Influenza Virus 2 08 16 Types of Influenza



























































- Slides: 86


Influenza Virus 2 08: 16

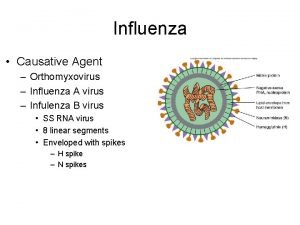
Types of Influenza Virus ¢ ¿ Three types: A, B, C Influenza Type A can infect: People, birds, pigs, horses, seals, whales and others Classified into subtypes ¿ Influenza Type B: Human virus Not classified according to Subtype Cause human epidemics but not pandemics ¿ Influenza Type C cause mild illness in humans Not classified according to subtype Do not cause epidemics or pandemics 3 08: 16

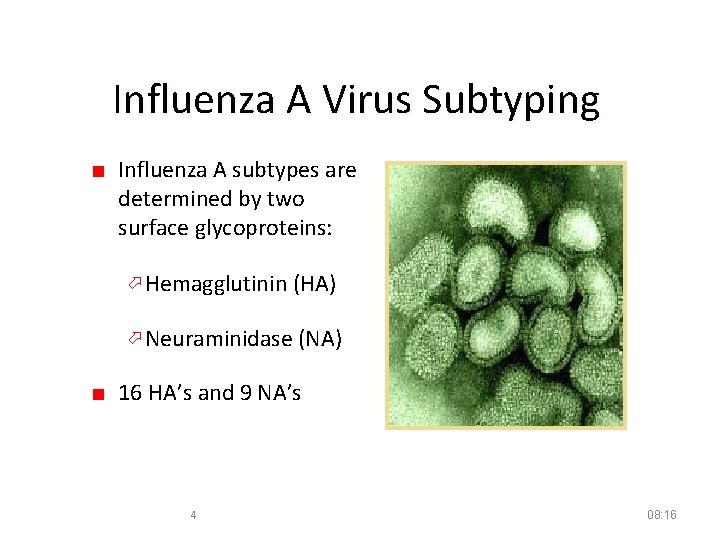
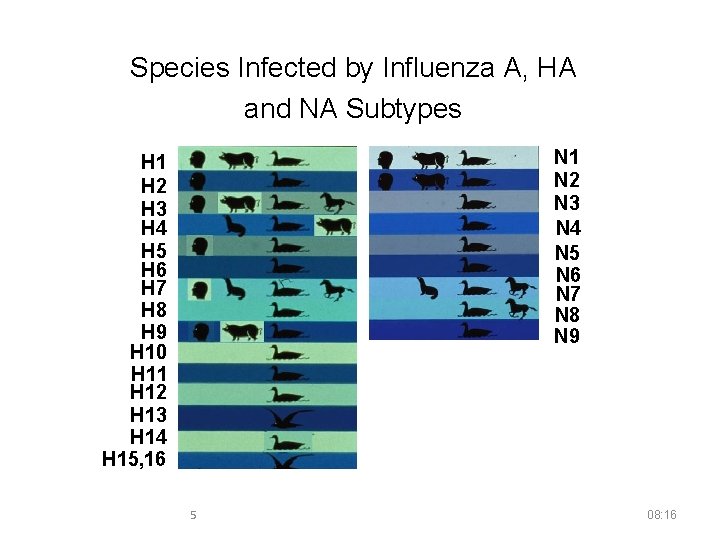
Influenza A Virus Subtyping ■ Influenza A subtypes are determined by two surface glycoproteins: Hemagglutinin (HA) Neuraminidase (NA) ■ 16 HA’s and 9 NA’s 4 08: 16

Species Infected by Influenza A, HA and NA Subtypes N 1 N 2 N 3 N 4 N 5 N 6 N 7 N 8 N 9 H 1 H 2 H 3 H 4 H 5 H 6 H 7 H 8 H 9 H 10 H 11 H 12 H 13 H 14 H 15, 16 5 08: 16

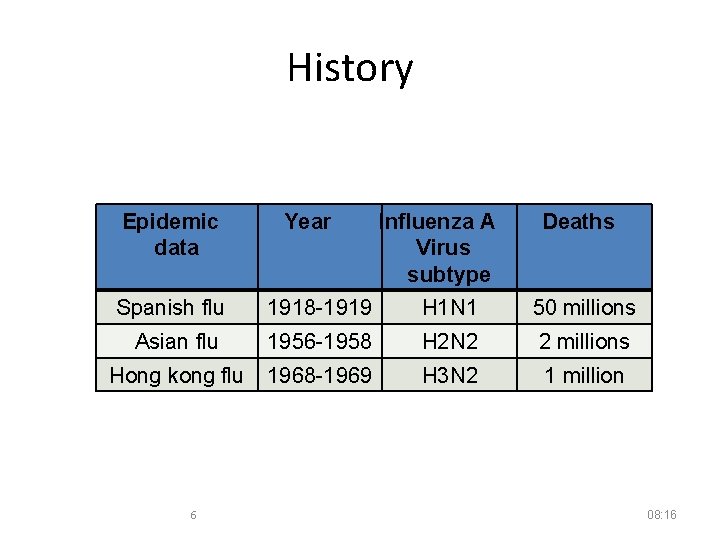
History Epidemic data Year Influenza A Virus subtype Deaths Spanish flu 1918 -1919 H 1 N 1 50 millions Asian flu 1956 -1958 H 2 N 2 2 millions Hong kong flu 1968 -1969 H 3 N 2 1 million 6 08: 16

