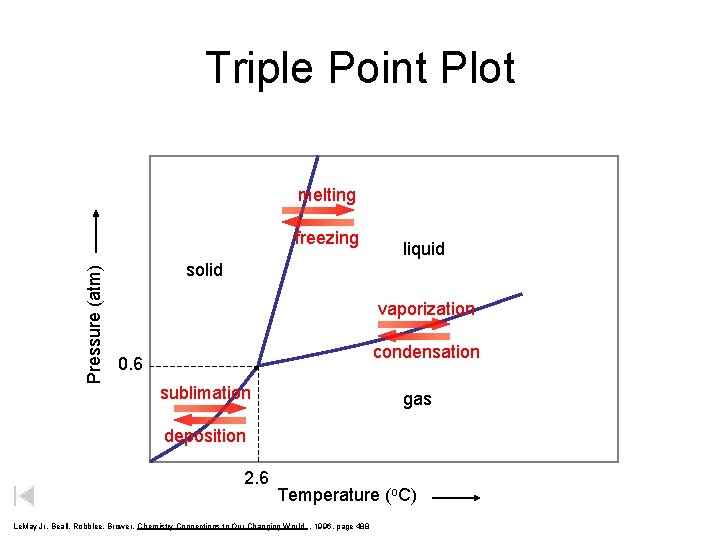
Triple Point Plot melting Pressure atm freezing liquid



- Slides: 16

Triple Point Plot melting Pressure (atm) freezing liquid solid vaporization condensation 0. 6 sublimation gas deposition 2. 6 Temperature (o. C) Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 488

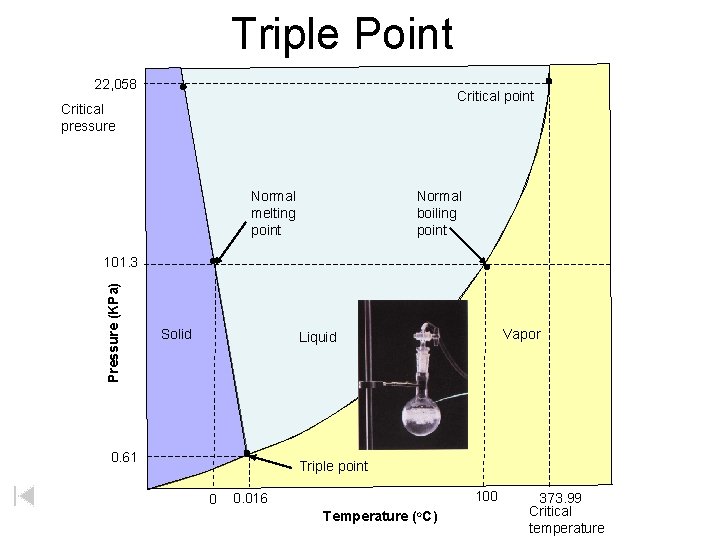
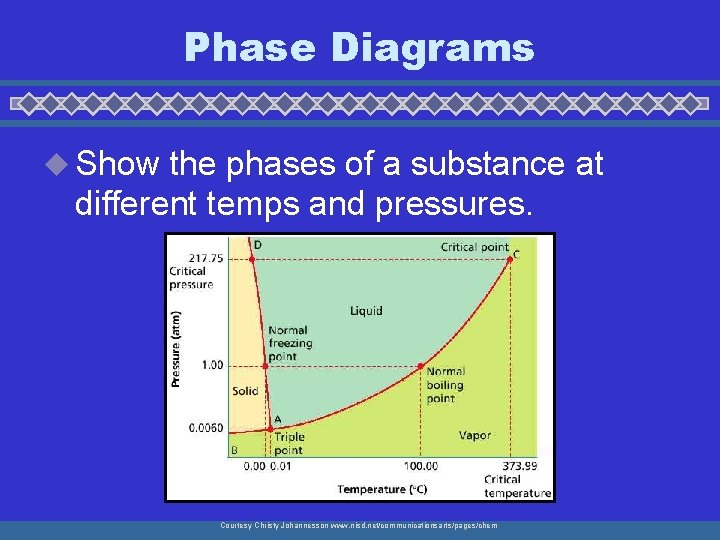
Triple Point 22, 058 Critical point Critical pressure Normal melting point Normal boiling point Pressure (KPa) 101. 3 Solid Vapor Liquid 0. 61 Triple point 0 100 0. 016 Temperature (o. C) 373. 99 Critical temperature

