODING the ELEMENT Atomic Number Symbol Name Atomic Mass

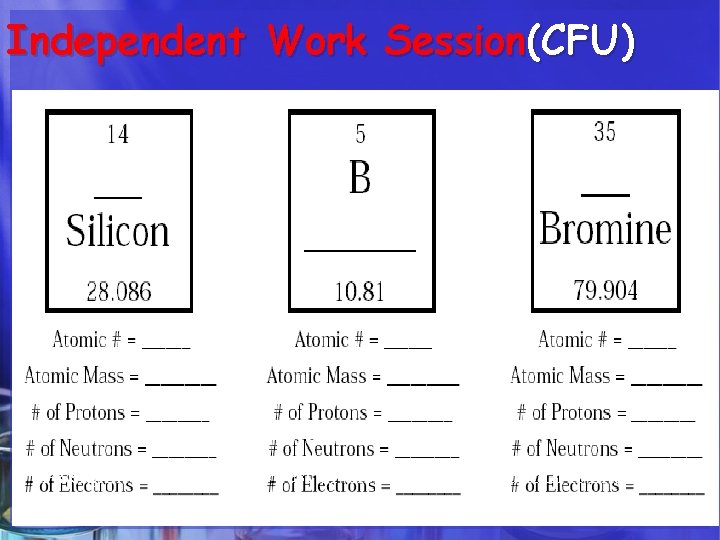
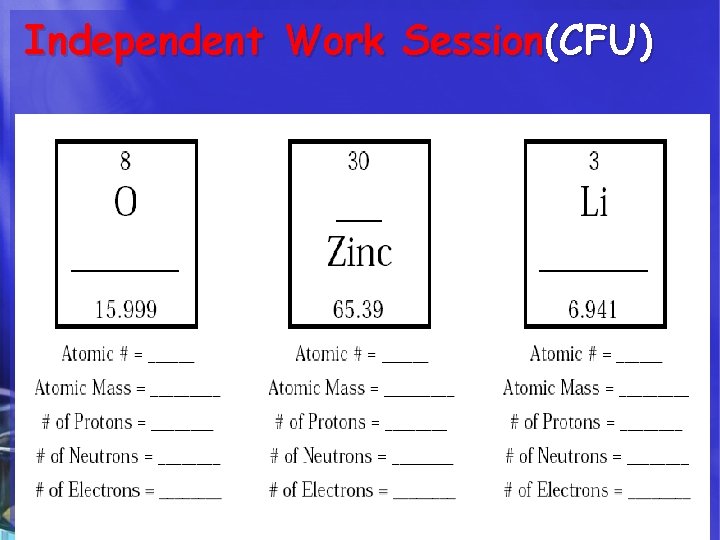
Independent Work Session(CFU) Complete Independently the “Decoding the Element” hand-out

CLOSING THINK…. . PAIR…. SHARE” What did you learn about the periodic table? Do you have any questions?

Homework • Study for Test on Tuesday • 7 th Period: your study guide graphic organizer is due tomorrow.

MATTER Classifying Matter: ELEMENTS & THE PERIODIC TABLE DAY FOUR

SPONGE 1. Decode the element. Can you find Sulfur? List the symbol, atomic number, and atomic mass. 2. How many elements are listed on the periodic table of elements? a. 28 b. 60 c. 72 d. more than 100

CONTENT STANDARD & ELEMENT Standard: S 8 P 1: Students will examine the scientific view of the nature of matter. Element: (f) Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements.

Language of the Standards • • • (Today's Vocabulary) Periodic Table Elements Atomic number Atomic mass Family Period Groups Metalloids Nonmetals

Why Is This Important ? Today I will learn about……. The Periodic Table and how it is arranged and classified. This is important because it will help me understand that the periodic table is arranged by physical and chemical properties/ characteristics. •

Essential Questions 1. What is an element? 2. How is the periodic table arranged?