Influenza Pandemics 20 th Century Credit: US National Museum of Health and Medicine 1918: “Spanish Flu” 1957: “Asian Flu” 50 million deaths 2 million deaths A(H 1 N 1) A(H 2 N 2) 7 1968: “Hong Kong Flu” 1 million deaths A(H 3 N 2) 08: 16

p(H 1 N 1) 2009

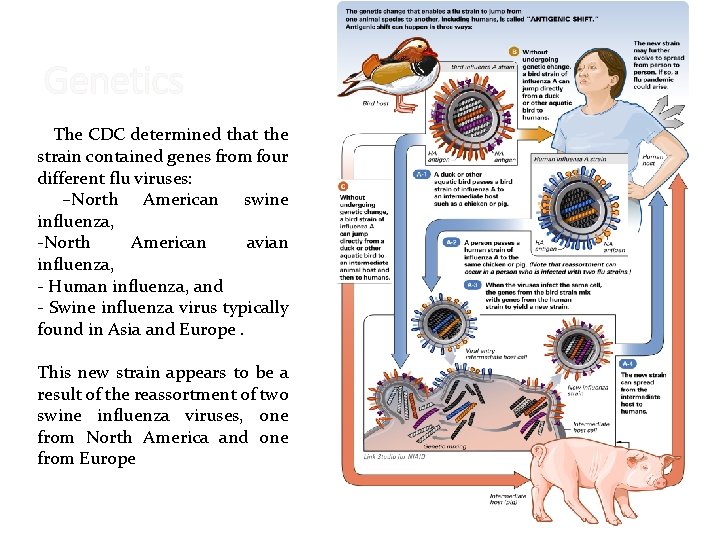
Genetics The CDC determined that the strain contained genes from four different flu viruses: –North American swine influenza, -North American avian influenza, - Human influenza, and - Swine influenza virus typically found in Asia and Europe. This new strain appears to be a result of the reassortment of two swine influenza viruses, one from North America and one from Europe

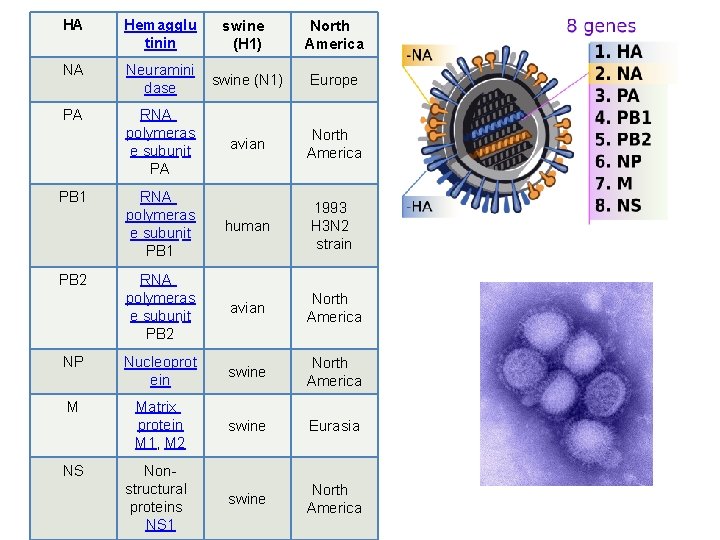
HA Hemagglu tinin swine (H 1) North America NA Neuramini dase swine (N 1) Europe PA RNA polymeras e subunit PA avian North America RNA polymeras e subunit PB 1 human 1993 H 3 N 2 strain RNA polymeras e subunit PB 2 avian North America NP Nucleoprot ein swine North America M Matrix protein M 1, M 2 swine Eurasia Nonstructural proteins NS 1 swine North America PB 1 PB 2 NS

Specimen Collection 11 ﺹ 8: 16

8: 16 ﺹ 12

Specimen Collection Kit • Personal protective equipment • Collection vials with VTM • Polyester fiber-tipped applicators • Tongue depressors • Items for blood collection 13 • Secondary container/ cooler • Ice packs • Suspect case forms • A pen or marker for labeling samples • Labels ﺹ 8: 16

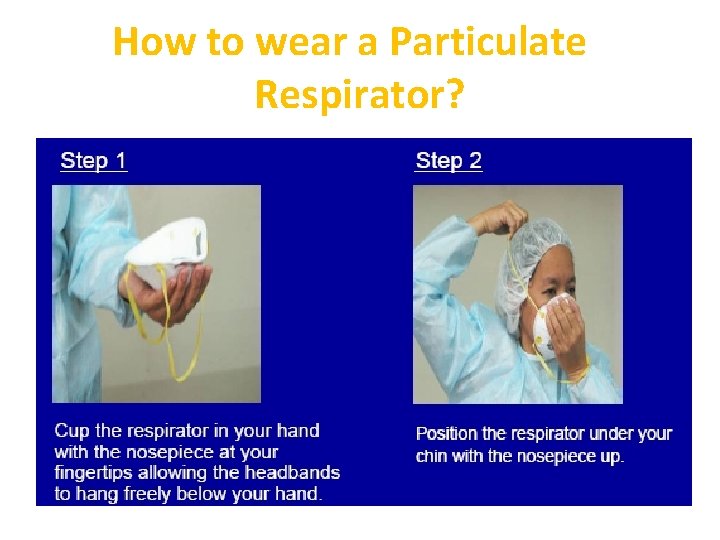
Personal Protective Equipment • • • Masks (N-95) Gloves Protective eyewear (goggles) Hair covers Boot or shoe covers Protective clothing (gown or apron) 14 ﺹ 8: 16

Safety Glasses


How to wear a Particulate Respirator?

How to wear a Particulate Respirator?

