Properties of Water freezing melting evaporation condensation cohesion


- Slides: 10

Properties of Water freezing melting evaporation condensation cohesion adhesion properties of water review 2

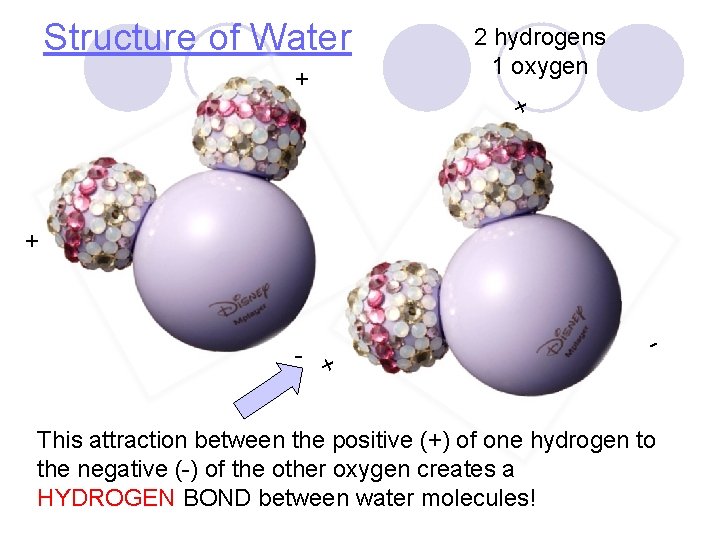
Structure of Water + 2 hydrogens 1 oxygen + + - This attraction between the positive (+) of one hydrogen to the negative (-) of the other oxygen creates a HYDROGEN BOND between water molecules!

Water Molecules are Held Together by Hydrogen Bonds • Hydrogen bonds are weak bonds between a hydrogen atom in one molecule and a negatively charged region of another molecule. • Hydrogen bonds are strong enough to cause water to cling to itself and other substances (ie. cohesion, adhesion).

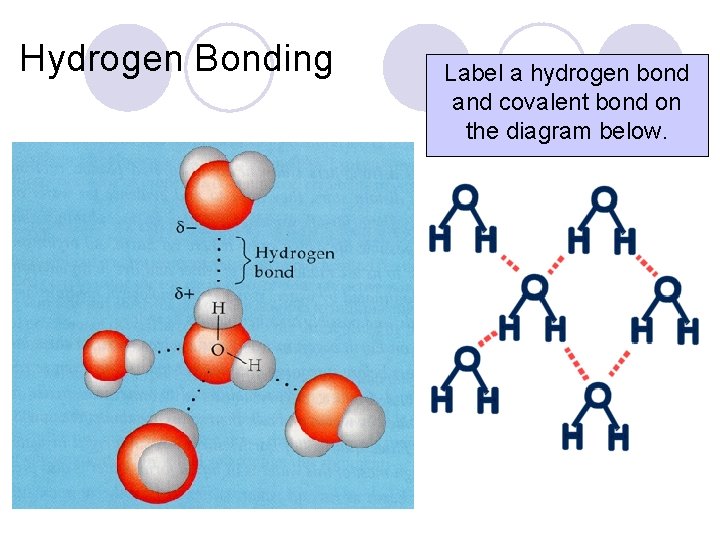
Hydrogen Bonding Label a hydrogen bond and covalent bond on the diagram below.

