Exp 12 A A Molar Mass from FreezingPoint




- Slides: 18

Exp 12 A: A Molar Mass from Freezing-Point Depression Purpose 1. Determine the freezing-point depression in a solution when a solute is added to a pure solvent 2. Use the freezing-point depression of the solution to determine the molar mass of the solute 3. Detemine the molecular formula of the solute based on the given empirical formula and the calculated molar mass • See also: Silberberg, Chapter 13, pp. 515 -520, and Sample problems 13. 7 and 13. 8

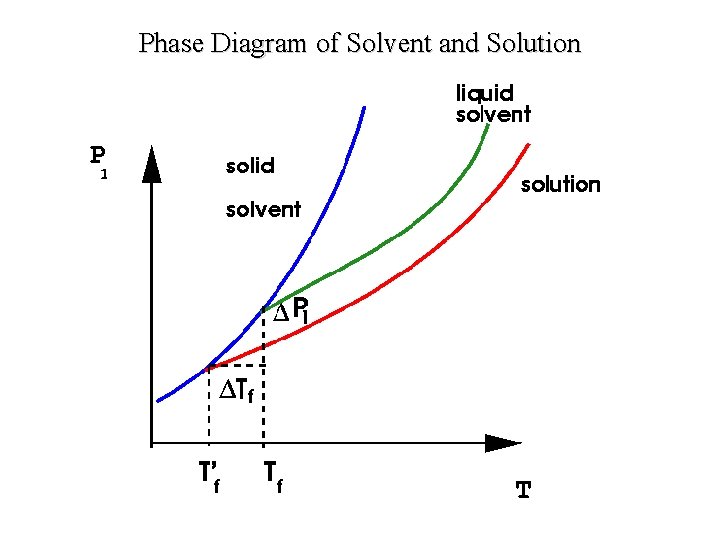
Exp 12 A: A Molar Mass from Freezing-Point Depression • The melting point is the temperature at which a solid changes to a liquid • The freezing point is the temperature at which a liquid changes to a solid • The freezing point of pure water is 0°C • The melting point can be depressed by adding a solute such as a salt. – Use of ordinary salt (sodium chloride, Na. Cl) on icy roads in the winter helps to melt the ice from the roads by lowering the melting point of the ice. – Making ice-cream by using a water-salt mixture to lower the freezing point of the solution • A solution (solvent + solute) typically has a measurably lower melting point than the pure solvent

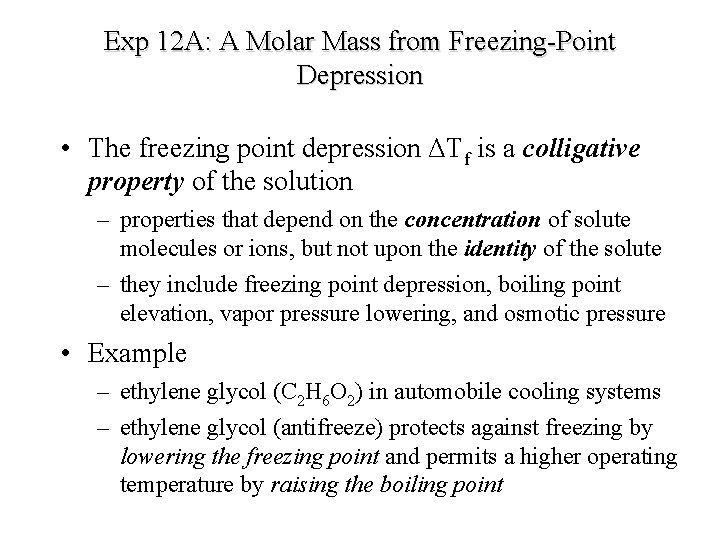
Exp 12 A: A Molar Mass from Freezing-Point Depression • The freezing point depression ΔTf is a colligative property of the solution – properties that depend on the concentration of solute molecules or ions, but not upon the identity of the solute – they include freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure • Example – ethylene glycol (C 2 H 6 O 2) in automobile cooling systems – ethylene glycol (antifreeze) protects against freezing by lowering the freezing point and permits a higher operating temperature by raising the boiling point