Types of Personal Protective Equipments - Cap - Plastic apron (splashing of blood, secretions, excretions)

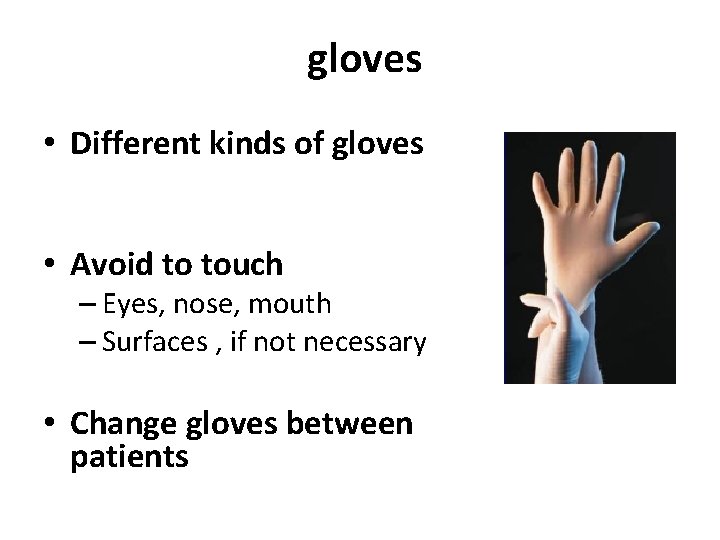
gloves • Different kinds of gloves • Avoid to touch – Eyes, nose, mouth – Surfaces , if not necessary • Change gloves between patients

Gowns An ideal gown must: • Fully cover the body • Have long sleeves • Fit snuggly at the wrists & neck Creative alternatives for Gown: laboratory coat

Boots or Shoe covers Creative alternatives for boots: plastic bags cover for regular shoes

Eye protection • Eye Protection 1. Face shields 2. Goggles

Viral Transport Media 24 ﺹ 8: 16

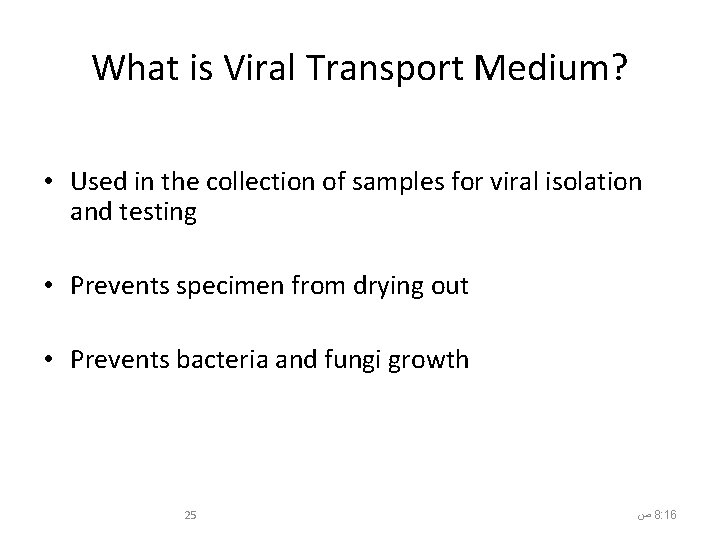
What is Viral Transport Medium? • Used in the collection of samples for viral isolation and testing • Prevents specimen from drying out • Prevents bacteria and fungi growth 25 ﺹ 8: 16

Storing VTM • Sterile collection vials containing 1 ml of VTM • Vials can be stored in a freezer at -20 ºC until use • Vials can be stored for short periods of time at : 4 - 8 ºC 26 ﺹ 8: 16

Phlebotomy Supplies • • Tourniquet Disposable needles Vacuum tubes with EDTA Plastic needle holder Alcohol and iodine swabs Gauze Band-aids Biohazard sharps container 27 ﺹ 8: 16

Polyester Fiber-Tipped Applicator • Should be drayon, or polyester fiber swabs • individually wrapped to ensure they are sterile • Do not use calcium alginated or cotton swabs ; they inhibit PCR 28 ﺹ 8: 17

How to Manage Kits • Store specimen collection kits “except VTM” in a dry, clean place • Store specimen collection kit where it will be accessible after hours and on weekends 29 ﺹ 8: 17







Methods of Collection • Oropharyngeal (Throat) swab • Nasopharyngeal aspirate • Blood sample 36 ﺹ 8: 17

Nasopharyngeal Swab 1. Insert dry swab into nostril and back to nasopharynx 2. Leave in place for a few seconds 3. Slowly remove swab while slightly rotating it 4. Use a different swab for the other nostril 5. Put tip of swab into vial containing VTM, breaking applicator’s stick 37 ﺹ 8: 17

Nasopharyngeal Aspirate Collection Process 1. 2. 3. 4. 5. 6. Attach mucus trap to vacuum source Place catheter into nostril parallel to palate Apply vacuum Slowly remove catheter while slightly rotating it Repeat with other nostril using the same catheter After collection, flush catheter with 3 ml VTM and return VTM to a plastic vial 38 ﺹ 8: 17

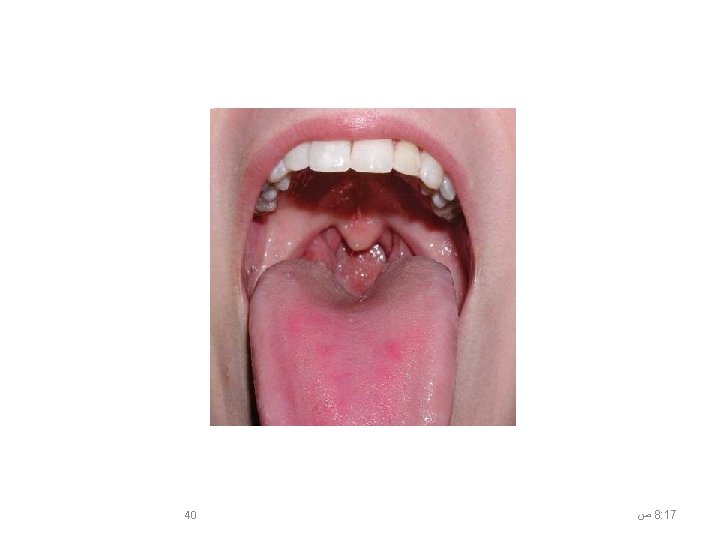
Oropharyngeal (Throat) Swab • Easy to do • Highest yield in detecting avian influenza in suspected cases • Have the patient open his/her mouth wide open. • The patient should try to resist gagging and closing the mouth while the swab touches the back of the throat near the tonsils. 39 ﺹ 8: 17

