Freezing Point Depression Experiment GOAL Determine the molecular


- Slides: 13

Freezing Point Depression Experiment GOAL: Determine the molecular weight of an unknown substance using freezing point depression. 1) Determine the FP temperature of a pure solvent. 2) Determine the FP temp of a solution made by dissolving a nonionic, nonvolatile unknown substance. 3) Calculate the molecular weight of the unk solute.

Low Down on Experiment Solvent – para-xylene: freezes between 10 - 15ºC Solute – unknown: will lower freezing point of para-xylene when in solution.

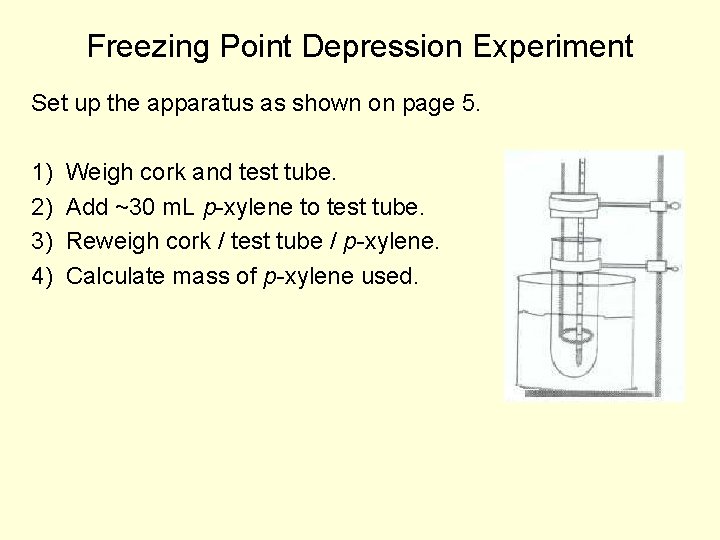
Freezing Point Depression Experiment Set up the apparatus as shown on page 5. 1) 2) 3) 4) Weigh cork and test tube. Add ~30 m. L p-xylene to test tube. Reweigh cork / test tube / p-xylene. Calculate mass of p-xylene used.

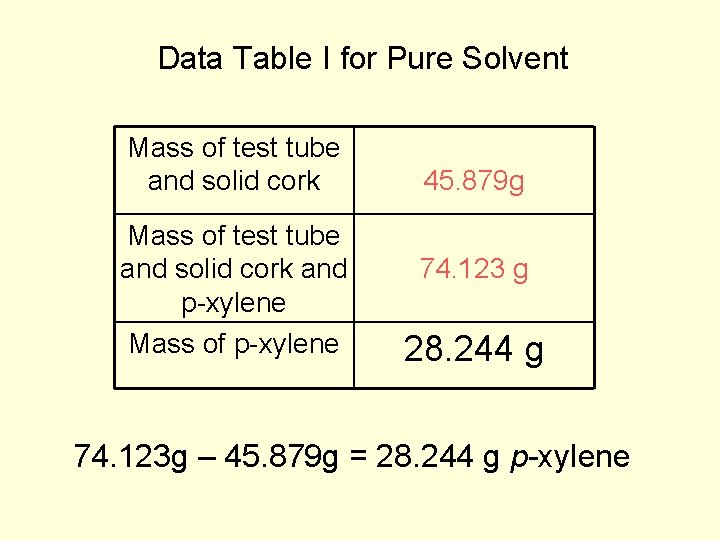
Data Table I for Pure Solvent Mass of test tube and solid cork and p-xylene Mass of p-xylene 45. 879 g 74. 123 g 28. 244 g 74. 123 g – 45. 879 g = 28. 244 g p-xylene

Time/Temp Data Freeze the pure p-xylene solvent. A device known as Vernier will gather time vs. temperature data throughout the experiment. The data will be transferred to YOUR USB memory jump drive to be used in generating a freezing point depression graph for the pure p-xylene solvent.

