How do transplants fit in the current therapeutic






- Slides: 42

How do transplants fit in the current therapeutic schema? Guenther Koehne, MD, Ph. D Adult Bone Marrow Transplant Service Division of Hematologic Oncology Department of Medicine Memorial Sloan-Kettering Cancer Center New York, New York

Autologous SCT Who? When? How? Maintenance? Allogeneic SCT Who? When? How? Maintenance?

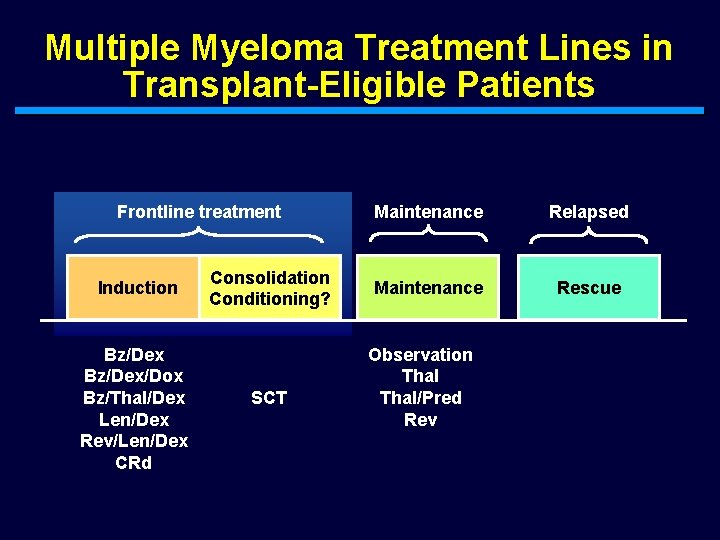
Multiple Myeloma Treatment Lines in Transplant-Eligible Patients Frontline treatment Induction Bz/Dex/Dox Bz/Thal/Dex Len/Dex Rev/Len/Dex CRd Consolidation Conditioning? SCT Maintenance Relapsed Maintenance Rescue Observation Thal/Pred Rev

Curr Opin Oncol, Sept 2012

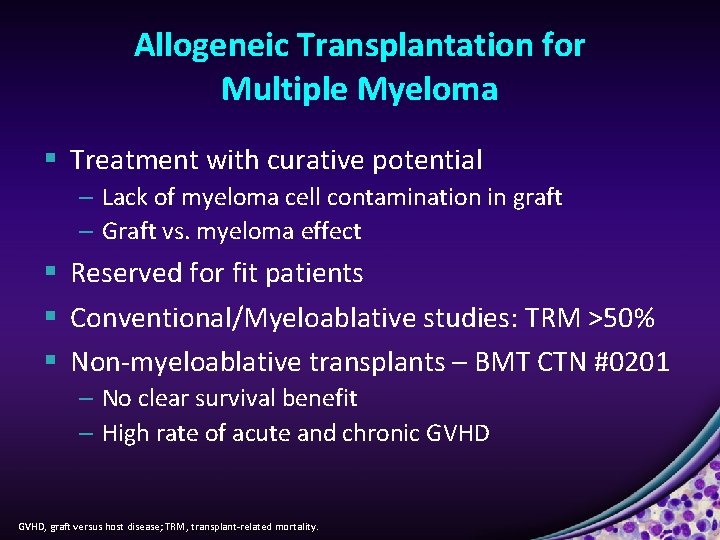
Allogeneic Transplantation for Multiple Myeloma § Treatment with curative potential – Lack of myeloma cell contamination in graft – Graft vs. myeloma effect § Reserved for fit patients § Conventional/Myeloablative studies: TRM >50% § Non-myeloablative transplants – BMT CTN #0201 – No clear survival benefit – High rate of acute and chronic GVHD, graft versus host disease; TRM, transplant-related mortality.

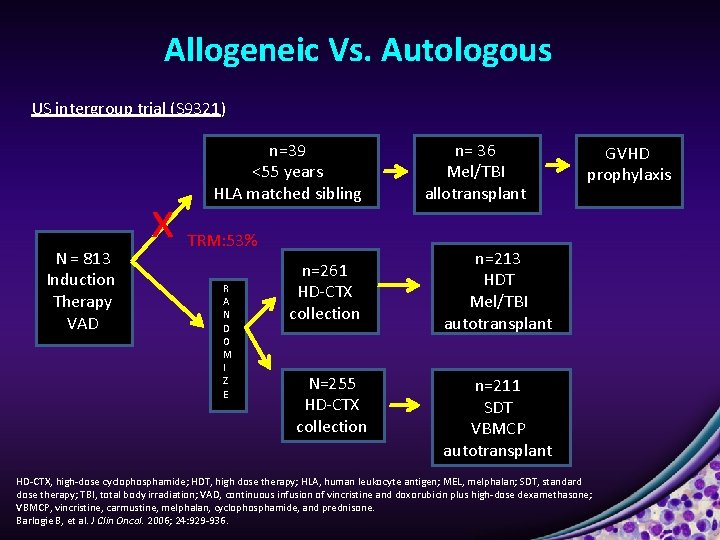
Allogeneic Vs. Autologous US intergroup trial (S 9321) N = 813 Induction Therapy VAD x n=39 <55 years HLA matched sibling TRM: 53% R A N D O M I Z E n=261 HD-CTX collection N=255 HD-CTX collection n= 36 Mel/TBI allotransplant GVHD prophylaxis n=213 HDT Mel/TBI autotransplant n=211 SDT VBMCP autotransplant HD-CTX, high-dose cyclophosphamide; HDT, high dose therapy; HLA, human leukocyte antigen; MEL, melphalan; SDT, standard dose therapy; TBI, total body irradiation; VAD, continuous infusion of vincristine and doxorubicin plus high-dose dexamethasone; VBMCP, vincristine, carmustine, melphalan, cyclophosphamide, and prednisone. Barlogie B, et al. J Clin Oncol. 2006; 24: 929 -936.

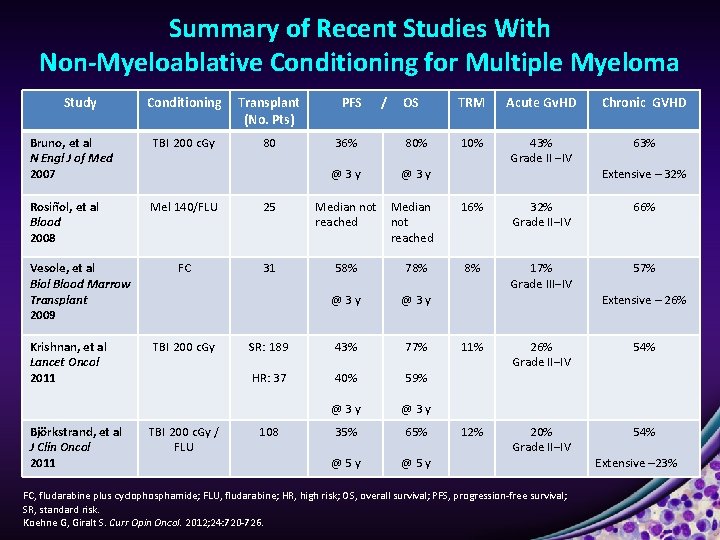
Summary of Recent Studies With Non-Myeloablative Conditioning for Multiple Myeloma Study Conditioning Transplant (No. Pts) Bruno, et al N Engl J of Med 2007 TBI 200 c. Gy 80 Rosiñol, et al Blood 2008 Mel 140/FLU 25 Median not reached FC 31 58% 78% @3 y SR: 189 43% 77% HR: 37 40% 59% @3 y 35% 65% @5 y Vesole, et al Biol Blood Marrow Transplant 2009 Krishnan, et al Lancet Oncol 2011 Björkstrand, et al J Clin Oncol 2011 TBI 200 c. Gy / FLU 108 PFS / OS TRM Acute Gv. HD Chronic GVHD 36% 80% 10% 63% @3 y 43% Grade II ‒IV Median not reached Extensive – 32% 16% 32% Grade II‒IV 66% 8% 17% Grade III‒IV 57% Extensive – 26% 11% 26% Grade II‒IV 54% 12% 20% Grade II‒IV 54% FC, fludarabine plus cyclophosphamide; FLU, fludarabine; HR, high risk; OS, overall survival; PFS, progression-free survival; SR, standard risk. Koehne G, Giralt S. Curr Opin Oncol. 2012; 24: 720 -726. Extensive – 23%