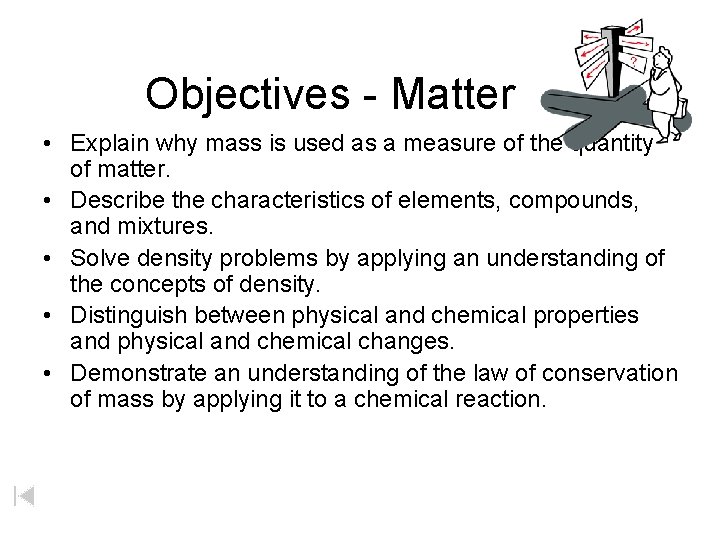
Objectives - Matter • Explain why mass is used as a measure of the quantity of matter. • Describe the characteristics of elements, compounds, and mixtures. • Solve density problems by applying an understanding of the concepts of density. • Distinguish between physical and chemical properties and physical and chemical changes. • Demonstrate an understanding of the law of conservation of mass by applying it to a chemical reaction.

Objectives - Energy • Identify various forms of energy. • Describe changes in energy that take place during a chemical reaction. • Distinguish between heat and temperature. • Solve calorimetry problems. • Describe the interactions that occur between electrostatic charges.

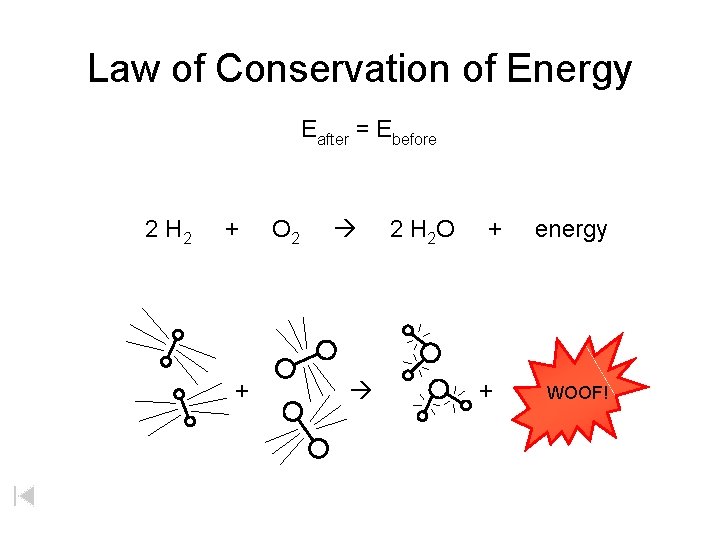
Law of Conservation of Energy Eafter = Ebefore 2 H 2 + + O 2 2 H 2 O + + energy WOOF!

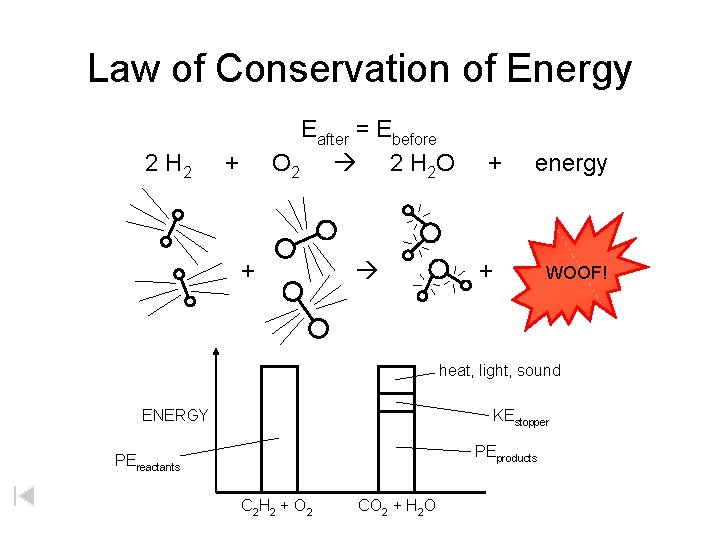
Law of Conservation of Energy 2 H 2 Eafter = Ebefore O 2 2 H 2 O + + + energy + WOOF! heat, light, sound ENERGY KEstopper PEproducts PEreactants C 2 H 2 + O 2 CO 2 + H 2 O

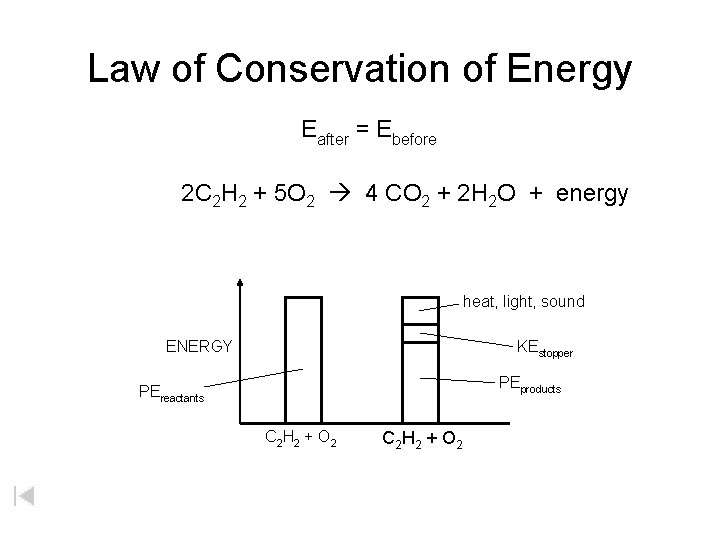
Law of Conservation of Energy Eafter = Ebefore 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O + energy heat, light, sound ENERGY KEstopper PEproducts PEreactants C 2 H 2 + O 2