8: 17 ﺹ 40

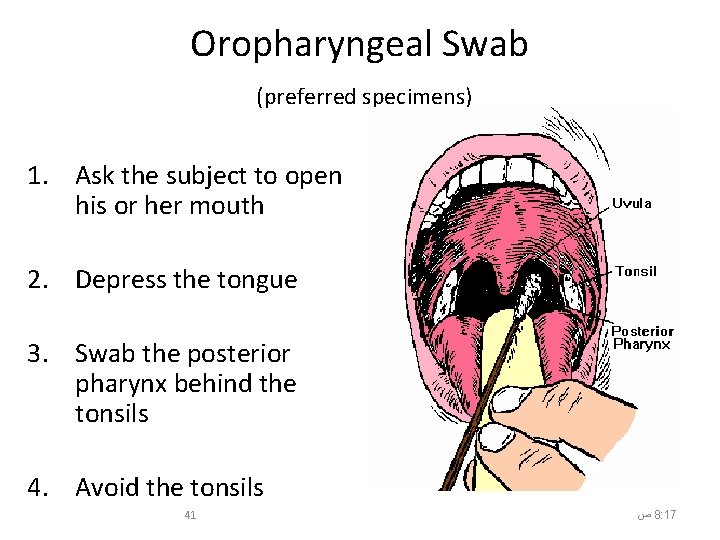
Oropharyngeal Swab (preferred specimens) 1. Ask the subject to open his or her mouth 2. Depress the tongue 3. Swab the posterior pharynx behind the tonsils 4. Avoid the tonsils 41 ﺹ 8: 17

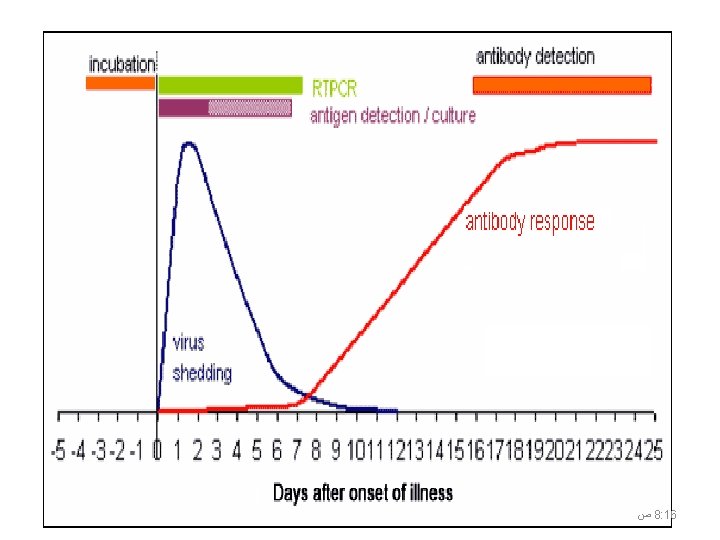
When to Collect Respiratory Specimens • As soon as possible after symptoms begin • before antiviral medications are administered • Even if symptoms began more than one week ago and antivirals administered but record both the time from symptom onset and the type and dosage of antivirals treatment • Multiple specimens on multiple days could be collected if you have access to patient 42 ﺹ 8: 17

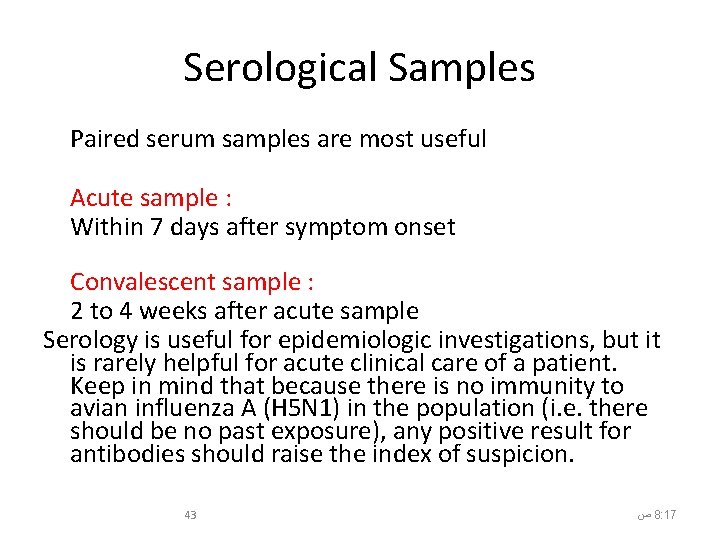
Serological Samples Paired serum samples are most useful Acute sample : Within 7 days after symptom onset Convalescent sample : 2 to 4 weeks after acute sample Serology is useful for epidemiologic investigations, but it is rarely helpful for acute clinical care of a patient. Keep in mind that because there is no immunity to avian influenza A (H 5 N 1) in the population (i. e. there should be no past exposure), any positive result for antibodies should raise the index of suspicion. 43 ﺹ 8: 17

How to Label Samples Use pre-printed barcode* labels: – On the specimen container – On the field data collection form – On the log book * If barcode labels are not available use Marker. Label each specimen with: – Subject’s name – Subject’s unique identification number 44 ﺹ 8: 17