Allogeneic SCT Who? Risk stratification based on high-risk factors When? Sooner than later How & Where? TCD HSCT & MSKCC Maintenance? Immunotherapeutic Approaches

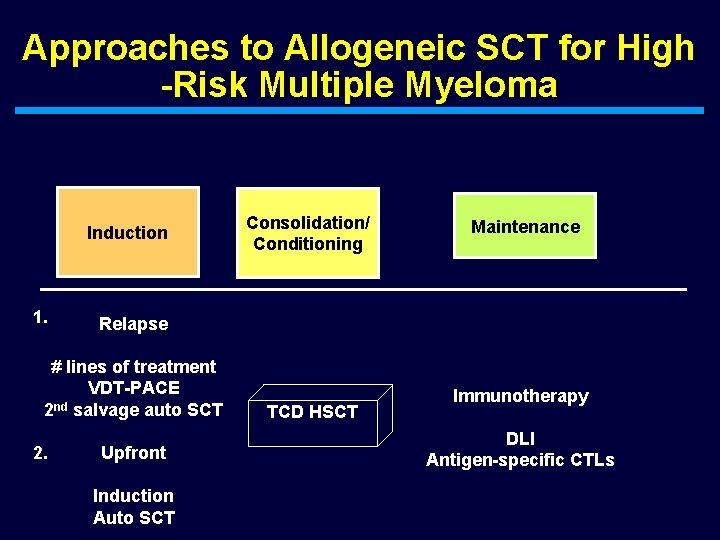
Approaches to Allogeneic SCT for High -Risk Multiple Myeloma Induction 1. Maintenance Relapse # lines of treatment VDT-PACE 2 nd salvage auto SCT 2. Consolidation/ Conditioning Upfront Induction Auto SCT TCD HSCT Immunotherapy DLI Antigen-specific CTLs

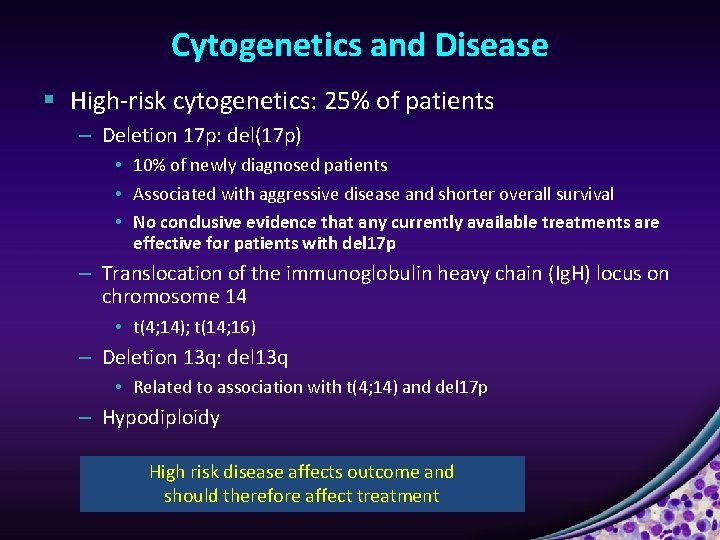
Cytogenetics and Disease § High-risk cytogenetics: 25% of patients – Deletion 17 p: del(17 p) • 10% of newly diagnosed patients • Associated with aggressive disease and shorter overall survival • No conclusive evidence that any currently available treatments are effective for patients with del 17 p – Translocation of the immunoglobulin heavy chain (Ig. H) locus on chromosome 14 • t(4; 14); t(14; 16) – Deletion 13 q: del 13 q • Related to association with t(4; 14) and del 17 p – Hypodiploidy High risk disease affects outcome and should therefore affect treatment

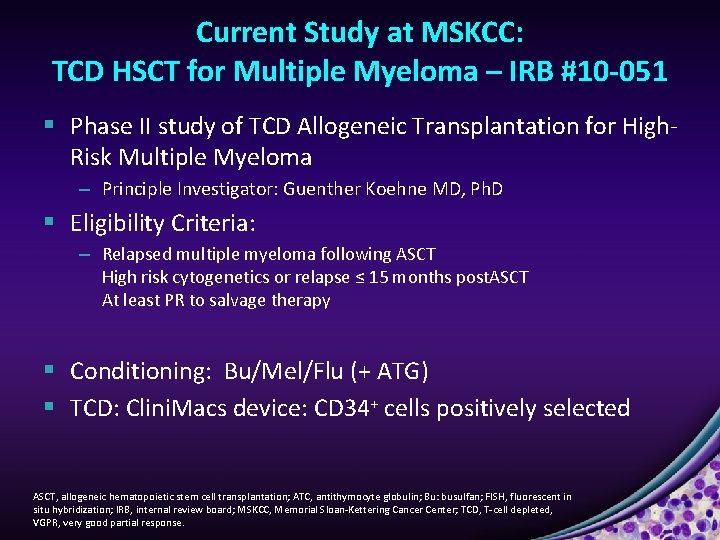
Current Study at MSKCC: TCD HSCT for Multiple Myeloma – IRB #10 -051 § Phase II study of TCD Allogeneic Transplantation for High. Risk Multiple Myeloma – Principle Investigator: Guenther Koehne MD, Ph. D § Eligibility Criteria: – Relapsed multiple myeloma following ASCT High risk cytogenetics or relapse ≤ 15 months post. ASCT At least PR to salvage therapy § Conditioning: Bu/Mel/Flu (+ ATG) § TCD: Clini. Macs device: CD 34+ cells positively selected ASCT, allogeneic hematopoietic stem cell transplantation; ATC, antithymocyte globulin; Bu: busulfan; FISH, fluorescent in situ hybridization; IRB, internal review board; MSKCC, Memorial Sloan-Kettering Cancer Center; TCD, T-cell depleted, VGPR, very good partial response.

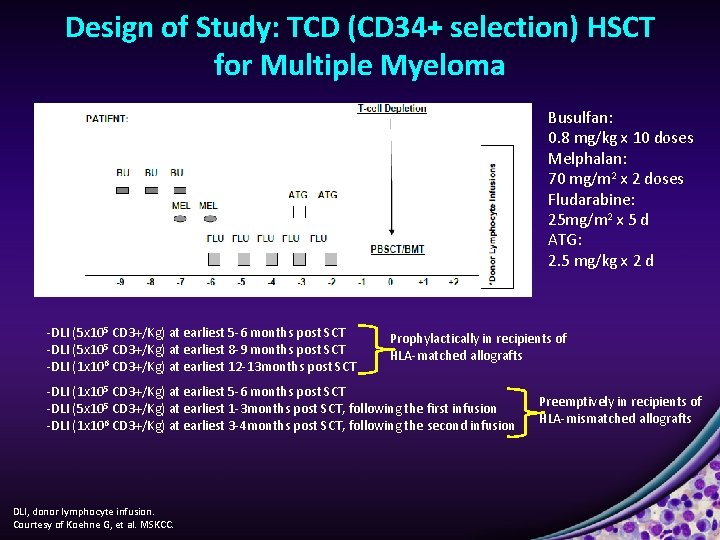
Design of Study: TCD (CD 34+ selection) HSCT for Multiple Myeloma Busulfan: 0. 8 mg/kg x 10 doses Melphalan: 70 mg/m 2 x 2 doses Fludarabine: 25 mg/m 2 x 5 d ATG: 2. 5 mg/kg x 2 d -DLI (5 x 105 CD 3+/Kg) at earliest 5 -6 months post SCT -DLI (5 x 105 CD 3+/Kg) at earliest 8 -9 months post SCT -DLI (1 x 106 CD 3+/Kg) at earliest 12 -13 months post SCT Prophylactically in recipients of HLA-matched allografts -DLI (1 x 105 CD 3+/Kg) at earliest 5 -6 months post SCT -DLI (5 x 105 CD 3+/Kg) at earliest 1 -3 months post SCT, following the first infusion -DLI (1 x 106 CD 3+/Kg) at earliest 3 -4 months post SCT, following the second infusion DLI, donor lymphocyte infusion. Courtesy of Koehne G, et al. MSKCC. Preemptively in recipients of HLA-mismatched allografts