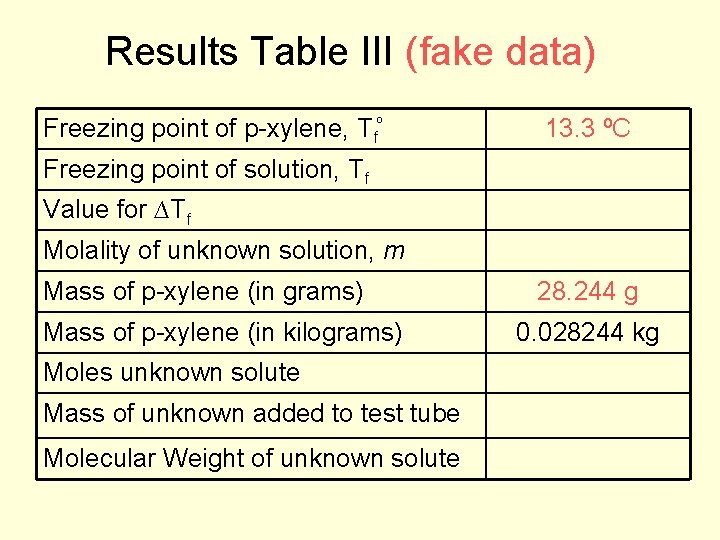
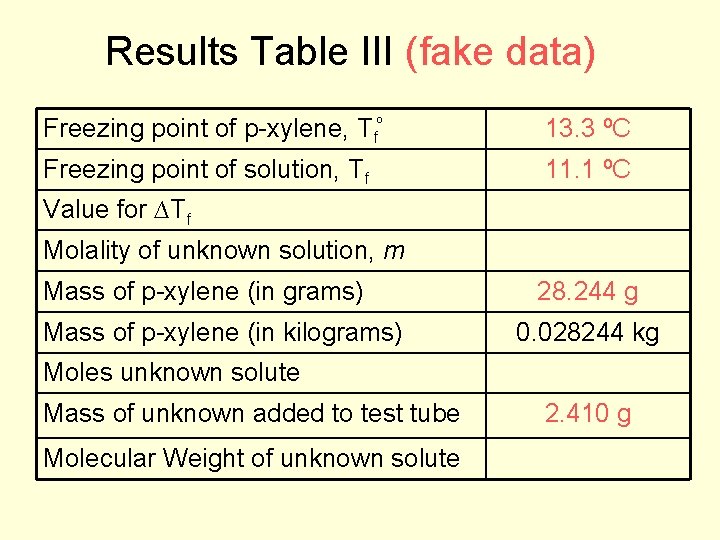
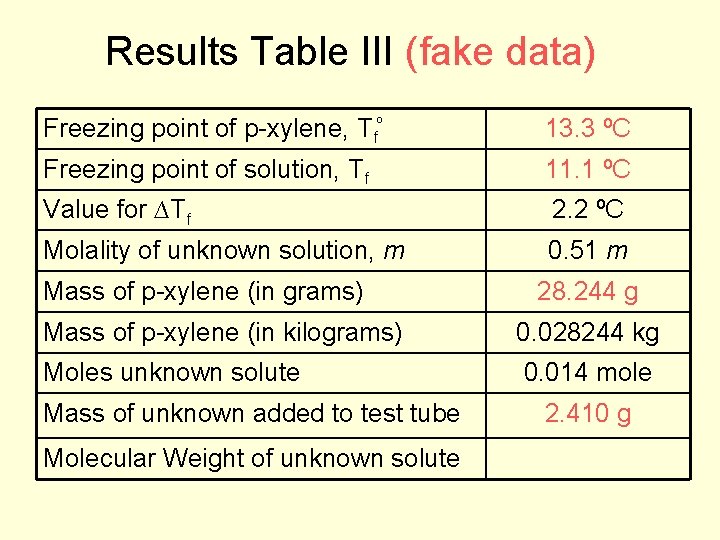
Results Table III (fake data) Freezing point of p-xylene, Tfº 13. 3 ºC Freezing point of solution, Tf Value for ∆Tf Molality of unknown solution, m Mass of p-xylene (in grams) Mass of p-xylene (in kilograms) Moles unknown solute Mass of unknown added to test tube Molecular Weight of unknown solute 28. 244 g 0. 028244 kg