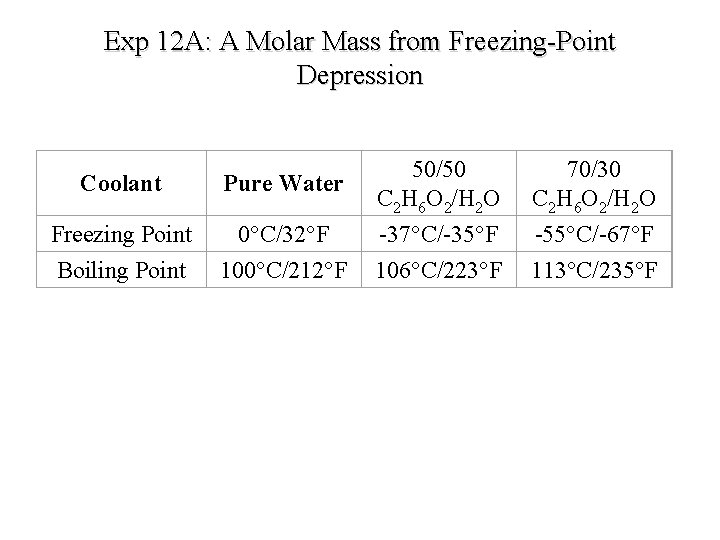
Exp 12 A: A Molar Mass from Freezing-Point Depression Coolant Pure Water Freezing Point Boiling Point 0°C/32°F 100°C/212°F 50/50 C 2 H 6 O 2/H 2 O -37°C/-35°F 106°C/223°F 70/30 C 2 H 6 O 2/H 2 O -55°C/-67°F 113°C/235°F

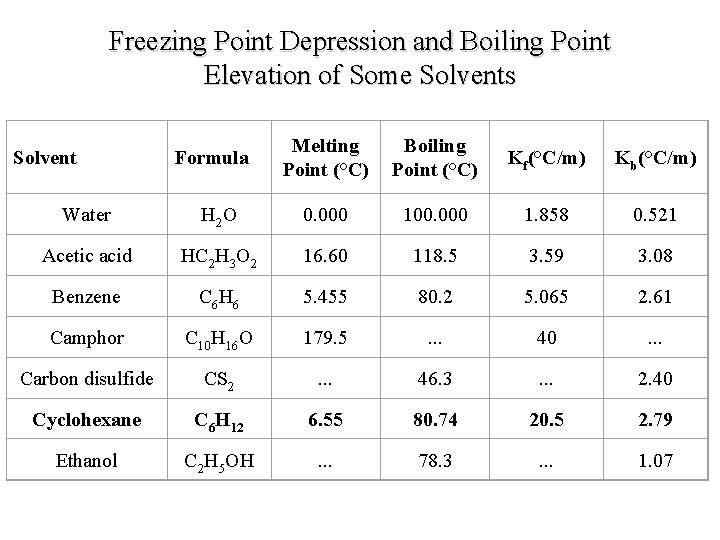
Freezing Point Depression and Boiling Point Elevation of Some Solvents Solvent Formula Melting Point (°C) Boiling Point (°C) Kf(°C/m) Kb(°C/m) Water H 2 O 0. 000 100. 000 1. 858 0. 521 Acetic acid HC 2 H 3 O 2 16. 60 118. 5 3. 59 3. 08 Benzene C 6 H 6 5. 455 80. 2 5. 065 2. 61 Camphor C 10 H 16 O 179. 5 . . . 40 . . . Carbon disulfide CS 2 . . . 46. 3 . . . 2. 40 Cyclohexane C 6 H 12 6. 55 80. 74 20. 5 2. 79 Ethanol C 2 H 5 OH . . . 78. 3 . . . 1. 07