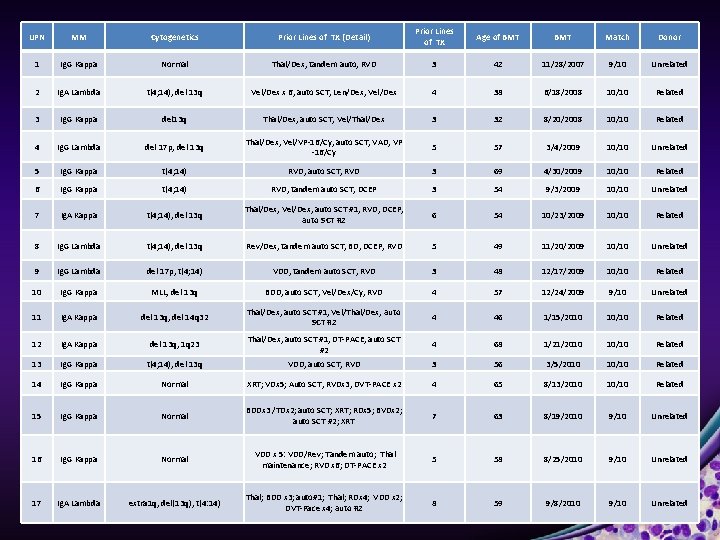
UPN MM Cytogenetics Prior Lines of TX (Detail) Prior Lines of TX Age of BMT Match Donor 1 Ig. G Kappa Normal Thal/Dex, tandem auto, RVD 3 42 11/28/2007 9/10 Unrelated 2 Ig. A Lambda t(4; 14), del 13 q Vel/Dex x 6, auto SCT, Len/Dex, Vel/Dex 4 38 6/18/2008 10/10 Related 3 Ig. G Kappa del 13 q Thal/Dex, auto SCT, Vel/Thal/Dex 3 32 8/20/2008 10/10 Related 4 Ig. G Lambda del 17 p, del 13 q Thal/Dex, Vel/VP-16/Cy, auto SCT, VAD, VP -16/Cy 5 57 3/4/2009 10/10 Unrelated 5 Ig. G Kappa t(4; 14) RVD, auto SCT, RVD 3 69 4/30/2009 10/10 Related 6 Ig. G Kappa t(4; 14) RVD, tandem auto SCT, DCEP 3 54 9/3/2009 10/10 Unrelated 7 Ig. A Kappa t(4; 14), del 13 q Thal/Dex, Vel/Dex, auto SCT #1, RVD, DCEP, auto SCT #2 6 54 10/23/2009 10/10 Related 8 Ig. G Lambda t(4; 14), del 13 q Rev/Dex, tandem auto SCT, BD, DCEP, RVD 5 49 11/20/2009 10/10 Unrelated 9 Ig. G Lambda del 17 p, t(4; 14) VDD, tandem auto SCT, RVD 3 48 12/17/2009 10/10 Related 10 Ig. G Kappa MLL, del 13 q BDD, auto SCT, Vel/Dex/Cy, RVD 4 57 12/24/2009 9/10 Unrelated 11 Ig. A Kappa del 13 q, del 14 q 32 Thal/Dex, auto SCT #1, Vel/Thal/Dex, auto SCT #2 4 46 1/15/2010 10/10 Related 12 Ig. A Kappa del 13 q, 1 q 23 Thal/Dex, auto SCT #1, DT-PACE, auto SCT #2 4 68 1/21/2010 10/10 Related 13 Ig. G Kappa t(4; 14), del 13 q VDD, auto SCT, RVD 3 56 3/5/2010 10/10 Related 14 Ig. G Kappa Normal XRT; VDx 5; Auto SCT, RVDx 3, DVT-PACE x 2 4 65 8/13/2010 10/10 Related 15 Ig. G Kappa Normal BDDx 3 /TDx 2; auto SCT; XRT; RDx 5; BVDx 2; auto SCT #2; XRT 7 63 8/19/2010 9/10 Unrelated 16 Ig. G Kappa Normal VDD x 5: VDD/Rev; Tandem auto; Thal maintenance; RVD x 6; DT-PACE x 2 5 58 8/25/2010 9/10 Unrelated 17 Ig. A Lambda extra 1 q, del(13 q), t(4: 14) Thal; BDD x 3; auto#1; Thal; RDx 4; VDD x 2; DVT-Pace x 4; auto #2 8 59 9/8/2010 9/10 Unrelated

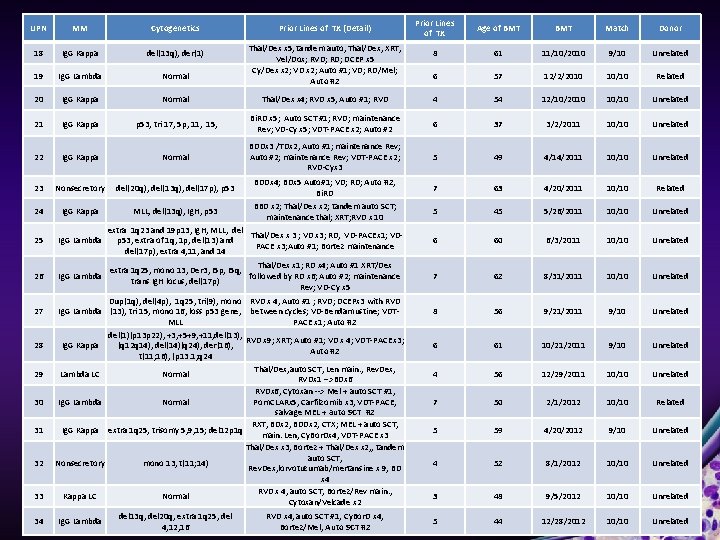
Prior Lines of TX Age of BMT Match Donor 8 61 11/10/2010 9/10 Unrelated 6 57 12/2/2010 10/10 Related Thal/Dex x 4; RVD x 5, Auto #1; RVD 4 54 12/10/2010 10/10 Unrelated p 53, tri 17, 5 p, 11, 15, Bi. RD x 5; Auto SCT #1; RVD; maintenance Rev; VD-Cy x 5; VDT-PACE x 2; Auto #2 6 37 3/2/2011 10/10 Unrelated Ig. G Kappa Normal BDDx 3 /TDx 2, Auto #1; maintenance Rev; Auto #2; maintenance Rev; VDT-PACE x 2; RVD-Cyx 3 5 49 4/14/2011 10/10 Unrelated 23 Nonsecretory del(20 q), del(13 q), del(17 p), p 53 7 63 4/20/2011 10/10 Related 24 Ig. G Kappa MLL, del(13 q), Ig. H, p 53 5 45 5/26/2011 10/10 Unrelated 25 Ig. G Lambda extra 1 q 23 and 19 p 13, Ig. H, MLL, del Thal/Dex x 3 ; VD x 3; RD, VD-PACEx 1; VDp 53, extra of 1 q, 1 p, del(13) and PACE x 3; Auto #1; Bortez maintenance del(17 p), extra 4, 11, and 14 6 60 6/3/2011 10/10 Unrelated 26 Ig. G Lambda Thal/Dex x 1; RD x 4; Auto #1 XRT/Dex extra 1 q 25, mono 13, Der 3, I 5 p, I 5 q, followed by RD x 6; Auto #2; maintenance trans Ig. H locus, del(17 p) Rev; VD-Cy x 5 7 62 8/31/2011 10/10 Unrelated 8 56 9/21/2011 9/10 Unrelated 6 61 10/21/2011 9/10 Unrelated 4 56 12/29/2011 10/10 Unrelated 7 50 2/1/2012 10/10 Related 5 59 4/20/2012 9/10 Unrelated 4 52 8/1/2012 10/10 Unrelated 3 48 9/5/2012 10/10 Unrelated 5 44 12/28/2012 10/10 Unrelated UPN MM Cytogenetics 18 Ig. G Kappa del(13 q), der(1) 19 Ig. G Lambda Normal 20 Ig. G Kappa Normal 21 Ig. G Kappa 22 27 28 29 30 31 32 33 34 Prior Lines of TX (Detail) Thal/Dex x 5, tandem auto, Thal/Dex, XRT, Vel/Dox; RVD; RD; DCEP x 5 Cy/Dex x 2; VD x 2; Auto #1; VD; RD/Mel; Auto #2 BDDx 4; BDx 5 Auto#1; VD; RD; Auto #2; Bi. RD BBD x 2; Thal/Dex x 2; tandem auto SCT; maintenance thal; XRT; RVD x 10 Dup(1 q), del(4 p), 1 q 25, tri(9), mono RVD x 4, Auto #1 ; RVD; DCEPx 3 with RVD Ig. G Lambda (13), tri 15, mono 16, loss p 53 gene, between cycles; VD-Bendamustine; VDTMLL PACE x 1; Auto #2 del(1)(p 13 p 22), +3, +5+9, +11, del(13), RVD x 9; XRT; Auto #1; VD x 4; VDT-PACE x 3; Ig. G Kappa (q 12 q 14), del(14)(q 24), der(16), Auto #2 t(11; 16), (p 13. 1; q 24 Thal/Dex, auto SCT, Len main. , Rev. Dex, Lambda LC Normal RVDx 1 -->BDx 6 RVDx 6, Cytoxan --> Mel + auto SCT #1, Ig. G Lambda Normal Pom. CLARx 5, Carfilzomib x 3, VDT-PACE, salvage MEL + auto SCT #2 RXT, BDx 2, BDDx 2, CTX; MEL + auto SCT, Ig. G Kappa extra 1 q 25, trisomy 5, 9, 15; del 12 p 1 q main. Len, Cy. Bor. Dx 4, VDT-PACE x 3 Thal/Dex x 3, Bortez + Thal/Dex x 2, , tandem auto SCT, Nonsecretory mono 13, t(11; 14) Rev. Dex, lorvotuzumab/mertansine x 9, BD x 4 RVD x 4, auto SCT, Bortez/Rev main. , Kappa LC Normal Cytoxan/Velcade x 2 Ig. G Lambda del 13 q, del 20 q, extra 1 q 25, del 4, 12, 16 RVD x 4, auto SCT #1, Cy. Bor. D x 4, Bortez/Mel, Auto SCT #2