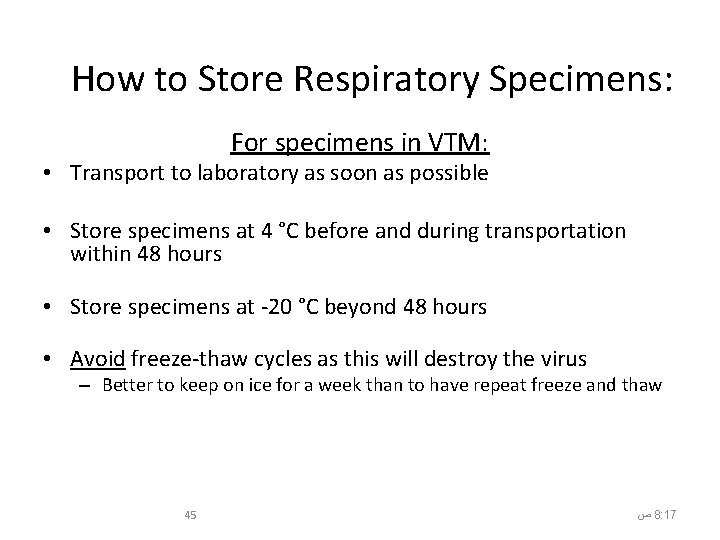
How to Store Respiratory Specimens: For specimens in VTM: • Transport to laboratory as soon as possible • Store specimens at 4 °C before and during transportation within 48 hours • Store specimens at -20 °C beyond 48 hours • Avoid freeze-thaw cycles as this will destroy the virus – Better to keep on ice for a week than to have repeat freeze and thaw 45 ﺹ 8: 17

How to Store serum Specimens: • Store specimen at 4 °C within 48 hours • Store specimens at -20 °C beyond 48 hours • Avoid repeated freeze-thaw cycles For both VTM specimens and sera 46 ﺹ 8: 17

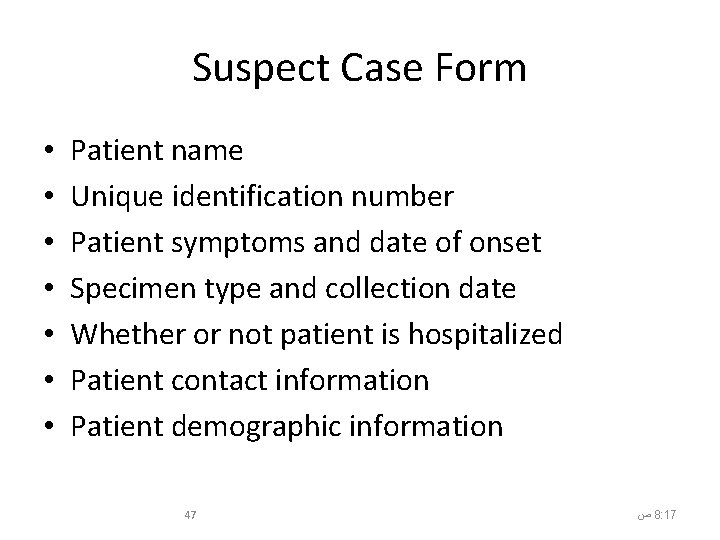
Suspect Case Form • • Patient name Unique identification number Patient symptoms and date of onset Specimen type and collection date Whether or not patient is hospitalized Patient contact information Patient demographic information 47 ﺹ 8: 17

Handling Infectious Materials in the Field • Always wear personal protective equipment • Be careful with sharp objects • Treat all clinical samples as potentially infected with influenza 48 ﺹ 8: 17

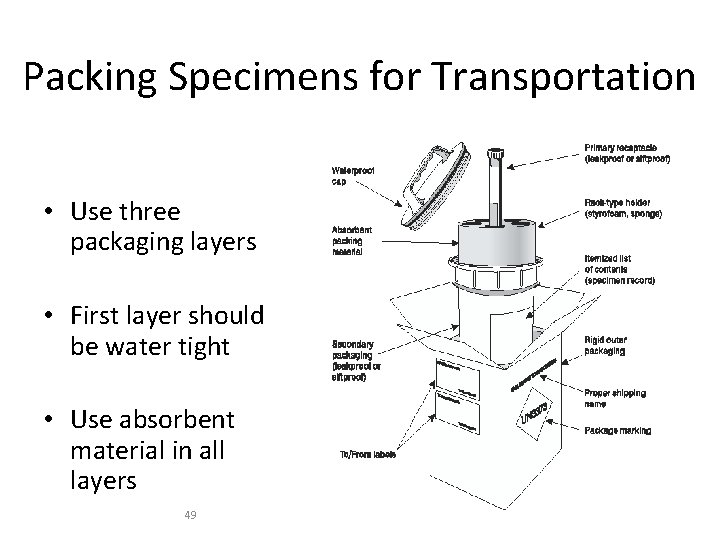
Packing Specimens for Transportation • Use three packaging layers • First layer should be water tight • Use absorbent material in all layers 49 ﺹ 8: 17

Packing Specimens for Transportation • Keep specimens at 4 ºC – Fill a cooler with ice packs or coolant packs but do not use dry ice unless the specimens are doublebagged and airtight; carbon dioxide from the dry ice can inactivate the virus. • Coordinate with the laboratory 50 ﺹ 8: 17

Summary 51 ﺹ 8: 17

Summary • Oropharyngeal swabs and lower respiratory specimens are the best specimens to collect for avian influenza A (H 5 N 1). • Nasopharyngeal swabs and nasal washes (for young children) are better for seasonal influenza and other pathogens. • Collect multiple specimens (respiratory and blood) on multiple days. • Properly dispose of any infectious material. 52 ﺹ 8: 17