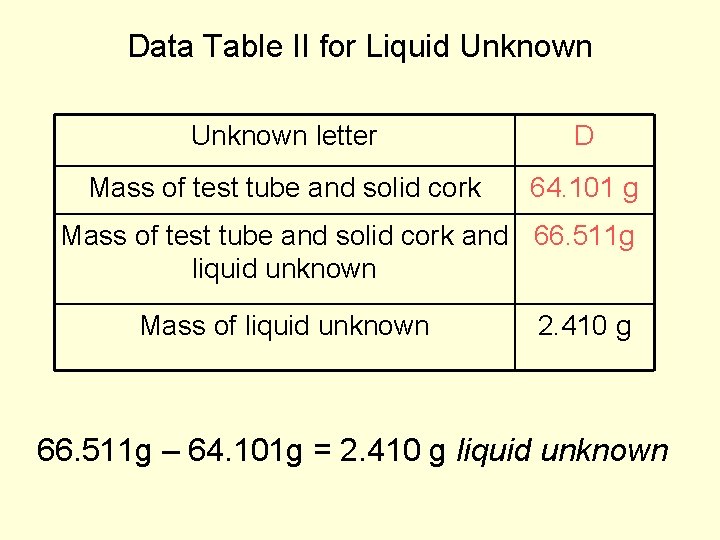
Data Table II for Liquid Unknown letter D Mass of test tube and solid cork 64. 101 g Mass of test tube and solid cork and 66. 511 g liquid unknown Mass of liquid unknown 2. 410 g 66. 511 g – 64. 101 g = 2. 410 g liquid unknown

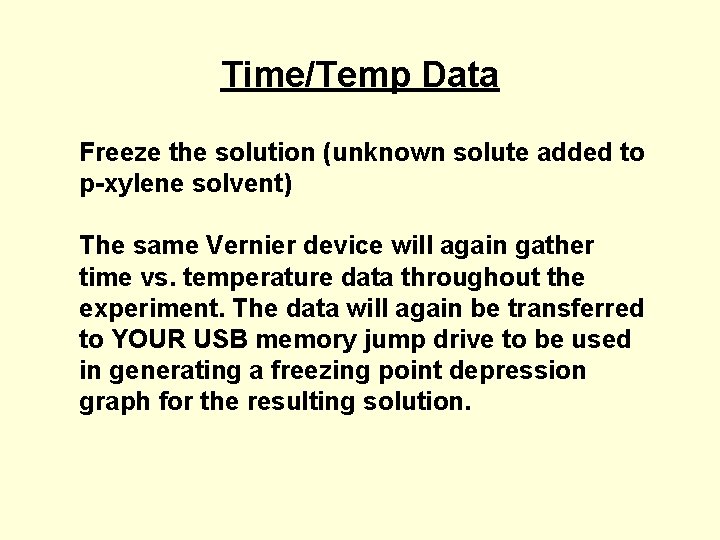
Time/Temp Data Freeze the solution (unknown solute added to p-xylene solvent) The same Vernier device will again gather time vs. temperature data throughout the experiment. The data will again be transferred to YOUR USB memory jump drive to be used in generating a freezing point depression graph for the resulting solution.

Results Table III (fake data) Freezing point of p-xylene, Tfº 13. 3 ºC Freezing point of solution, Tf 11. 1 ºC Value for ∆Tf Molality of unknown solution, m Mass of p-xylene (in grams) Mass of p-xylene (in kilograms) 28. 244 g 0. 028244 kg Moles unknown solute Mass of unknown added to test tube Molecular Weight of unknown solute 2. 410 g

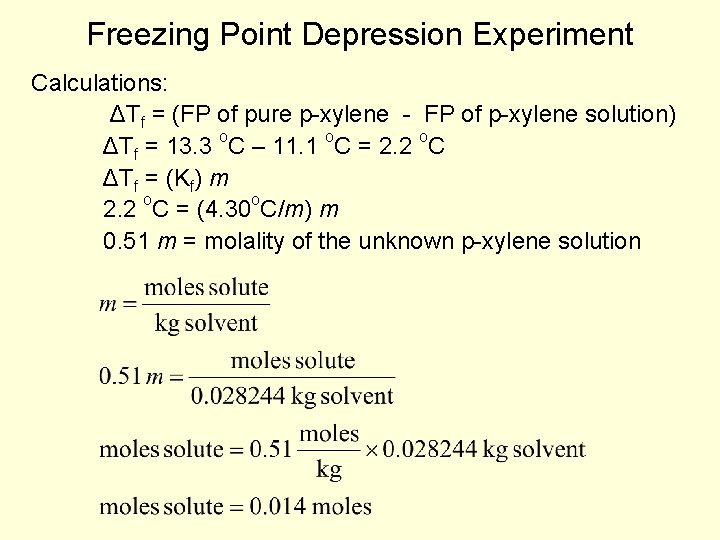
Freezing Point Depression Experiment Calculations: ΔTf = (FP of pure p-xylene - FP of p-xylene solution) ΔTf = 13. 3 o. C – 11. 1 o. C = 2. 2 o. C ΔTf = (Kf) m 2. 2 o. C = (4. 30 o. C/m) m 0. 51 m = molality of the unknown p-xylene solution