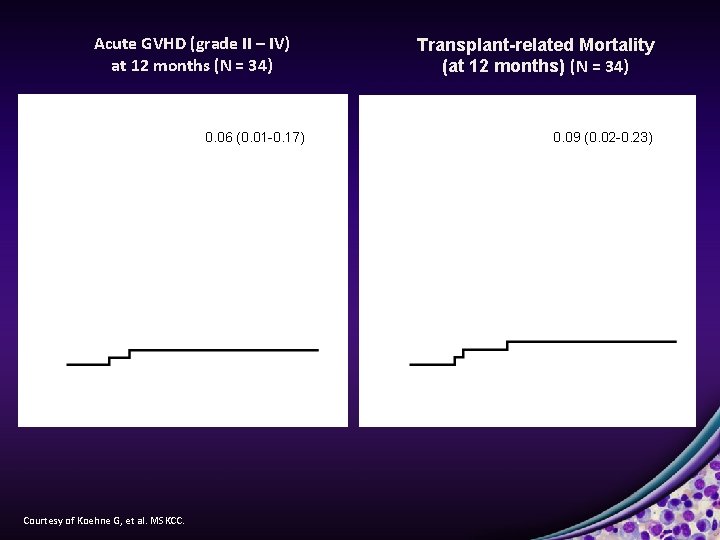
Acute GVHD (grade II – IV) at 12 months (N = 34) 0. 06 (0. 01 -0. 17) Courtesy of Koehne G, et al. MSKCC. Transplant-related Mortality (at 12 months) (N = 34) 0. 09 (0. 02 -0. 23)

Graft failure or rejection Chronic GVHD None observed Courtesy of Koehne G, et al. MSKCC.

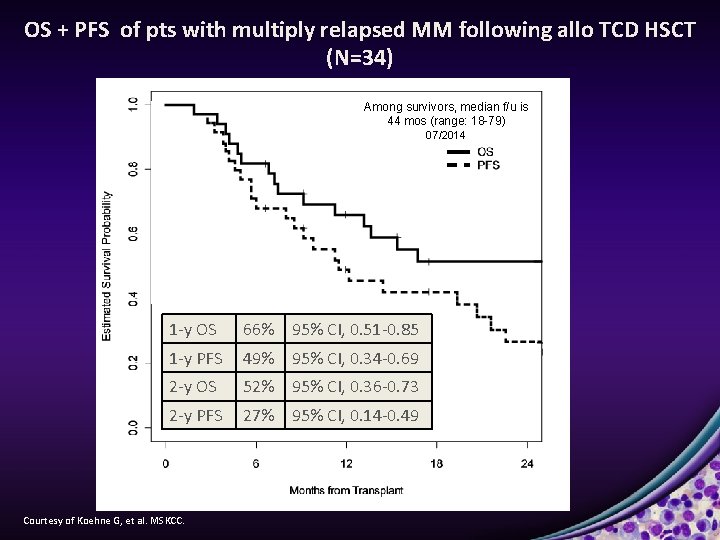
OS + PFS of pts with multiply relapsed MM following allo TCD HSCT (N=34) Among survivors, median f/u is 44 mos (range: 18 -79) 07/2014 1 -y OS 66% 95% CI, 0. 51 -0. 85 1 -y PFS 49% 95% CI, 0. 34 -0. 69 2 -y OS 52% 95% CI, 0. 36 -0. 73 2 -y PFS 27% 95% CI, 0. 14 -0. 49 Courtesy of Koehne G, et al. MSKCC.

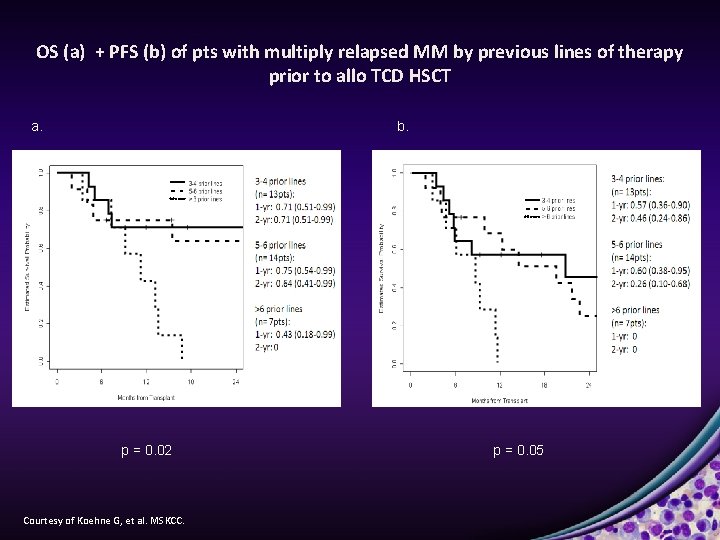
OS (a) + PFS (b) of pts with multiply relapsed MM by previous lines of therapy prior to allo TCD HSCT a. b. p = 0. 02 Courtesy of Koehne G, et al. MSKCC. p = 0. 05

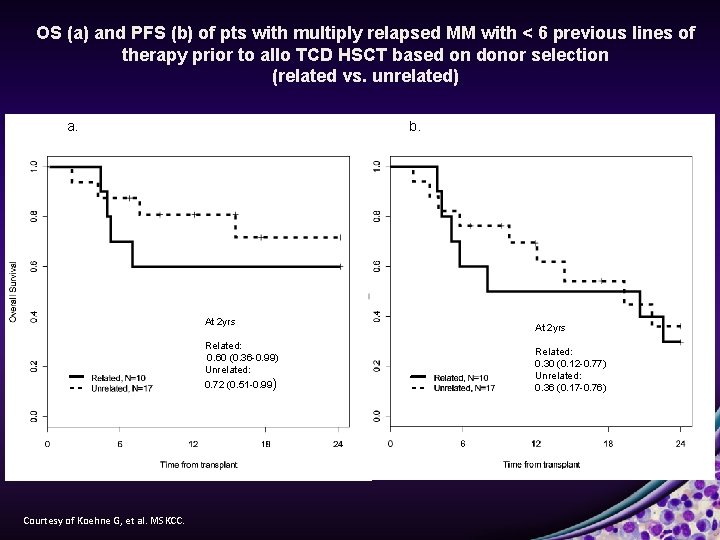
OS (a) and PFS (b) of pts with multiply relapsed MM with < 6 previous lines of therapy prior to allo TCD HSCT based on donor selection (related vs. unrelated) a. b. At 2 yrs Related: 0. 60 (0. 36 -0. 99) Unrelated: 0. 72 (0. 51 -0. 99) Courtesy of Koehne G, et al. MSKCC. At 2 yrs Related: 0. 30 (0. 12 -0. 77) Unrelated: 0. 36 (0. 17 -0. 76)

