Answer the following question For each answer explain




















- Slides: 35

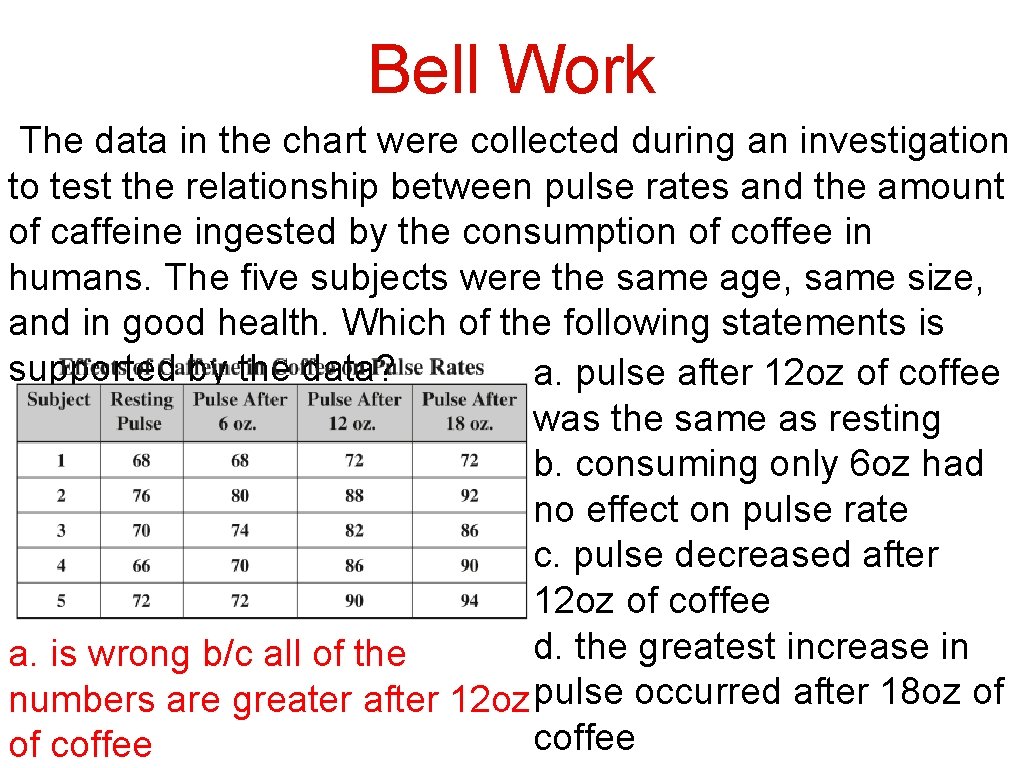
Answer the following question. For each answer, explain why it is correct or incorrect. Bell Work The data in the chart were collected during an investigation to test the relationship between pulse rates and the amount of caffeine ingested by the consumption of coffee in humans. The five subjects were the same age, same size, and in good health. Which of the following statements is supported by the data? a. pulse after 12 oz of coffee was the same as resting b. consuming only 6 oz had no effect on pulse rate c. pulse decreased after 12 oz of coffee d. the greatest increase in pulse occurred after 18 oz of coffee

Bell Work The data in the chart were collected during an investigation to test the relationship between pulse rates and the amount of caffeine ingested by the consumption of coffee in humans. The five subjects were the same age, same size, and in good health. Which of the following statements is supported by the data? a. pulse after 12 oz of coffee was the same as resting b. consuming only 6 oz had no effect on pulse rate c. pulse decreased after 12 oz of coffee d. the greatest increase in a. is wrong b/c all of the numbers are greater after 12 oz pulse occurred after 18 oz of coffee

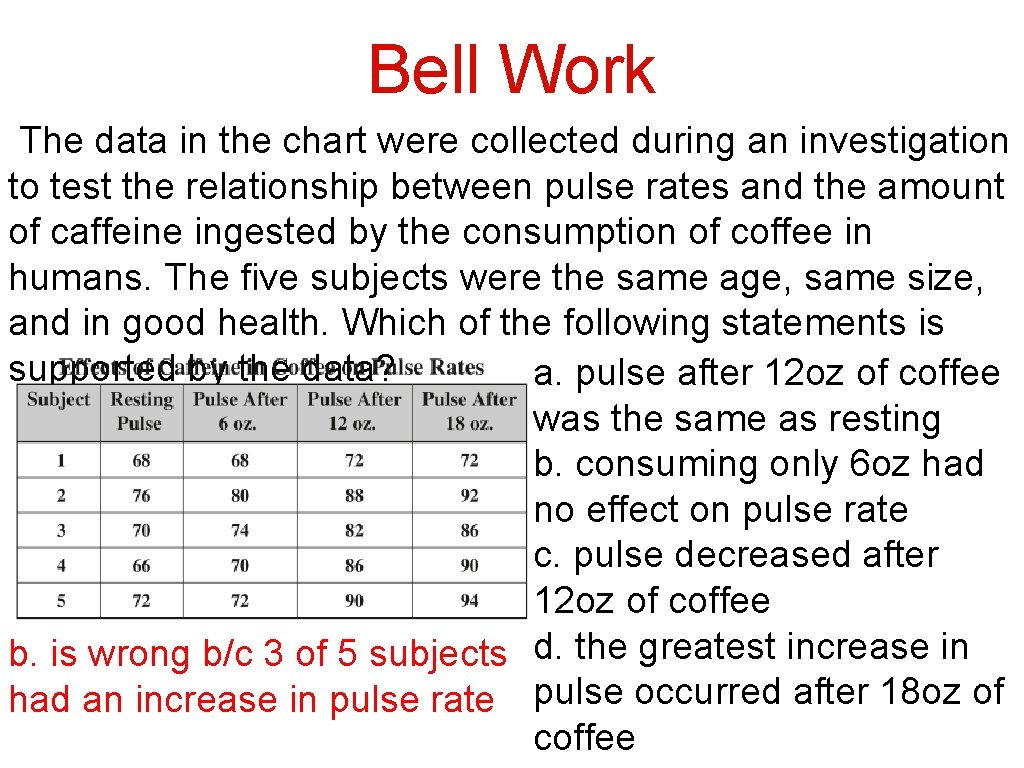
Bell Work The data in the chart were collected during an investigation to test the relationship between pulse rates and the amount of caffeine ingested by the consumption of coffee in humans. The five subjects were the same age, same size, and in good health. Which of the following statements is supported by the data? a. pulse after 12 oz of coffee was the same as resting b. consuming only 6 oz had no effect on pulse rate c. pulse decreased after 12 oz of coffee b. is wrong b/c 3 of 5 subjects d. the greatest increase in had an increase in pulse rate pulse occurred after 18 oz of coffee