Exp 12 A: A Molar Mass from Freezing-Point Depression • For dilute solutions freezing-point depression is found to be proportional to the molal concentration cm of the solution: • ΔTf = Kf cm where Kf is called the freezing-point-depression constant • Kf (cyclohexane) = 20. 5 o. C/m • Molality (m) equals the number of moles of a given substance per kilogram of solvent (mol/kg) • it does not change with the temperature, as it deals with the mass of solvent rather than the volume of solution molality of a solution is always constant irrespective of the physical conditions like temperature and pressure

Exp 12 A: A Molar Mass from Freezing-Point Depression - Experimental Part A: Measuring the freezing point of cyclohexane • Pipet exactly 20. 0 ml cyclohexane in a large test tube. Close immediately • Calculate mass of cyclohexane – d = 0. 779 g/ml – 20. 0 ml = 20. 0 ml x 0. 779 g/ml = 15. 58 g = 15. 6 g • Record volume and calculated mass on your worksheet

Exp 12 A: A Molar Mass from Freezing-Point Depression - Experimental Part A: Measuring the freezing point of cyclohexane • Place the test tube with cylcohexane in a beaker with crushed ice and water • Stir gently, but constantly • When the temperature gets close to the freezing point of cyclohexane (melting point = 6. 6 o. C), take temperature readings every 15 sec. This will take 10 -15 min • Record the temperature change to the nearest 0. 1 o. C. Stop the experiment once all cyclohexane is frozen and the temperature decreases further. • Allow cyclohexane to melt completely. • Repeat the freezing point determination • Plot the data, determine the freezing point in each case, and calculate the mean freezing point

Exp 12 A: A Molar Mass from Freezing-Point Depression - Experimental Part B: Measuring the freezing point of solutions • Weigh 0. 24 - 0. 25 g of the solute on weighing paper (sample 1). Determine the mass exactly (record in 4 decimals) • Weigh 0. 10 - 0. 11 g of solute on a 2 nd piece of weighing paper (sample 2). Weigh in 4 decimals • Transfer sample 1 to the solvent in freezing point depression apparatus • Stir until the solute is completely dissolved. All of the solute must be dissolved • Stir gently but constantly and cool the solution as before • Determine the temperature as described under (A) until the entire solution is frozen • Remove the apparatus and allow the frozen solution to melt completely. • Graph your data! • How do your data look?

Exp 12 A: A Molar Mass from Freezing-Point Depression - Experimental Part B: Measuring the freezing point of solutions • Add sample 2 to the solution containing sample 1 and repeat the freezing process • Plot data for both experiments • Calculate the change in freezing point, DTf • Calculate the molal mass of the solute from each DTf. Mind the significant figures • Obtain the mean molar mass • Share data with the rest of the class • Calculate the molecular formula of the solute from the grand average and the empirical formula (C 3 H 2 Cl)

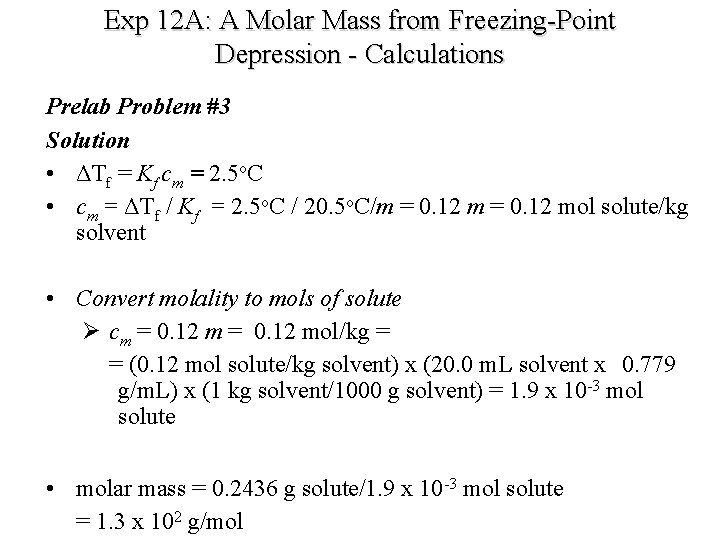
Exp 12 A: A Molar Mass from Freezing-Point Depression - Calculations Prelab Problem #3 - a 0. 2436 -g sample of an unknown substance was dissolved in 20. 0 m. L cyclohexane. - The density of cyclohxane = 0. 779 g/m. L. - The freezing point depression was 2. 5 o. C. - Kf = 20. 5 o. C/m - Calculate the molar mass of the unknown substance.