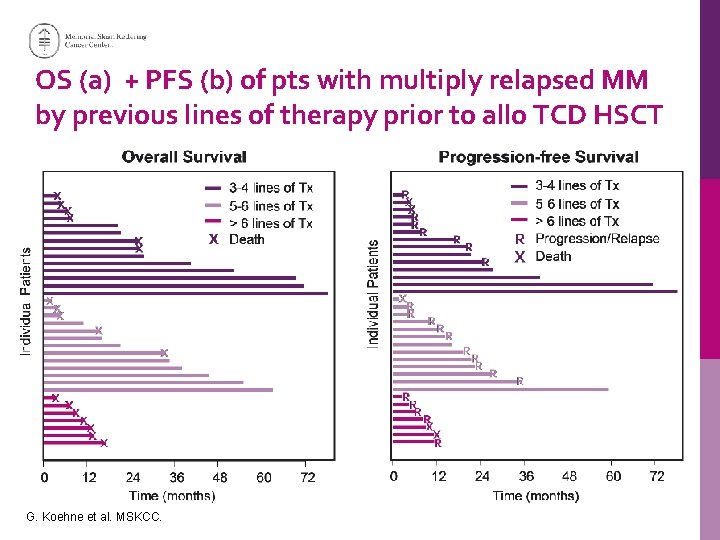
OS (a) + PFS (b) of pts with multiply relapsed MM by previous lines of therapy prior to allo TCD HSCT G. Koehne et al. MSKCC.

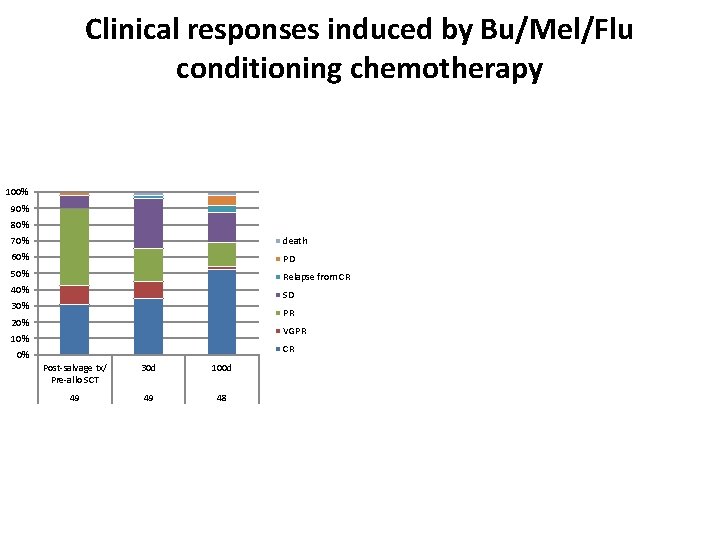
Clinical responses induced by Bu/Mel/Flu conditioning chemotherapy 100% 90% 80% 70% death 60% PD 50% Relapse from CR 40% SD 30% PR 20% VGPR 10% CR 0% Post-salvage tx/ Pre-allo SCT 30 d 100 d 49 49 48

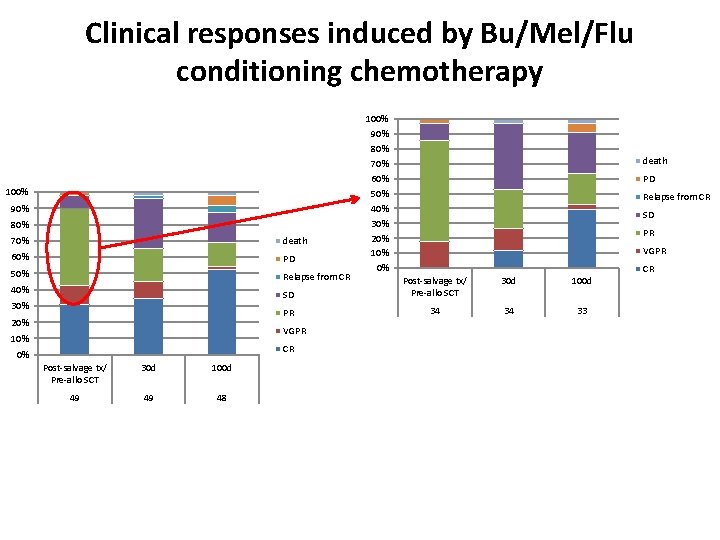
Clinical responses induced by Bu/Mel/Flu conditioning chemotherapy 100% 90% 80% 70% death 60% PD 50% Relapse from CR 40% 30% 20% Post-salvage tx/ Pre-allo SCT 30 d 100 d 49 49 48 PD Relapse from CR SD PR VGPR 30 d 100 d SD PR 34 34 33 CR 0% death Post-salvage tx/ Pre-allo SCT VGPR 10% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% CR

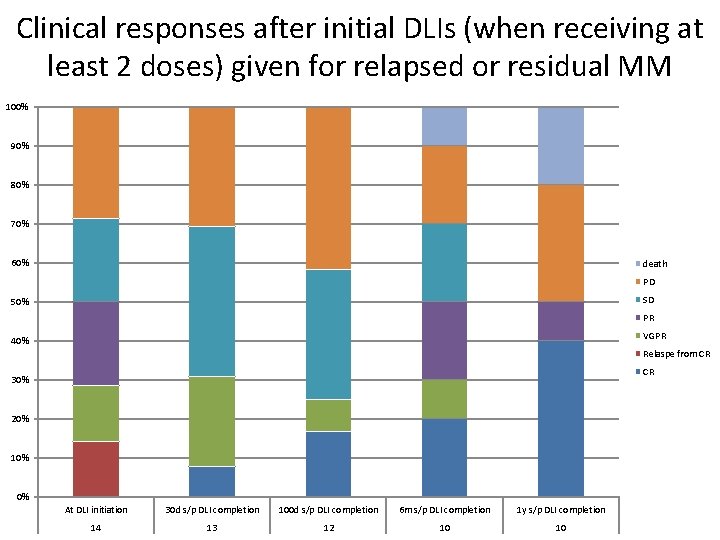
Clinical responses after initial DLIs (when receiving at least 2 doses) given for relapsed or residual MM 100% 90% 80% 70% 60% death PD SD 50% PR VGPR 40% Relaspe from CR CR 30% 20% 10% 0% At DLI initiation 30 d s/p DLI completion 100 d s/p DLI completion 6 m s/p DLI completion 1 y s/p DLI completion 14 13 12 10 10