ACTIVATOR “Lets Sort This Out” Directions Sort the parts of the element : • • Atomic Number Atomic Mass Element Symbol Element Name Music Link http: //www. schooltube. com/video/7 f 5 dc 6746 a 0 f 4482 a 0 ae/P eriodic-Table-Song-1

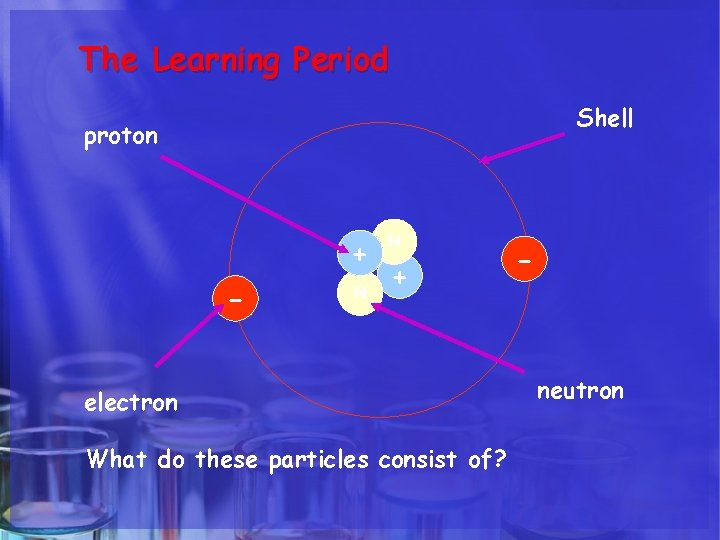
The Learning Period. ATOM HELIUM Shell proton + - N N + electron What do these particles consist of? - neutron

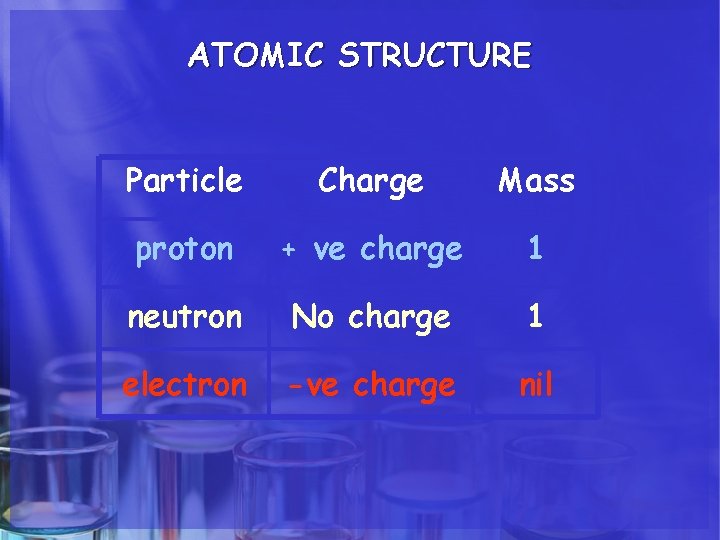
ATOMIC STRUCTURE Particle Charge Mass proton + ve charge 1 neutron No charge 1 electron -ve charge nil

ATOMIC STRUCTURE He 2 4 Atomic number the number of protons in an atom Atomic mass the number of protons and neutrons in an atom number of electrons = number of protons

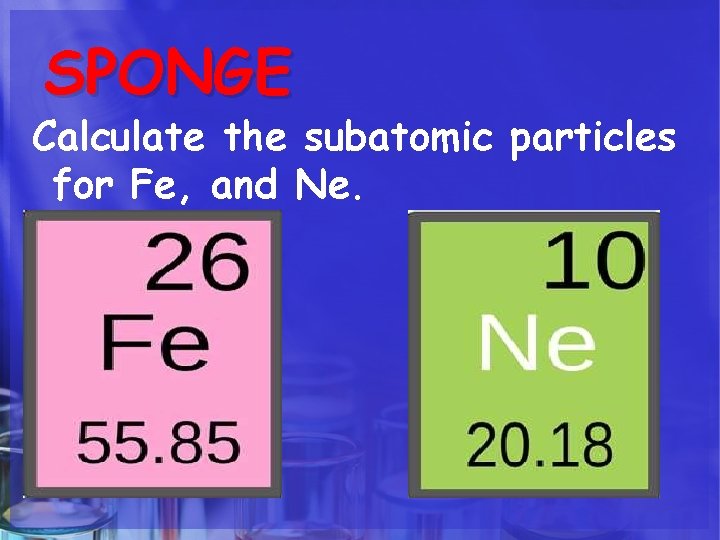
HOW DO WE CALCULATE ? ? ? 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The number of Protons = Number of Electrons. 3. The number of Neutrons= Subtract the atomic number from the rounded atomic mass. 4. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus.