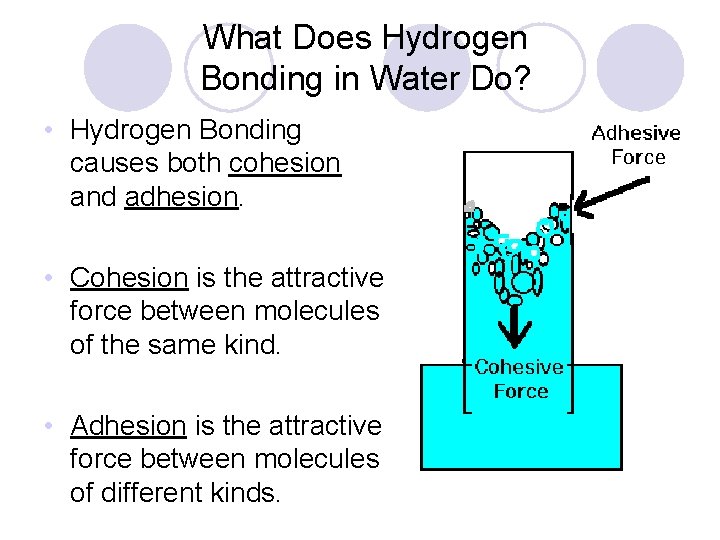
What Does Hydrogen Bonding in Water Do? • Hydrogen Bonding causes both cohesion and adhesion. • Cohesion is the attractive force between molecules of the same kind. • Adhesion is the attractive force between molecules of different kinds.

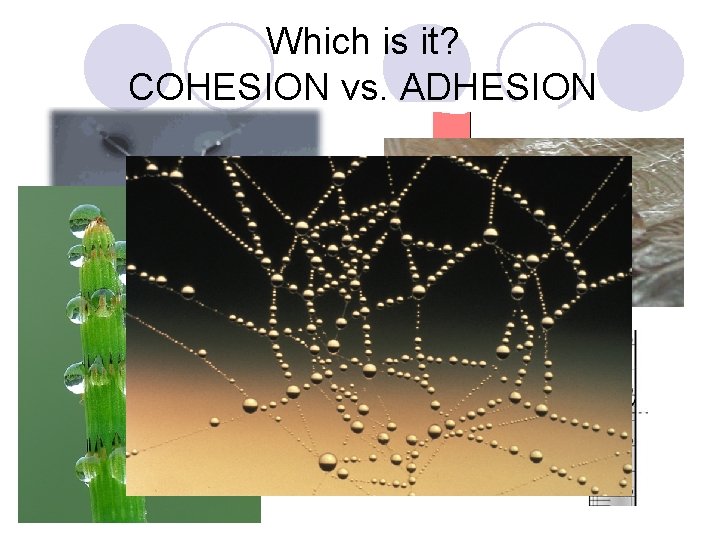
Which is it? COHESION vs. ADHESION

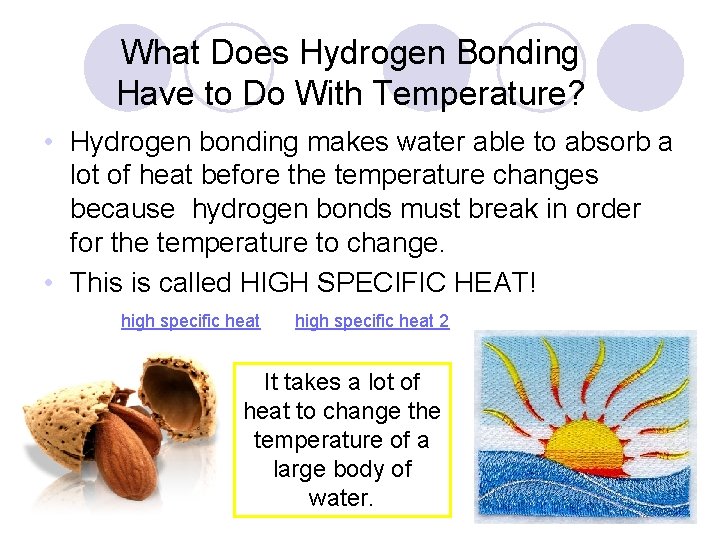
What Does Hydrogen Bonding Have to Do With Temperature? • Hydrogen bonding makes water able to absorb a lot of heat before the temperature changes because hydrogen bonds must break in order for the temperature to change. • This is called HIGH SPECIFIC HEAT! high specific heat 2 It takes a lot of heat to change the temperature of a large body of water.