Exp 12 A: A Molar Mass from Freezing-Point Depression - Calculations Prelab Problem #3 Solution • ΔTf = Kf cm = 2. 5 o. C • cm = ΔTf / Kf = 2. 5 o. C / 20. 5 o. C/m = 0. 12 mol solute/kg solvent • Convert molality to mols of solute Ø cm = 0. 12 mol/kg = = (0. 12 mol solute/kg solvent) x (20. 0 m. L solvent x 0. 779 g/m. L) x (1 kg solvent/1000 g solvent) = 1. 9 x 10 -3 mol solute • molar mass = 0. 2436 g solute/1. 9 x 10 -3 mol solute = 1. 3 x 102 g/mol

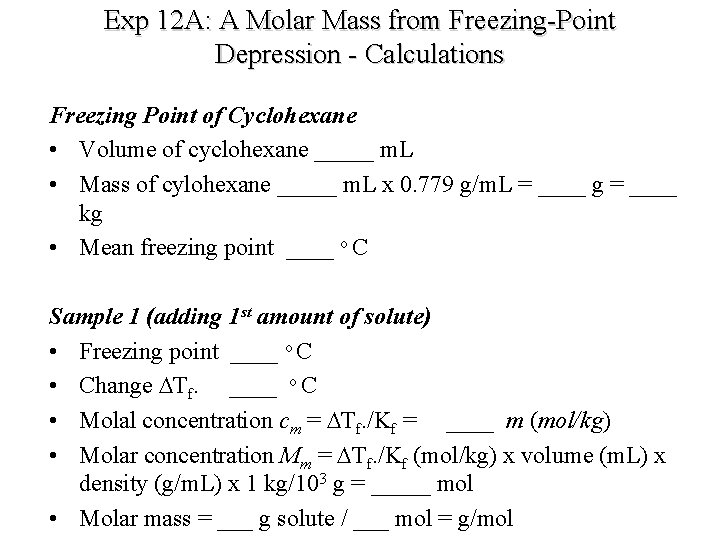
Exp 12 A: A Molar Mass from Freezing-Point Depression - Calculations Freezing Point of Cyclohexane • Volume of cyclohexane _____ m. L • Mass of cylohexane _____ m. L x 0. 779 g/m. L = ____ g = ____ kg • Mean freezing point ____ o C Sample 1 (adding 1 st amount of solute) • Freezing point ____ o C • Change DTf. ____ o C • Molal concentration cm = DTf. /Kf = ____ m (mol/kg) • Molar concentration Mm = DTf. /Kf (mol/kg) x volume (m. L) x density (g/m. L) x 1 kg/103 g = _____ mol • Molar mass = ___ g solute / ___ mol = g/mol