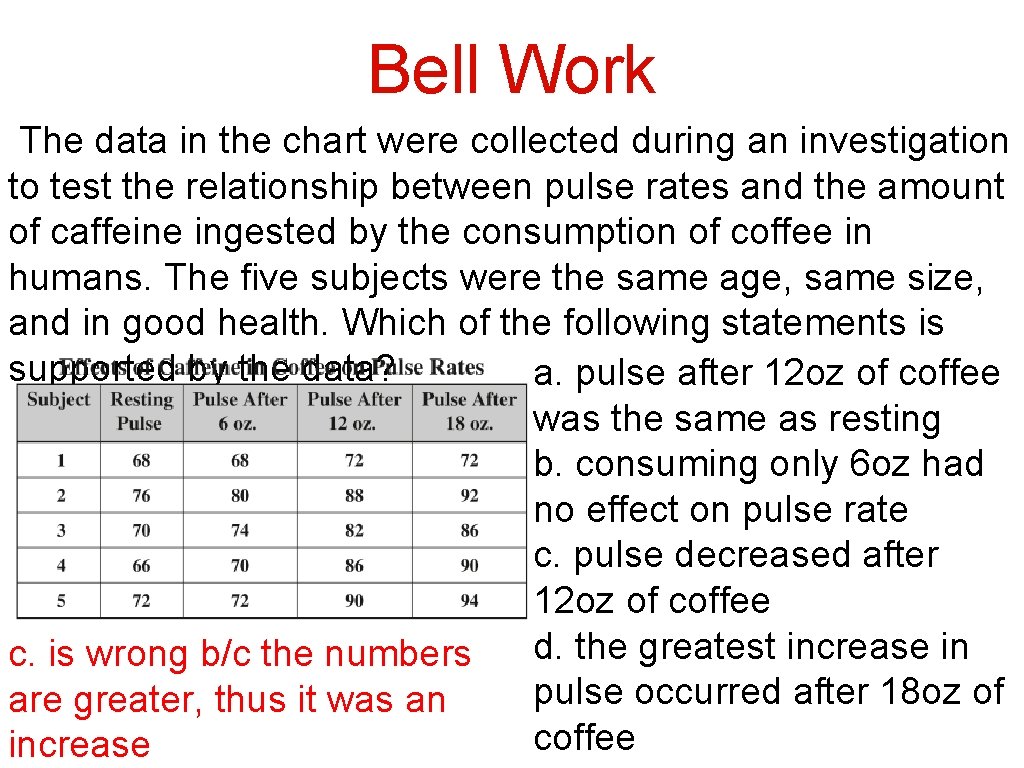
Bell Work The data in the chart were collected during an investigation to test the relationship between pulse rates and the amount of caffeine ingested by the consumption of coffee in humans. The five subjects were the same age, same size, and in good health. Which of the following statements is supported by the data? a. pulse after 12 oz of coffee was the same as resting b. consuming only 6 oz had no effect on pulse rate c. pulse decreased after 12 oz of coffee d. the greatest increase in c. is wrong b/c the numbers pulse occurred after 18 oz of are greater, thus it was an coffee increase