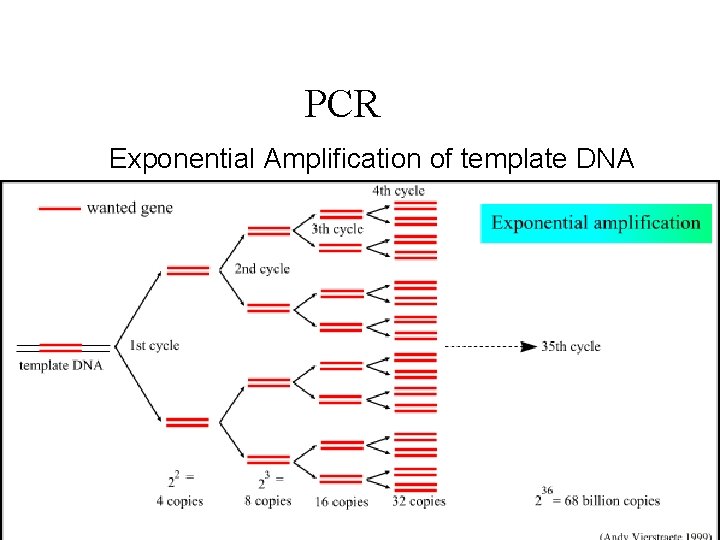
PCR Exponential Amplification of template DNA 59 ﺩﻭﺭ ﻣﺨﺎﺑﺮ ﺍﻟﺼﺤﺔ ﺍﻟﻌﺎﻣﺔ 6/7/2021 8: 18 AM


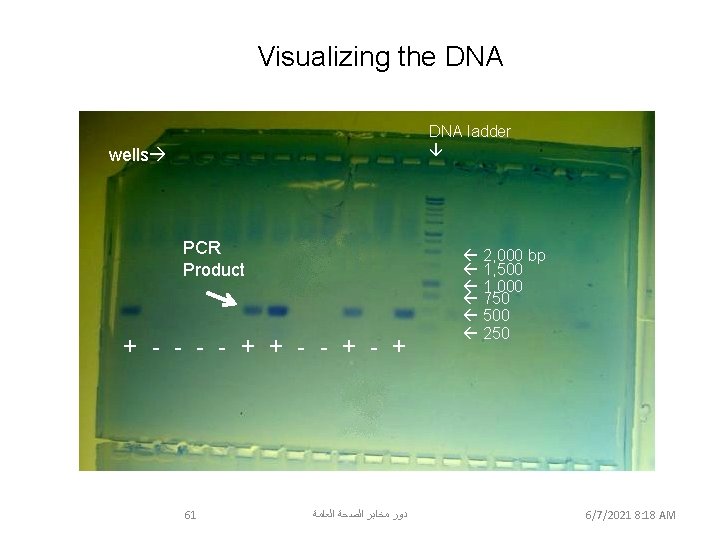
Visualizing the DNA ladder wells PCR Product + - - + 2, 000 bp 1, 500 1, 000 750 500 250 Samples # 1, 6, 7, 10 & 12 were positive for Wolbachia DNA 61 ﺩﻭﺭ ﻣﺨﺎﺑﺮ ﺍﻟﺼﺤﺔ ﺍﻟﻌﺎﻣﺔ 6/7/2021 8: 18 AM March 12, 2006

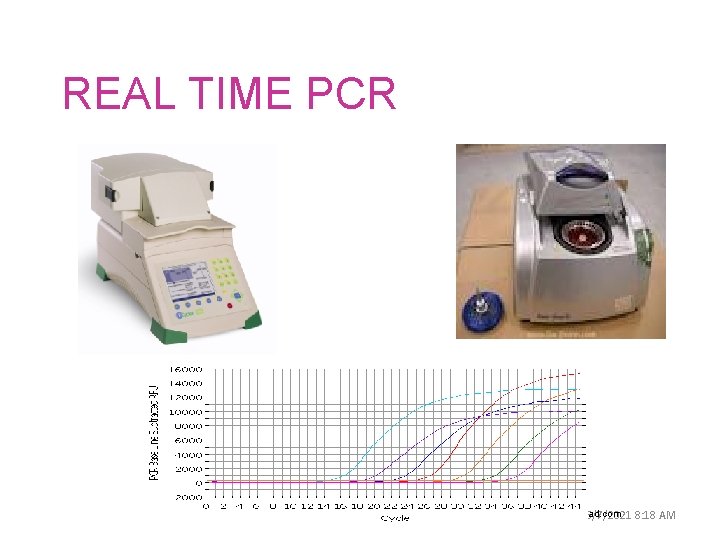
REAL TIME PCR 62 ﺩﻭﺭ ﻣﺨﺎﺑﺮ ﺍﻟﺼﺤﺔ ﺍﻟﻌﺎﻣﺔ www. biorad. com 6/7/2021 8: 18 AM

CDC Realtime RTPCR protocol for Detection of Influenza A (H 1 N 1) of swine origin

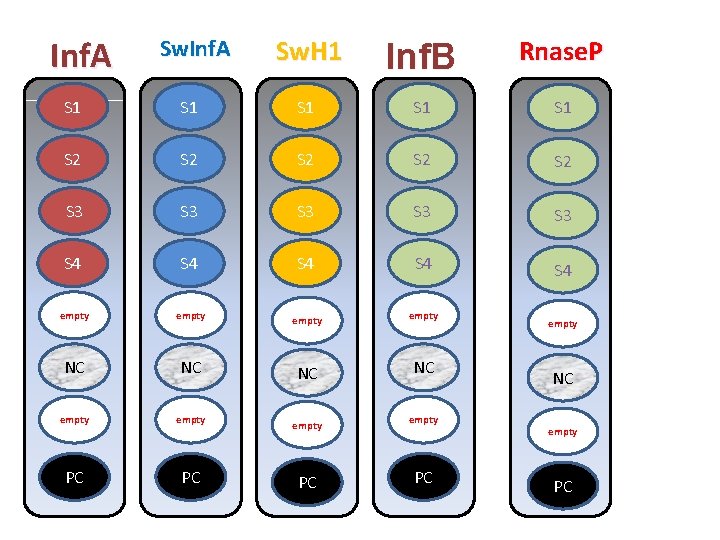
q. RT-PCR • Used to determine whether sample is Influenza A – M gene primers and probes (segment 7) • Tested for Influenza B – B NS (segment 8) • Tested for H 5, H 1, H 3 (others if thought likely) on segment 4 primers and probes • Tested for RNA integrity based on human ribonuclease P sequence.