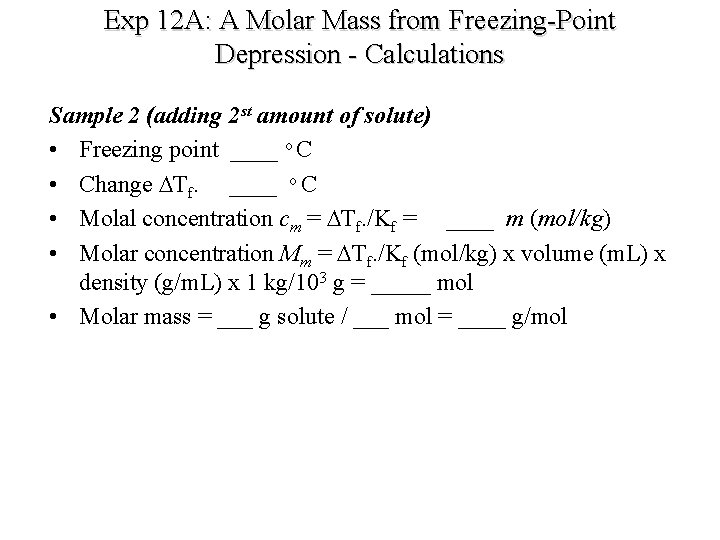
Exp 12 A: A Molar Mass from Freezing-Point Depression - Calculations Sample 2 (adding 2 st amount of solute) • Freezing point ____ o C • Change DTf. ____ o C • Molal concentration cm = DTf. /Kf = ____ m (mol/kg) • Molar concentration Mm = DTf. /Kf (mol/kg) x volume (m. L) x density (g/m. L) x 1 kg/103 g = _____ mol • Molar mass = ___ g solute / ___ mol = ____ g/mol

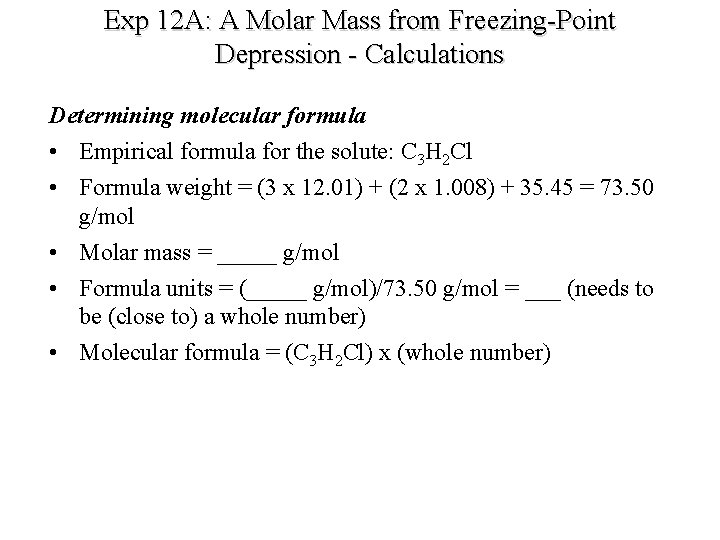
Exp 12 A: A Molar Mass from Freezing-Point Depression - Calculations Determining molecular formula • Empirical formula for the solute: C 3 H 2 Cl • Formula weight = (3 x 12. 01) + (2 x 1. 008) + 35. 45 = 73. 50 g/mol • Molar mass = _____ g/mol • Formula units = (_____ g/mol)/73. 50 g/mol = ___ (needs to be (close to) a whole number) • Molecular formula = (C 3 H 2 Cl) x (whole number)

This Thursday • Exp. 12 A: Postlab • Exp. 13: Rate of an Iodine Clock Reaction – Prelab preparations and – Prelab assignment

Phase Diagram of Solvent and Solution

Exp 12 A: A Molar Mass from Freezing- Point Depression Molarity • molarity (M) equals the number of moles of a given substance per liter of solution (mol/L) • measurement of the absolute number of particles in a solution, irrespective of their weight and volume Molality • molality (m) equals the number of moles of a given substance per kilogram of solvent (mol/kg) • it does not change with the temperature, as it deals with the mass of solvent rather than the volume of solution molality of a solution is always constant irrespective of the physical conditions like temperature and pressure