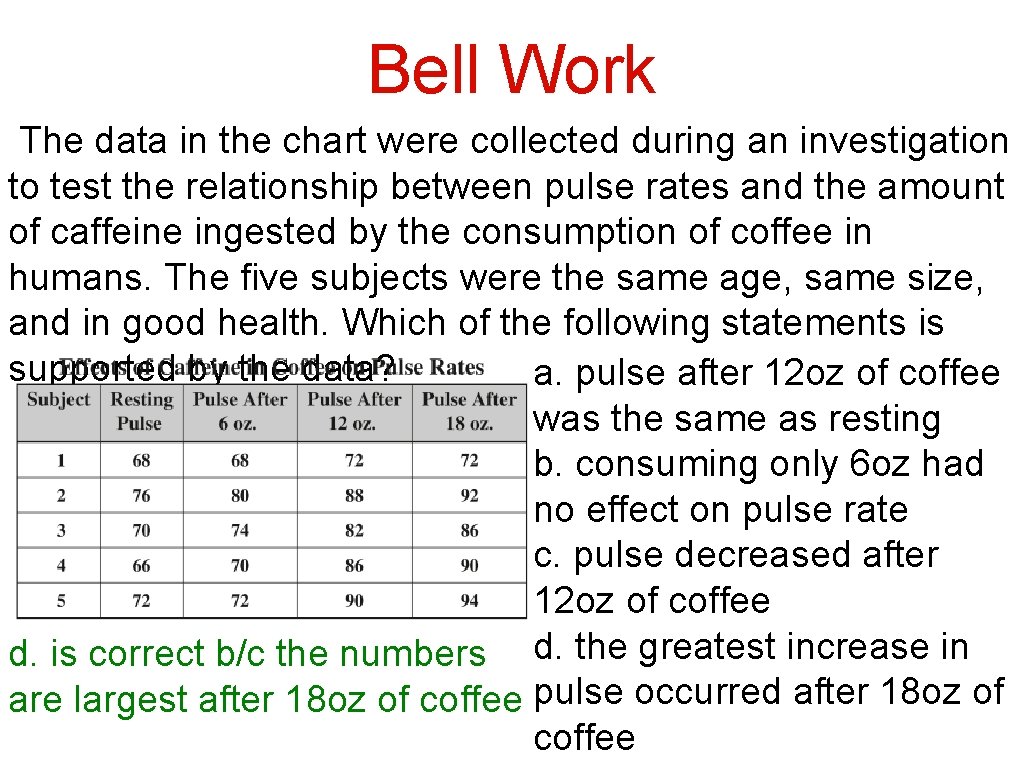
Bell Work The data in the chart were collected during an investigation to test the relationship between pulse rates and the amount of caffeine ingested by the consumption of coffee in humans. The five subjects were the same age, same size, and in good health. Which of the following statements is supported by the data? a. pulse after 12 oz of coffee was the same as resting b. consuming only 6 oz had no effect on pulse rate c. pulse decreased after 12 oz of coffee d. is correct b/c the numbers d. the greatest increase in are largest after 18 oz of coffee pulse occurred after 18 oz of coffee

Bell Work Vocab • Bar Graph • Line Graph • Dependent Variable • Independent Variable • Control Group

What does a peanut M&M and an egg have in common?

Biochemistry Unit Page R 11

Atoms, Atomic Bonding, and Properties of Water August 19, 2013

Atoms • smallest unit of matter • matter: anything that has mass & takes up space • made of three parts called subatomic particles

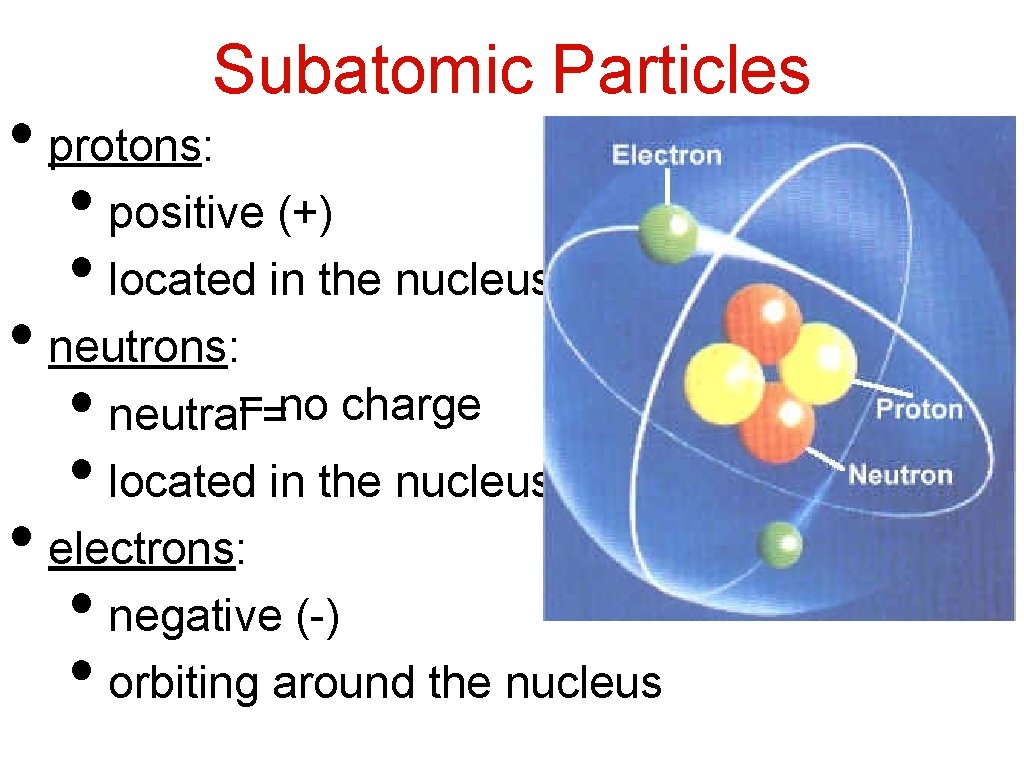
Subatomic Particles • protons: • positive (+) • located in the nucleus • neutrons: • neutral==no charge • located in the nucleus • electrons: • negative (-) • orbiting around the nucleus