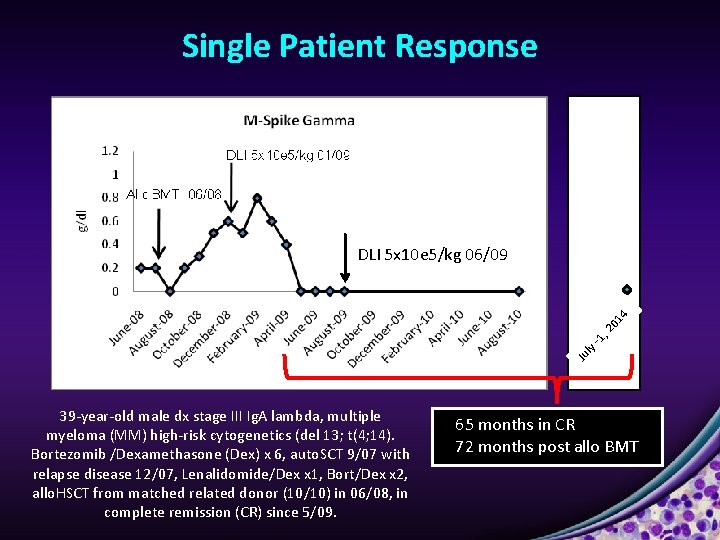
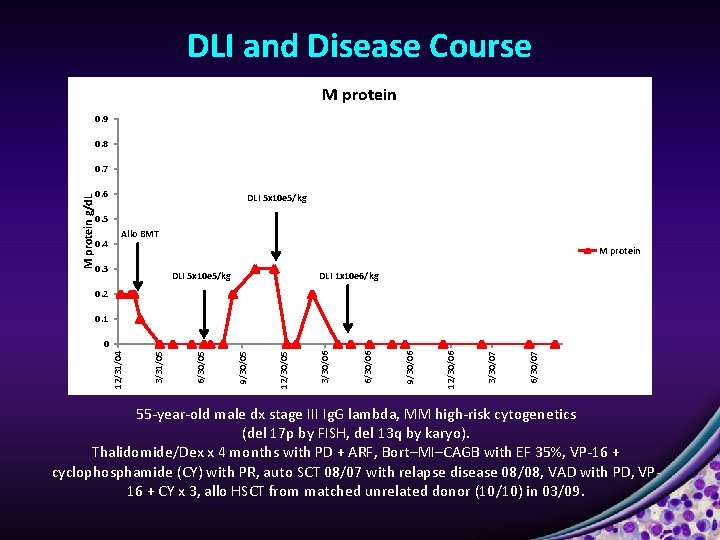
Single Patient Response Ju ly -1 , 2 01 4 DLI 5 x 10 e 5/kg 06/09 39 -year-old male dx stage III Ig. A lambda, multiple myeloma (MM) high-risk cytogenetics (del 13; t(4; 14). Bortezomib /Dexamethasone (Dex) x 6, auto. SCT 9/07 with relapse disease 12/07, Lenalidomide/Dex x 1, Bort/Dex x 2, allo. HSCT from matched related donor (10/10) in 06/08, in complete remission (CR) since 5/09. 65 months in CR 72 months post allo BMT

DLI and Disease Course M protein 0. 9 0. 8 M protein g/d. L 0. 7 0. 6 DLI 5 x 10 e 5/kg 0. 5 0. 4 Allo BMT M protein 0. 3 DLI 5 x 10 e 5/kg DLI 1 x 10 e 6/kg 0. 2 0. 1 6/30/07 3/30/07 12/30/06 9/30/06 6/30/06 3/30/06 12/30/05 9/30/05 6/30/05 3/31/05 12/31/04 0 55 -year-old male dx stage III Ig. G lambda, MM high-risk cytogenetics (del 17 p by FISH, del 13 q by karyo). Thalidomide/Dex x 4 months with PD + ARF, Bort–MI–CAGB with EF 35%, VP-16 + cyclophosphamide (CY) with PR, auto SCT 08/07 with relapse disease 08/08, VAD with PD, VP 16 + CY x 3, allo HSCT from matched unrelated donor (10/10) in 03/09.

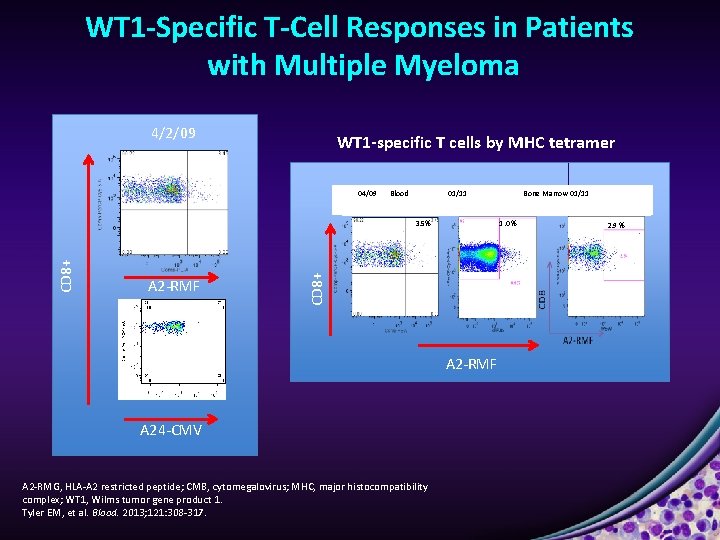
WT 1 -Specific T-Cell Responses in Patients with Multiple Myeloma 4/2/09 WT 1 -specific T cells by MHC tetramer 04/09 Blood 01/11 A 2 -RMF 1. 0 % CD 8+ 3. 5% A 2 -RMF A 24 -CMV A 2 -RMG, HLA-A 2 restricted peptide; CMB, cytomegalovirus; MHC, major histocompatibility complex; WT 1, Wilms tumor gene product 1. Tyler EM, et al. Blood. 2013; 121: 308 -317. Bone Marrow 01/11 2. 9 %

WT 1—A Potential Target for Multiple Myeloma? § WT 1: zinc finger transcription factor — Roles in cell proliferation, differentiation, apoptosis and organ development § Preferentially expressed during embryogenesis, but also at low levels in kidney, ovary, endometrium, testis and spleen of adults § Frequently overexpressed in a number of solid and hematologic malignancies — Expression correlates with disease progression in MDS, ALL, & CML — Molecular marker for risk assessment § Emergence of WT 1 -specific T cells correlates with better relapse-free survival post allogeneic transplant in leukemia 1 § MM cells are efficiently lysed by WT 1 -specific cytotoxic T lymphocytes 2 § WT 1 expression in the BM of myeloma patients correlates with disease stage 3 ALL, acute lymphocytic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome. 1. Rezvani K, et al. Blood. 2007; 110: 1924 -1932; 2. Azuma T, et al. Clin Cancer Res. 2004; 10: 7402 -7412; 3. Hatta Y, et al. J Exp Clin Cancer Res. 2005; 24: 595 -599.

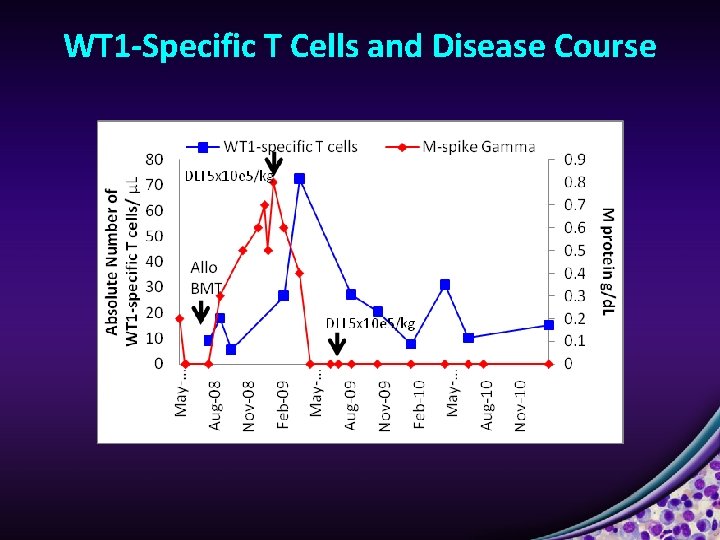
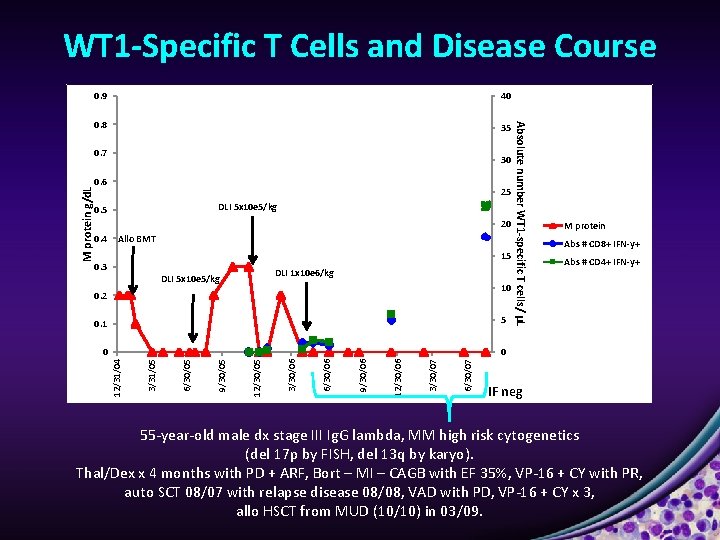
WT 1 -Specific T Cells and Disease Course