The Atoms Family - Atomic Math Challenge Atomic Number Symbol Name Atomic Mass 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The number of Protons = Number of Electrons. 3. The number of Neutrons= Subtract the atomic number from the rounded atomic mass. 4. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus.

Independent Work Session(CFU) Assignment: Finish the rest of the worksheet and turn it in to your teacher.

Independent Work Session(CFU)

HOMEWORK CONTINUE TO REVIEW YOUR STUDY GUIDE FOR THE Unit Test

CLOSING “THINK…. . PAIR…. SHARE” What did you learn about subatomic particles? Do you have any questions?

MATTER Classifying Matter: ELEMENTS & THE PERIODIC TABLE DAY 5

SPONGE Calculate the subatomic particles for Fe, and Ne.

CONTENT STANDARD & ELEMENT Standard: S 8 P 1: Students will examine the scientific view of the nature of matter. Element: (f) Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements.

Language of the Standards • • • (Today's Vocabulary) Periodic Table Elements Atomic number Atomic mass Family Period Groups Metalloids Nonmetals

Why Is This Important ? Today I will learn about……. The Periodic Table and how it is arranged and classified. This is important because it will help me understand that the periodic table is arranged by physical and chemical properties/ characteristics. •

Essential Questions 1. What is an element? 2. How is the periodic table arranged?

Learning Period Family Game: • Non-metals, metalloids, and noble gases. • Each student will receive a card and much match that card with the correct group/family.

Independent Work Session(CFU) Foldable Instructions DIRECTIONS: Construct an Interest Bound Book Foldable and title it “Periodic Table”. Include: • • • NAME DEFINITION EXAMPLE OF … Metals, Non-Metals, Metalloids & Nobel Gases. 1. Hamburger fold the foldable handouts 2. Cut 2 cm slits from the edges of the fold of the handout that has page 2 (as marked) 3. Cut along the fold line of the other sheet of paper beginning and ending about 2 cm from each end (as marked). 4. Burrito fold and insert paper from step 2 into the hole and open, forming a book.

Independent Work Session(CFU) “FOLDABLE” http: //foldables. wikispaces. com/Foldables