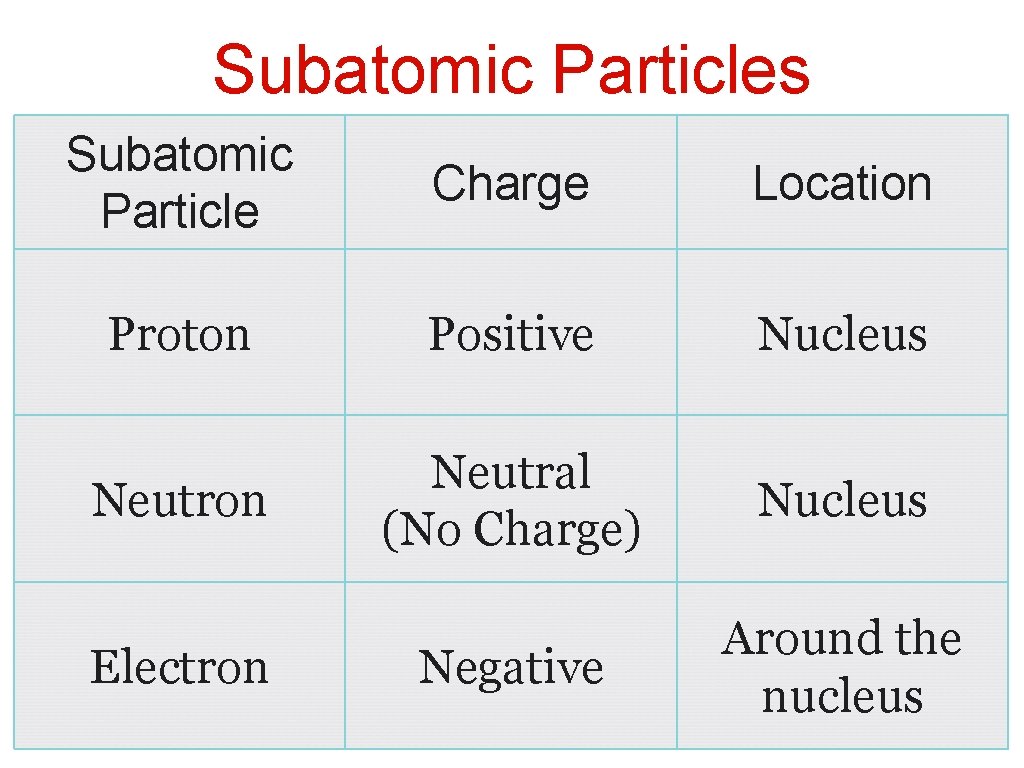
Subatomic Particles Subatomic Particle Charge Location Proton Positive Nucleus Neutron Neutral (No Charge) Nucleus Negative Around the nucleus Electron