RNA Extraction Layout Sample A Sample B Sample C Sample D





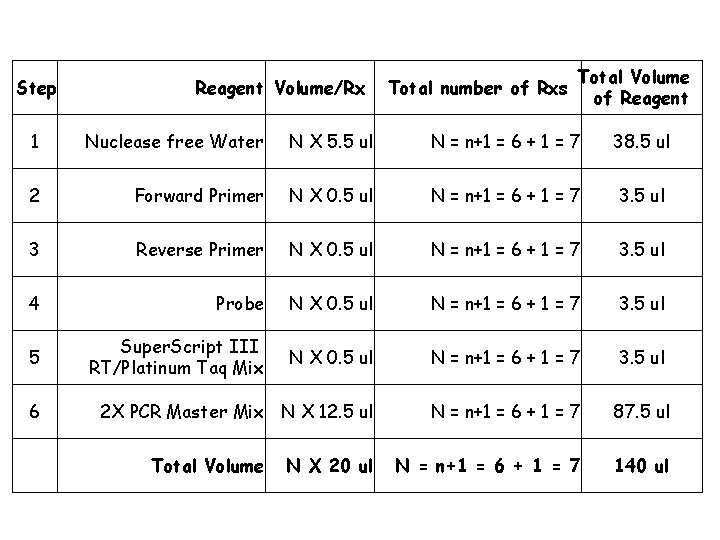
Invitrogen Superscript III Platinum One Step Quantitative Kit Forward Primer (40 µM) Reverse Primer (40 µM) Dual Labeled Probe (10 µM)

Step Reagent Volume/Rx Total number of Rxs Total Volume of Reagent 1 Nuclease free Water N X 5. 5 ul N = n+1 = 6 + 1 = 7 38. 5 ul 2 Forward Primer N X 0. 5 ul N = n+1 = 6 + 1 = 7 3. 5 ul 3 Reverse Primer N X 0. 5 ul N = n+1 = 6 + 1 = 7 3. 5 ul 4 Probe N X 0. 5 ul N = n+1 = 6 + 1 = 7 3. 5 ul 5 Super. Script III RT/Platinum Taq Mix N X 0. 5 ul N = n+1 = 6 + 1 = 7 3. 5 ul 2 X PCR Master Mix N X 12. 5 ul N = n+1 = 6 + 1 = 7 87. 5 ul N = n+1 = 6 + 1 = 7 140 ul 6 Total Volume N X 20 ul


Inf. A Sw. H 1 Inf. B Rnase. P S 1 S 1 S 1 S 2 S 2 S 2 S 3 S 3 S 3 S 4 S 4 S 4 empty NC NC empty PC PC Z 4 empty NC empty PC

RP control (human Rnase P gene) • Each specimen should be tested for RNP gene. This reaction serves as an internal pos control for the presence of human nucleic acid in the extract as well as a control for inhibition of the reaction.

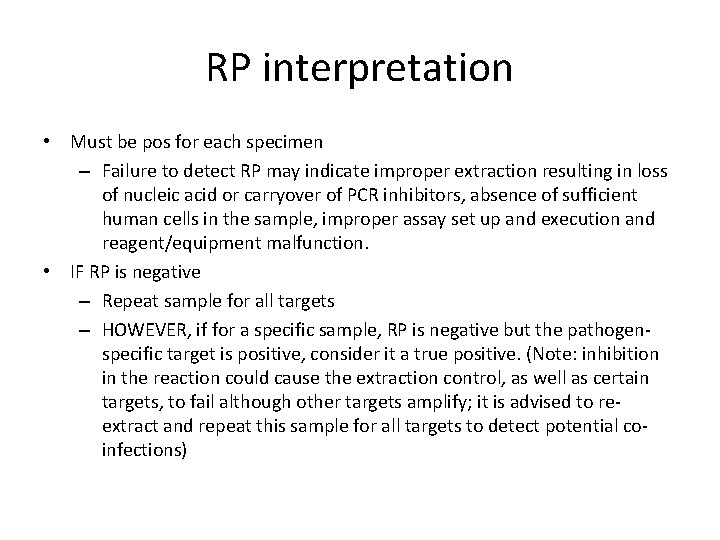
RP interpretation • Must be pos for each specimen – Failure to detect RP may indicate improper extraction resulting in loss of nucleic acid or carryover of PCR inhibitors, absence of sufficient human cells in the sample, improper assay set up and execution and reagent/equipment malfunction. • IF RP is negative – Repeat sample for all targets – HOWEVER, if for a specific sample, RP is negative but the pathogenspecific target is positive, consider it a true positive. (Note: inhibition in the reaction could cause the extraction control, as well as certain targets, to fail although other targets amplify; it is advised to reextract and repeat this sample for all targets to detect potential coinfections)