HOMEWORK CONTINUE TO REVIEW YOUR STUDY GUIDE FOR THE Unit Test

CLOSING “ Think About It” Question: If you were a family on the periodic table, which one would you be and why?
 Condensation phase change
Condensation phase change Vaporization particles
Vaporization particles Evaporation condensation melting freezing
Evaporation condensation melting freezing Incongruent melting
Incongruent melting Change in state of matter
Change in state of matter Elizabeth mulroney
Elizabeth mulroney Physicl change
Physicl change Properties and changes of matter worksheet
Properties and changes of matter worksheet Chemical properties of air
Chemical properties of air 5 phases of matter
5 phases of matter Definition of substance
Definition of substance Matter-properties and changes answer key
Matter-properties and changes answer key Two types of changes
Two types of changes Which is a big idea for matter and change
Which is a big idea for matter and change True or false: chemical and physical changes alter matter.
True or false: chemical and physical changes alter matter. Phase changes of matter
Phase changes of matter Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas Lesson 6 changes of state answer key
Lesson 6 changes of state answer key Cool chilly cold freezing
Cool chilly cold freezing Donate eggs nashville
Donate eggs nashville Expansion upon freezing
Expansion upon freezing Expansion upon freezing
Expansion upon freezing Mobile tcp in mobile computing
Mobile tcp in mobile computing What are the market form of meat
What are the market form of meat Air freezing point
Air freezing point Pham method freezing
Pham method freezing Fluidized bed freezers
Fluidized bed freezers Planck's equation for freezing time
Planck's equation for freezing time Preservation by freezing
Preservation by freezing Indirect contact freezing
Indirect contact freezing What is vapor pressure lowering
What is vapor pressure lowering Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Colligative properties
Colligative properties Expansion upon freezing
Expansion upon freezing Cool treeless and dry
Cool treeless and dry Expansion upon freezing
Expansion upon freezing δ+
δ+ Upper air soundings
Upper air soundings Fahrenheit brine
Fahrenheit brine Dehydrofreezing
Dehydrofreezing Transmission/timeout freezing
Transmission/timeout freezing Freezing point of xylene
Freezing point of xylene How to calculate molar mass from freezing point
How to calculate molar mass from freezing point Aimlab freezing
Aimlab freezing Winter personifications
Winter personifications Freezing of fish
Freezing of fish Freezing point chapter 21
Freezing point chapter 21 Kinetic particle theory o level questions
Kinetic particle theory o level questions Freezing point chapter 12
Freezing point chapter 12 Verbal escalation continuum
Verbal escalation continuum Ions in aqueous solutions and colligative properties
Ions in aqueous solutions and colligative properties Freezing point chapter 13
Freezing point chapter 13 Freezing point chapter 12
Freezing point chapter 12 Tyndall effect
Tyndall effect 32 fahrenheit to celsius
32 fahrenheit to celsius Freezing point chapter 8
Freezing point chapter 8 Vapor pressure lowering
Vapor pressure lowering Hardness texture color and freezing point are examples of
Hardness texture color and freezing point are examples of Cell freezing
Cell freezing Advantages of freezing
Advantages of freezing Section 1 composition of matter
Section 1 composition of matter Meysam golmohammadi
Meysam golmohammadi Composition of matter section 1
Composition of matter section 1 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Cerebral aqueduct
Cerebral aqueduct Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Grey matter and white matter in brain
Grey matter and white matter in brain Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter State of matter
State of matter Properties of liquid state of matter
Properties of liquid state of matter Thermal energy in states of matter
Thermal energy in states of matter Sixth state of matter
Sixth state of matter øis
øis Pepsi mentos
Pepsi mentos Physical state of matter
Physical state of matter Vaporization matter
Vaporization matter Conduction convection and radiation venn diagram
Conduction convection and radiation venn diagram Meaning of colloidal suspension
Meaning of colloidal suspension What is the particle theory of matter
What is the particle theory of matter Law of constancy of interfacial angle
Law of constancy of interfacial angle Compressible state of matter
Compressible state of matter State of matter
State of matter Liquid homogeneous mixture
Liquid homogeneous mixture 6th state of matter
6th state of matter Liquid particle diagram
Liquid particle diagram Ionic bond melting point
Ionic bond melting point Melting point trend
Melting point trend Physical properties of group 2 elements
Physical properties of group 2 elements Why is america called a melting pot
Why is america called a melting pot Oxbridge melting pot
Oxbridge melting pot Congruent melting point definition
Congruent melting point definition Melting point of group 2
Melting point of group 2 Our iceberg is melting 8 steps
Our iceberg is melting 8 steps Batch melting equation
Batch melting equation Compare the properties of metals and nonmetals
Compare the properties of metals and nonmetals Melting pot vs salad
Melting pot vs salad Tungsten melting pot
Tungsten melting pot Melting cubes
Melting cubes How to find the specific heat capacity
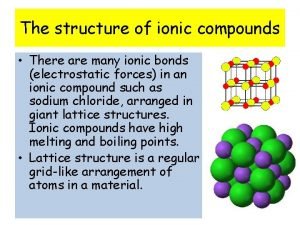
How to find the specific heat capacity Why do ionic compounds have high melting and boiling points
Why do ionic compounds have high melting and boiling points