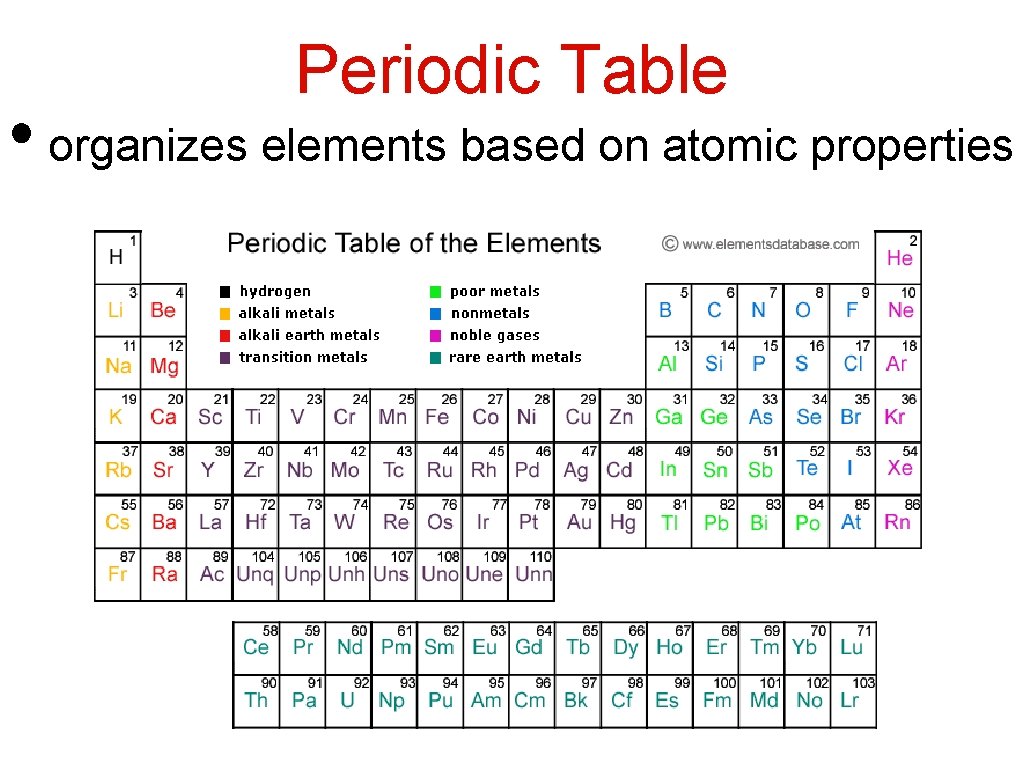
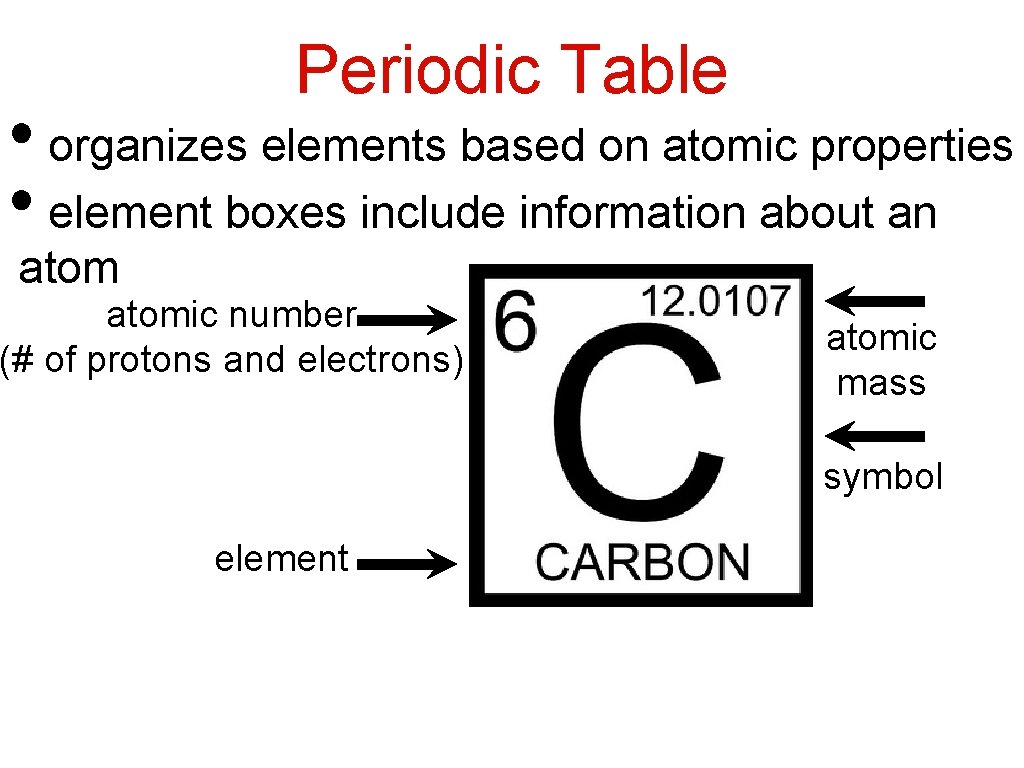
Periodic Table • organizes elements based on atomic properties

Periodic Table • organizes elements based on atomic properties • element boxes include information about an atomic number (# of protons and electrons) atomic mass symbol element

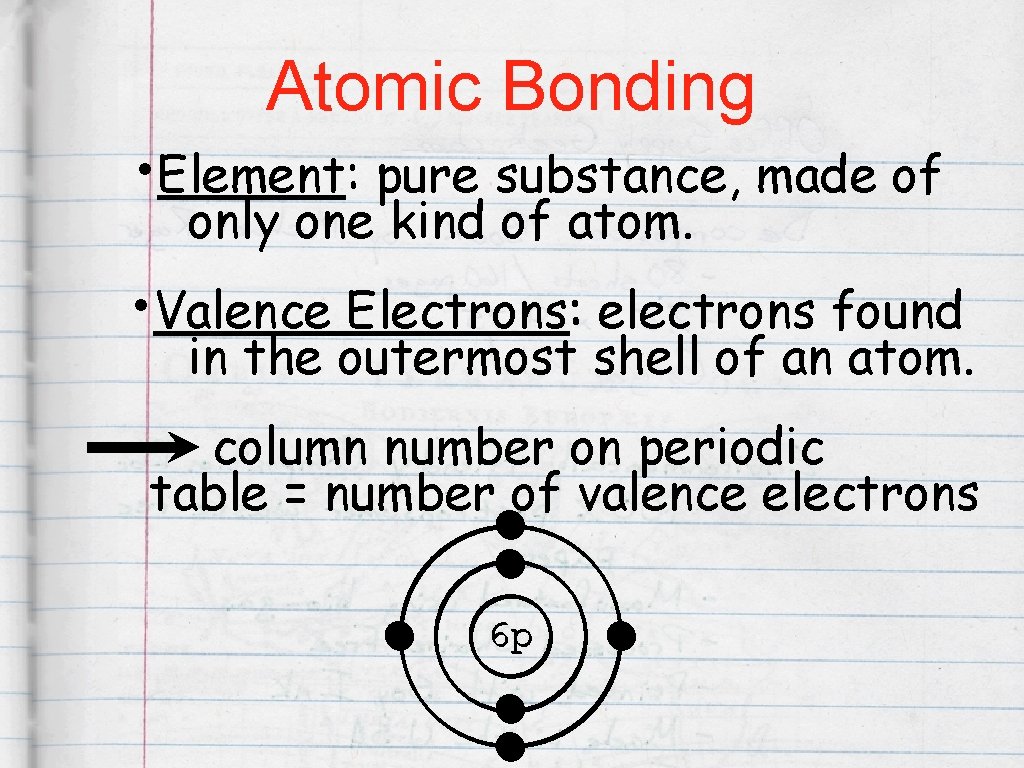
Atomic Bonding • Element: : pure substance, made of only one kind of atom. • Valence Electrons: : electrons found in the outermost shell of an atom. column number on periodic table = number of valence electrons 6 p