FREEZING water (ice) traps the heat energy under the surface stabilizing the temperature of the ocean for living things to live there successfully!

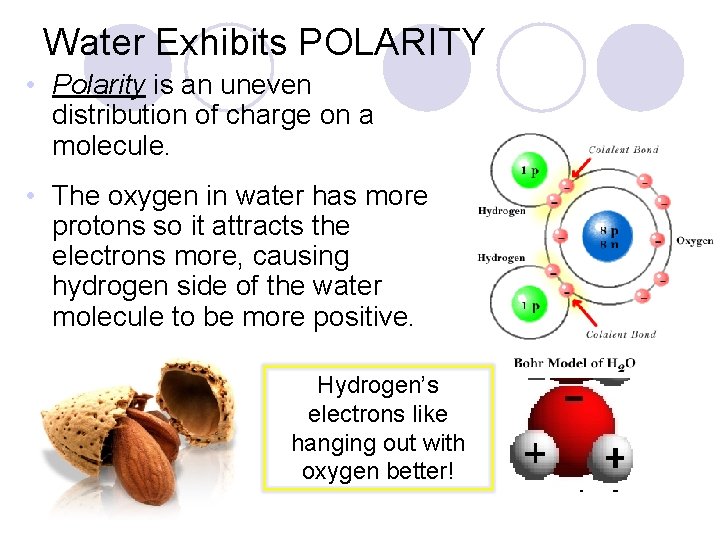
Water Exhibits POLARITY • Polarity is an uneven distribution of charge on a molecule. • The oxygen in water has more protons so it attracts the electrons more, causing hydrogen side of the water molecule to be more positive. Hydrogen’s electrons like hanging out with oxygen better!

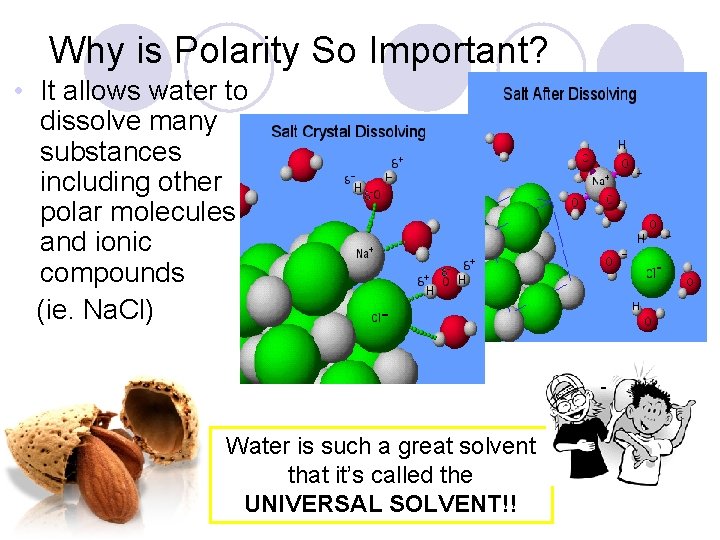
Why is Polarity So Important? • It allows water to dissolve many substances including other polar molecules and ionic compounds (ie. Na. Cl) - Water is such a great solvent that it’s called the UNIVERSAL SOLVENT!!
 Examples of phase change
Examples of phase change Solid to liquid endothermic or exothermic
Solid to liquid endothermic or exothermic Evaporation condensation melting freezing
Evaporation condensation melting freezing Evaporation condensation precipitation transpiration
Evaporation condensation precipitation transpiration Water cycle and transpiration
Water cycle and transpiration Fusion evaporation condensation
Fusion evaporation condensation Prep 1
Prep 1 Evaporation mind map
Evaporation mind map Difference between congruent and incongruent melting point
Difference between congruent and incongruent melting point Water and water and water water
Water and water and water water Water
Water
