Results Table III (fake data) Freezing point of p-xylene, Tfº 13. 3 ºC Freezing point of solution, Tf 11. 1 ºC Value for ∆Tf 2. 2 ºC Molality of unknown solution, m 0. 51 m Mass of p-xylene (in grams) 28. 244 g Mass of p-xylene (in kilograms) 0. 028244 kg Moles unknown solute 0. 014 mole Mass of unknown added to test tube Molecular Weight of unknown solute 2. 410 g

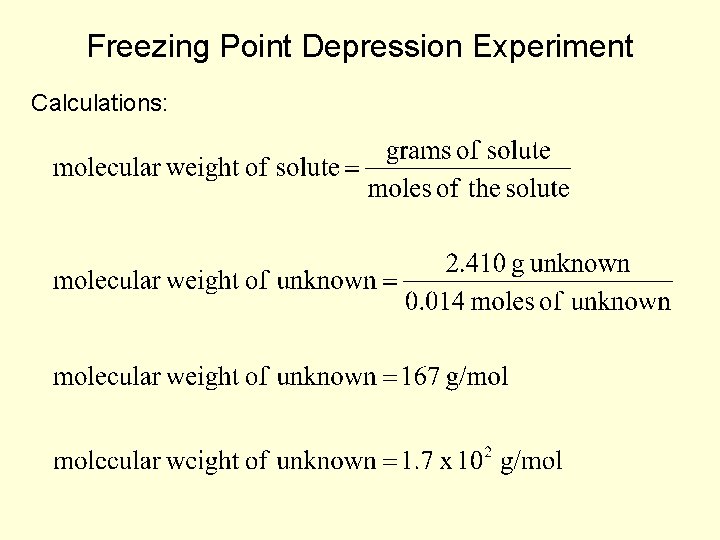
Freezing Point Depression Experiment Calculations:

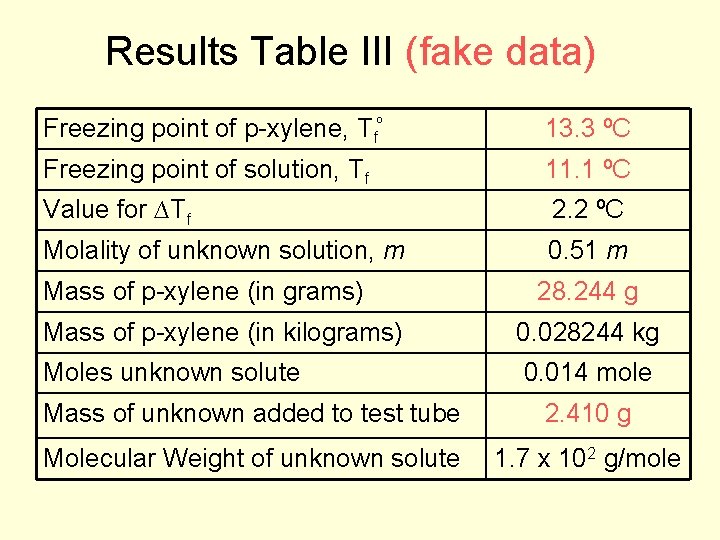
Results Table III (fake data) Freezing point of p-xylene, Tfº 13. 3 ºC Freezing point of solution, Tf 11. 1 ºC Value for ∆Tf 2. 2 ºC Molality of unknown solution, m 0. 51 m Mass of p-xylene (in grams) 28. 244 g Mass of p-xylene (in kilograms) 0. 028244 kg Moles unknown solute 0. 014 mole Mass of unknown added to test tube 2. 410 g Molecular Weight of unknown solute 1. 7 x 102 g/mole