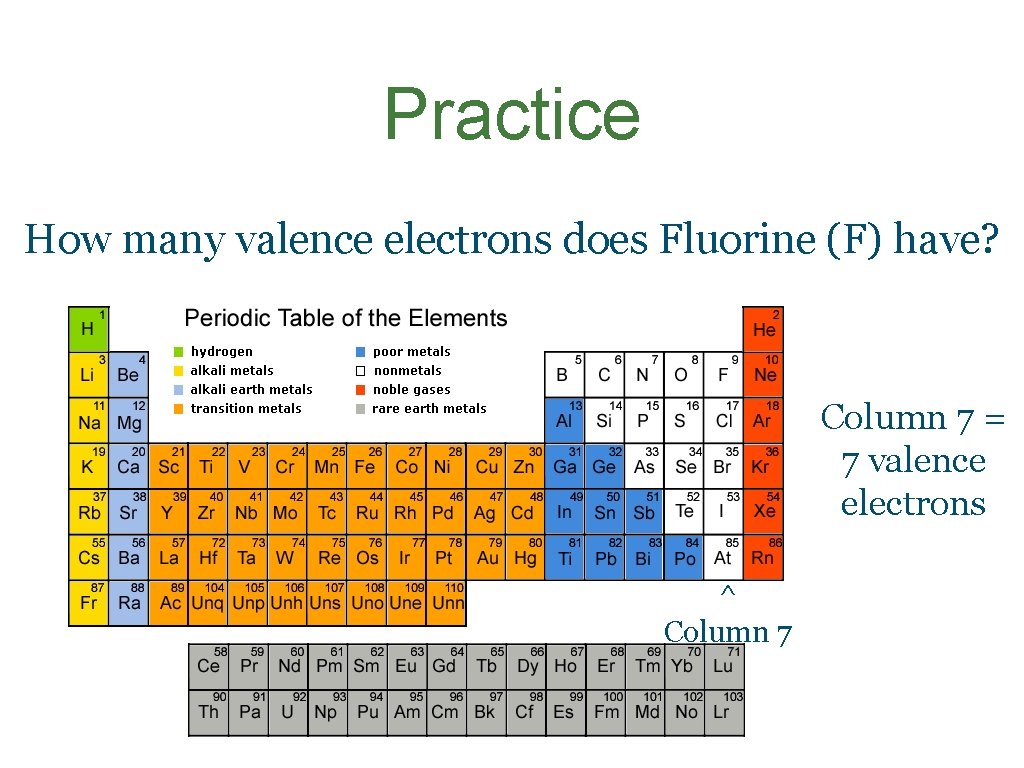
Practice How many valence electrons does Fluorine (F) have? Column 7 = 7 valence electrons ^ Column 7

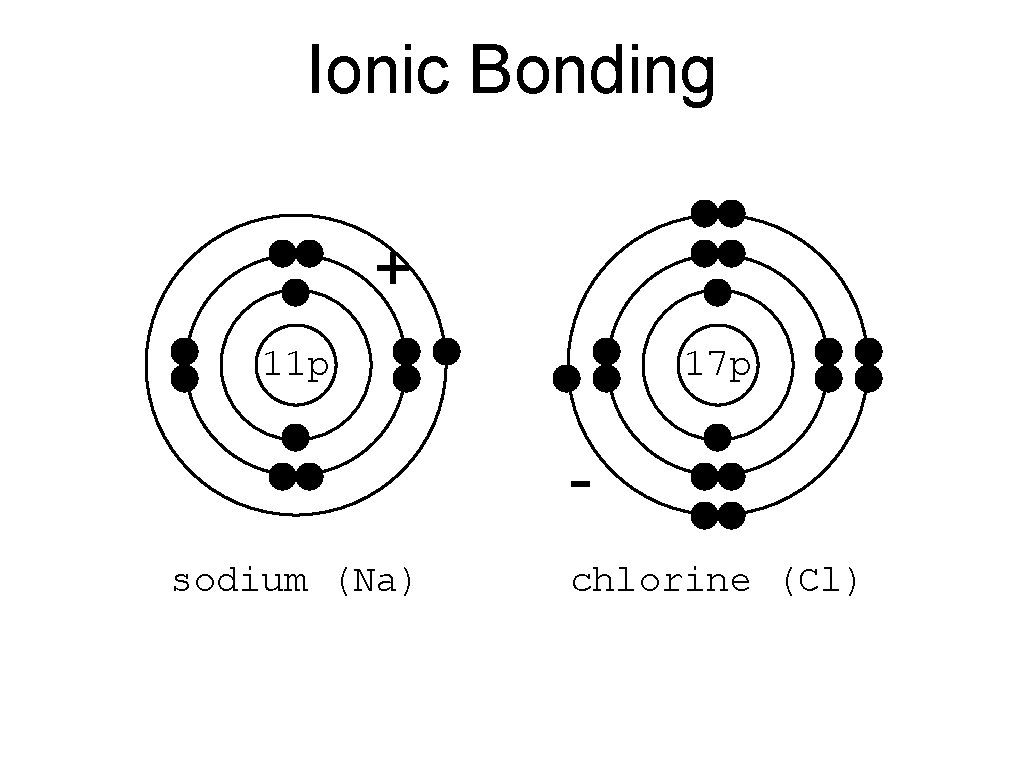
• Atomic Bonding: : two or more atoms joined to form a molecule. • Atoms bond because. . . ATOMS WANT 8 VALENCE ELECTRONS **exception: hydrogen and helium only want 2 • Ionic Bonding: : bond where electrons are stolen. **THINK: ionic = “I want it” • held together by (+) and (-) attraction • example: salt (Na. Cl)