WT 1 -Specific T Cells and Disease Course 40 0. 8 35 M protein g/d. L 0. 7 30 0. 6 25 DLI 5 x 10 e 5/kg 0. 5 20 0. 4 Allo BMT 15 0. 3 DLI 1 x 10 e 6/kg DLI 5 x 10 e 5/kg 10 0. 2 6/30/07 3/30/07 12/30/06 9/30/06 6/30/06 3/30/06 12/30/05 9/30/05 0 6/30/05 0 3/31/05 5 12/31/04 0. 1 Absolute number WT 1 -specific T cells/ μL 0. 9 M protein Abs # CD 8+ IFN-y+ Abs # CD 4+ IFN-y+ IF neg 55 -year-old male dx stage III Ig. G lambda, MM high risk cytogenetics (del 17 p by FISH, del 13 q by karyo). Thal/Dex x 4 months with PD + ARF, Bort – MI – CAGB with EF 35%, VP-16 + CY with PR, auto SCT 08/07 with relapse disease 08/08, VAD with PD, VP-16 + CY x 3, allo HSCT from MUD (10/10) in 03/09.

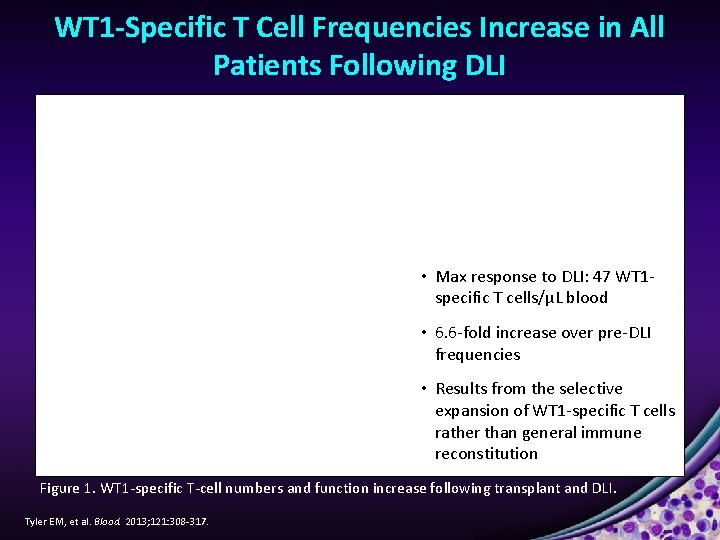
WT 1 -Specific T Cell Frequencies Increase in All Patients Following DLI • Max response to DLI: 47 WT 1 specific T cells/μL blood • 6. 6 -fold increase over pre-DLI frequencies • Results from the selective expansion of WT 1 -specific T cells rather than general immune reconstitution Figure 1. WT 1 -specific T-cell numbers and function increase following transplant and DLI. Tyler EM, et al. Blood. 2013; 121: 308 -317.

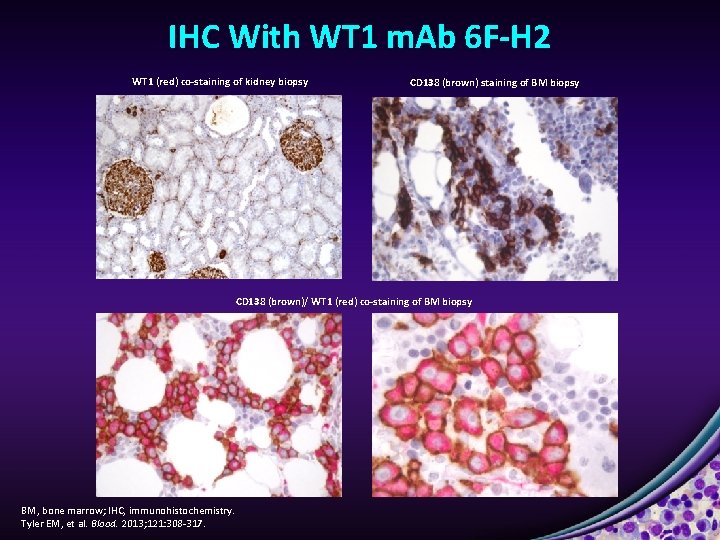
IHC With WT 1 m. Ab 6 F-H 2 WT 1 (red) co-staining of kidney biopsy CD 138 (brown) staining of BM biopsy CD 138 (brown)/ WT 1 (red) co-staining of BM biopsy BM, bone marrow; IHC, immunohistochemistry. Tyler EM, et al. Blood. 2013; 121: 308 -317.

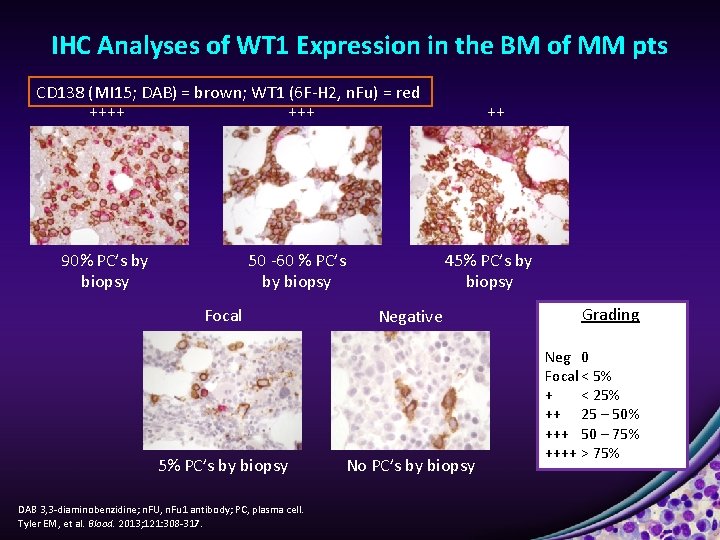
IHC Analyses of WT 1 Expression in the BM of MM pts CD 138 (MI 15; DAB) = brown; WT 1 (6 F-H 2, n. Fu) = red ++++ 90% PC’s by biopsy 50 -60 % PC’s by biopsy Focal 5% PC’s by biopsy DAB 3, 3 -diaminobenzidine; n. FU, n. Fu 1 antibody; PC, plasma cell. Tyler EM, et al. Blood. 2013; 121: 308 -317. ++ 45% PC’s by biopsy Negative No PC’s by biopsy Grading Neg 0 Focal < 5% + < 25% ++ 25 – 50% +++ 50 – 75% ++++ > 75%

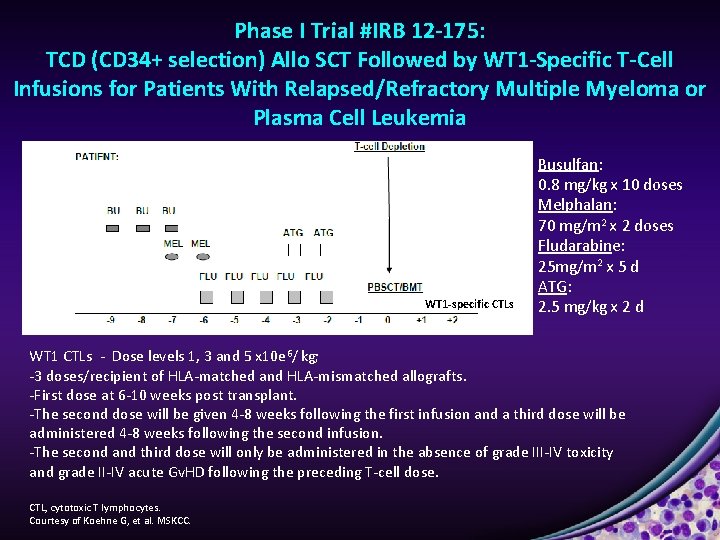
Phase I Trial #IRB 12 -175: TCD (CD 34+ selection) Allo SCT Followed by WT 1 -Specific T-Cell Infusions for Patients With Relapsed/Refractory Multiple Myeloma or Plasma Cell Leukemia WT WT 1 -specific CTLs W Busulfan: 0. 8 mg/kg x 10 doses Melphalan: 70 mg/m 2 x 2 doses Fludarabine: 25 mg/m 2 x 5 d ATG: 2. 5 mg/kg x 2 d WT 1 CTLs - Dose levels 1, 3 and 5 x 10 e 6/ kg; -3 doses/recipient of HLA-matched and HLA-mismatched allografts. -First dose at 6 -10 weeks post transplant. -The second dose will be given 4 -8 weeks following the first infusion and a third dose will be administered 4 -8 weeks following the second infusion. -The second and third dose will only be administered in the absence of grade III-IV toxicity and grade II-IV acute Gv. HD following the preceding T-cell dose. CTL, cytotoxic T lymphocytes. Courtesy of Koehne G, et al. MSKCC.