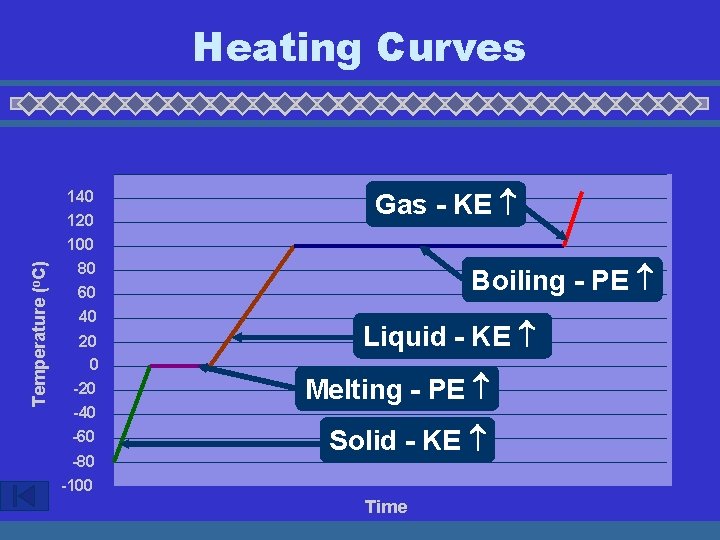
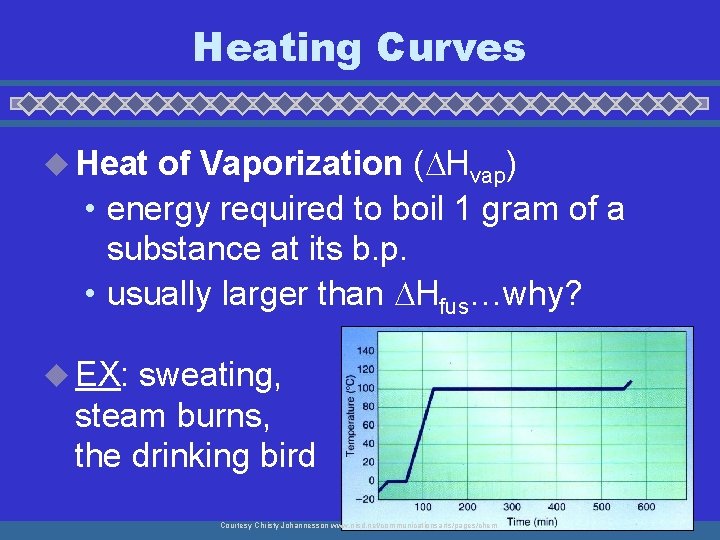
Temperature (o. C) Heating Curves 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE Time

Heating Curves u Temperature Change • change in KE (molecular motion) • depends on heat capacity u Heat Capacity • energy required to raise the temp of 1 gram of a substance by 1°C • “Volcano” clip - water has a very high heat capacity Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Heating Curves u Phase Change • change in PE (molecular arrangement) • temp remains constant u Heat of Fusion ( Hfus) • energy required to melt 1 gram of a substance at its m. p. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Heating Curves u Heat of Vaporization ( Hvap) • energy required to boil 1 gram of a substance at its b. p. • usually larger than Hfus…why? u EX: sweating, steam burns, the drinking bird Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Phase Diagrams u Show the phases of a substance at different temps and pressures. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem


Resources - Matter and Energy Objectives - matter and energy Objectives - measurement Objectives - phases of matter Worksheet - vocabulary Activity - chromatography Worksheet - percentage composition Outline - causes of change - calorimetry Worksheet - properties Worksheet - calorimetry problems 1 Worksheet - density problems Worksheet - calorimetry problems 2 Activity - density blocks Worksheet - heat energy problems Lab - golf ball lab Worksheet - conversion factors Worksheet - classifying matter Worksheet - atoms, mass, and the mole Article - buckeyball & questions (video) activity - mole pattern Article - buried in ice Outline (general)

Resources - Matter and Energy Objectives - matter and energy Objectives - measurement Objectives - phases of matter Worksheet - vocabulary Activity - chromatography Worksheet - percentage composition Outline - causes of change - calorimetry Worksheet - properties Worksheet - calorimetry problems 1 Worksheet - density problems Worksheet - calorimetry problems 2 Activity - density blocks Worksheet - heat energy problems Lab - golf ball lab Worksheet - conversion factors Worksheet - classifying matter Worksheet - atoms, mass, and the mole Article - buckeyball & questions (video) activity - mole pattern Article - buried in ice Outline (general)