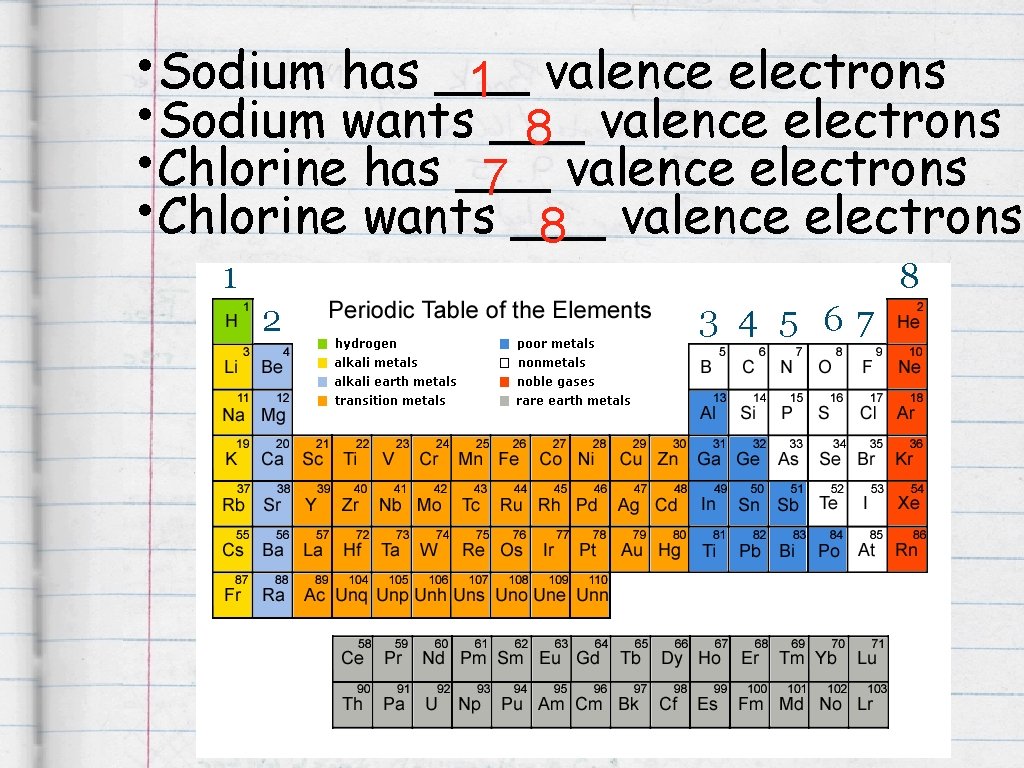
• Sodium has ___ 1 valence electrons • Sodium wants ___ 8 valence electrons • Chlorine has ___ 7 valence electrons • Chlorine wants ___ 8 valence electrons 1 8 2 3 4 5 67

Ionic Bonding + 11 p 17 p sodium (Na) chlorine (Cl)

Atomic Bonding • Covalent Bonding: : bond where electrons are shared. **THINK: covalent = “cooperate” • example: water (H 2 O)

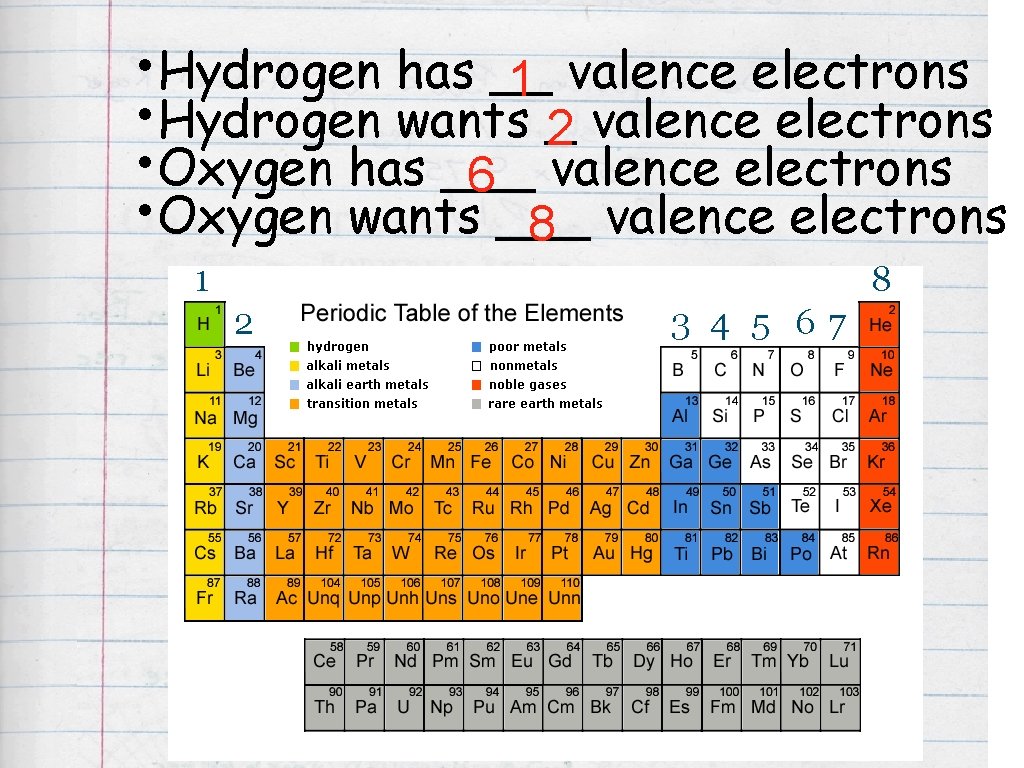
• Hydrogen has __ 1 valence electrons • Hydrogen wants _2 valence electrons • Oxygen has ___ 6 valence electrons • Oxygen wants ___ 8 valence electrons 1 8 2 3 4 5 67