96 wells plate


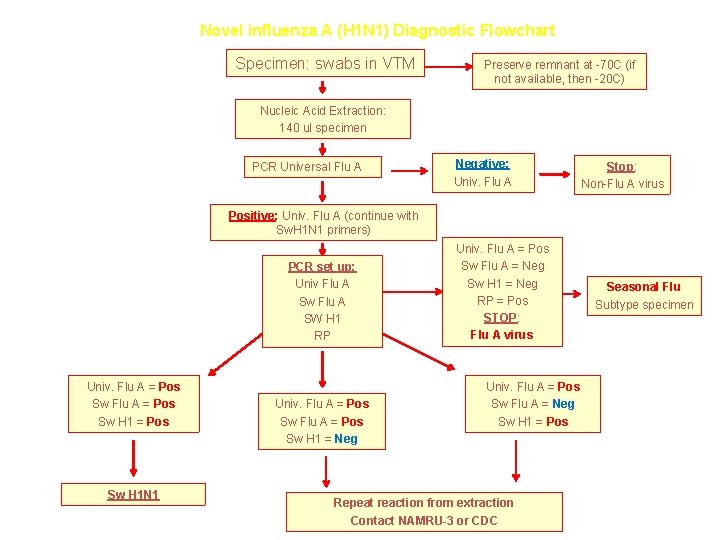
Novel influenza A (H 1 N 1) Diagnostic Flowchart Specimen: swabs in VTM Preserve remnant at -70 C (if not available, then -20 C) Nucleic Acid Extraction: 140 ul specimen PCR Universal Flu A Negative: Univ. Flu A Stop: Non-Flu A virus Positive: Univ. Flu A (continue with Sw. H 1 N 1 primers) PCR set up: Univ Flu A Sw Flu A SW H 1 RP Univ. Flu A = Pos Sw H 1 N 1 Univ. Flu A = Pos Sw H 1 = Neg Univ. Flu A = Pos Sw Flu A = Neg Sw H 1 = Neg RP = Pos STOP: Flu A virus Univ. Flu A = Pos Sw Flu A = Neg Sw H 1 = Pos Repeat reaction from extraction Contact NAMRU-3 or CDC Seasonal Flu Subtype specimen






 Rimantidina
Rimantidina Influenza virus replication
Influenza virus replication Piano di divisione delle staffe
Piano di divisione delle staffe Influenza a causative agent
Influenza a causative agent The great influenza rhetorical analysis
The great influenza rhetorical analysis Olfactory mucosa
Olfactory mucosa Influenza
Influenza Stomach flu vs influenza
Stomach flu vs influenza Is influenza a airborne disease
Is influenza a airborne disease Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Influenza ww1
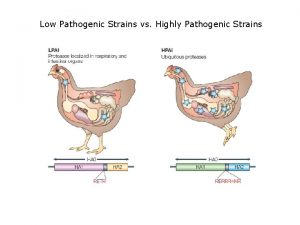
Influenza ww1 Low pathogenic avian influenza
Low pathogenic avian influenza T4 page virus
T4 page virus Trojanski konj virus
Trojanski konj virus Dermotropos
Dermotropos Virus dermatrópicos ejemplos
Virus dermatrópicos ejemplos Ce este un virus informatic?
Ce este un virus informatic? Veoh virus
Veoh virus Grayware virus
Grayware virus Rabies virus incubation period
Rabies virus incubation period Lytta virus is real
Lytta virus is real Pox virus ailesi
Pox virus ailesi Mumps virus
Mumps virus Mumps medicine
Mumps medicine Adware virus informatico
Adware virus informatico Contoh virus rna
Contoh virus rna Como se clasifica los virus
Como se clasifica los virus Estructuras reproductivas de los hongos
Estructuras reproductivas de los hongos Pepino mozaïek virus
Pepino mozaïek virus Growth curve of virus
Growth curve of virus Virus cultivation in embryonated eggs
Virus cultivation in embryonated eggs Mcafee anti-virus
Mcafee anti-virus Virus polimorfos o mutantes
Virus polimorfos o mutantes Jason
Jason Virus
Virus Stomach virus
Stomach virus Sofiacruzv
Sofiacruzv Ppt virus kelas 10
Ppt virus kelas 10 Rage
Rage Duhare
Duhare Growth curve of virus
Growth curve of virus Hepanda virus
Hepanda virus Merilyn merisalu
Merilyn merisalu Trap door adalah
Trap door adalah Virus trojanski konj
Virus trojanski konj Que son los virus
Que son los virus Virus multiple sclerosis
Virus multiple sclerosis Coder un virus
Coder un virus Virus
Virus Japanese encephalitis virus
Japanese encephalitis virus Snopes
Snopes Virus mnemonic
Virus mnemonic Chemical nature of virus
Chemical nature of virus Enurese
Enurese Concatenamero significato
Concatenamero significato Endositosis yang diperantarai reseptor
Endositosis yang diperantarai reseptor Bacteria virus fungi and parasites
Bacteria virus fungi and parasites Rna virus
Rna virus Karina brevis
Karina brevis Tiara virus
Tiara virus Como se tranmite el sida
Como se tranmite el sida Biosynthesis of rna viruses
Biosynthesis of rna viruses Virus respiratorios
Virus respiratorios World first computer virus
World first computer virus Overview of transcription and translation
Overview of transcription and translation Causal agent of tobacco mosaic virus
Causal agent of tobacco mosaic virus Polyproteins
Polyproteins Klasifikasi virus
Klasifikasi virus Lytta virus
Lytta virus Taksonomi virus dengue
Taksonomi virus dengue What is the destructive event or prank the virus delivers
What is the destructive event or prank the virus delivers Halimbawa ng virus sa computer
Halimbawa ng virus sa computer Is the zika virus lytic or lysogenic
Is the zika virus lytic or lysogenic Pearson education, inc
Pearson education, inc Virus capsid
Virus capsid File infector virus example
File infector virus example Biosafety of recombinant adeno associated virus vectors
Biosafety of recombinant adeno associated virus vectors West nyle virus
West nyle virus Properties of viruses
Properties of viruses Latin word for poison
Latin word for poison Chrysophyta
Chrysophyta Una bacteria y sus partes
Una bacteria y sus partes Virus vs antivirus
Virus vs antivirus Dermotropicos
Dermotropicos Autorreplicables
Autorreplicables Virus analysis tools
Virus analysis tools Virus infectie
Virus infectie