Covalent Bonding 1 p 8 p 1 p

What happens when you put a water bottle or coke into the freezer?

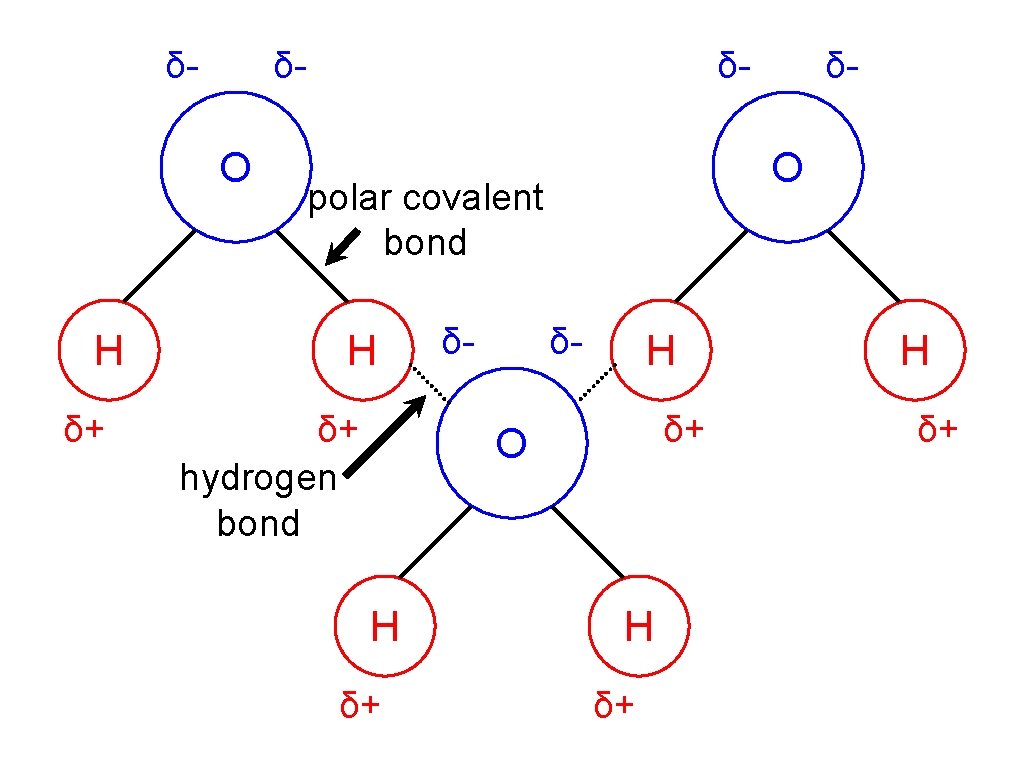
Water (H 20) • • water is made up of __ 3 atoms 2 ____, hydrogen 1 ____ oxygen • held together by polar______ covalent bonds • • polar covalent: _______ unequal sharing oxygen holds the ____ electrons more • • oxygen slightly ____ negative δhydrogen slightly _______ positive δ+

• an Hydrogen Bonding: interaction that holds polar molecules together • • opposite charges ____ attract like charges ____ repel • for Hydrogen bonds are responsible all of water’s unique properties

δ- δ- O H δ+ δ- O polar covalent bond H δ+ hydrogen bond δ- δ- H δ+ O H δ+ δ- H δ+

Properties of Water • expansion upon freezing • high specific heat • cohesion • adhesion

4 Special Properties of Water Expansion Upon Freezing as water freezes, the molecules rearrange based on δ- and δ+ charges. crystal structure less dense than water, so ice floats. 1 • ★life can survive beneath ice ★Earth is not frozen solid!

High Specific Heat 2 • Specific Heat: : heat required to raise temperature. • Hydrogen bonds hold water molecules together, so it takes a lot of energy to raise temperature ★maintains a moderate temperature on Earth

Properties of Water • expansion upon freezing • high specific heat • cohesion • adhesion

3 4 Special Properties of Water Cohesion hydrogen bonding causes water molecules to stick to other water molecules water sticking to water •

surface tension: cohesion at the surface allows water to resist other forces

Adhesion hydrogen bonding causes wat molecules to stick to other substanceswater sticking to something else 4

Transportation of water in plants: adhesion and cohesion allow water to move from the roots to the leaves

Homework • Pg. 39 1 -5 • Pg. 43 1 -5 Write question. Make sure homework has proper heading in case it’s taken up.