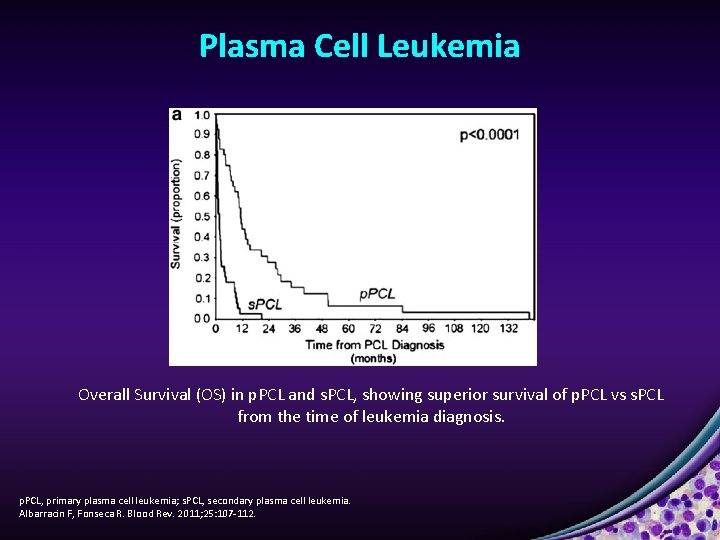
Plasma Cell Leukemia Overall Survival (OS) in p. PCL and s. PCL, showing superior survival of p. PCL vs s. PCL from the time of leukemia diagnosis. p. PCL, primary plasma cell leukemia; s. PCL, secondary plasma cell leukemia. Albarracin F, Fonseca R. Blood Rev. 2011; 25: 107 -112.

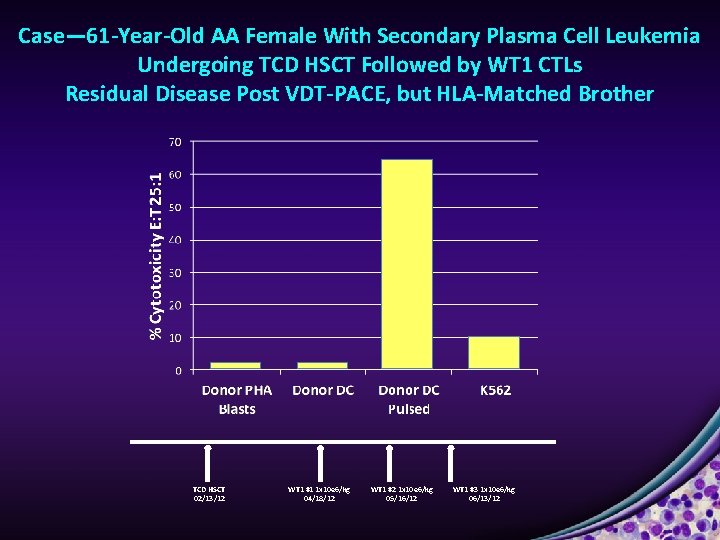
Case― 61 -Year-Old AA Female With Secondary Plasma Cell Leukemia Undergoing TCD HSCT Followed by WT 1 CTLs Residual Disease Post VDT-PACE, but HLA-Matched Brother TCD HSCT 02/13/12 WT 1 #1 1 x 10 e 6/kg 04/18/12 WT 1 #2 1 x 10 e 6/kg 05/16/12 WT 1 #3 1 x 10 e 6/kg 06/13/12

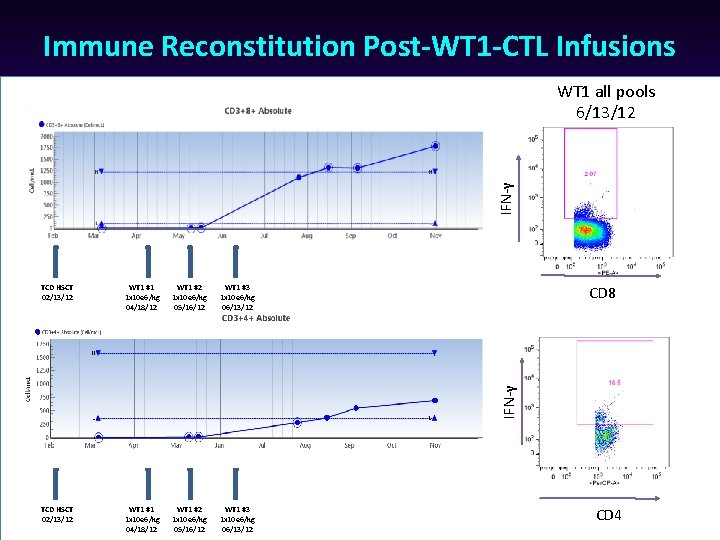
Immune Reconstitution Post-WT 1 -CTL Infusions IFN-γ WT 1 all pools 6/13/12 WT 1 #1 1 x 10 e 6/kg 04/18/12 WT 1 #2 1 x 10 e 6/kg 05/16/12 WT 1 #3 1 x 10 e 6/kg 06/13/12 TCD HSCT 02/13/12 WT 1 #1 1 x 10 e 6/kg 04/18/12 WT 1 #2 1 x 10 e 6/kg 05/16/12 WT 1 #3 1 x 10 e 6/kg 06/13/12 CD 8 IFN-γ TCD HSCT 02/13/12 CD 4

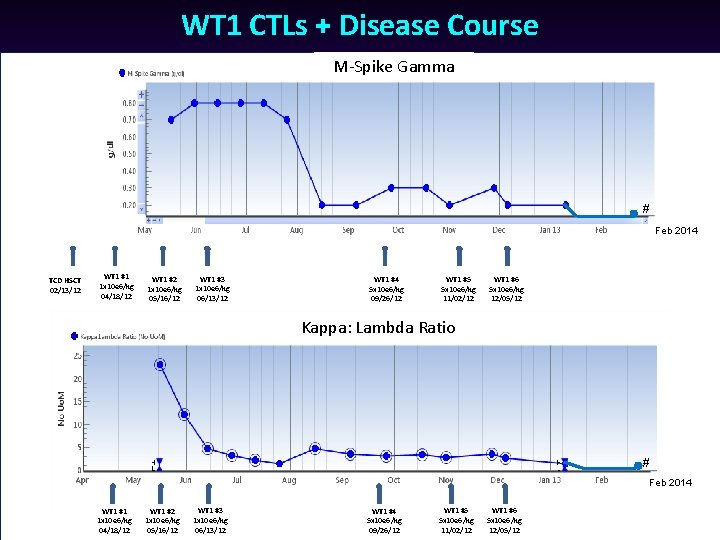
WT 1 CTLs + Disease Course M-Spike Gamma # Feb 2014 TCD HSCT 02/13/12 WT 1 #1 1 x 10 e 6/kg 04/18/12 WT 1 #2 1 x 10 e 6/kg 05/16/12 WT 1 #3 1 x 10 e 6/kg 06/13/12 WT 1 #4 5 x 10 e 6/kg 09/26/12 WT 1 #5 5 x 10 e 6/kg 11/02/12 WT 1 #6 5 x 10 e 6/kg 12/05/12 Kappa: Lambda Ratio # Feb 2014 WT 1 #1 1 x 10 e 6/kg 04/18/12 WT 1 #2 1 x 10 e 6/kg 05/16/12 WT 1 #3 1 x 10 e 6/kg 06/13/12 WT 1 #4 5 x 10 e 6/kg 09/26/12 WT 1 #5 5 x 10 e 6/kg 11/02/12 WT 1 #6 5 x 10 e 6/kg 12/05/12


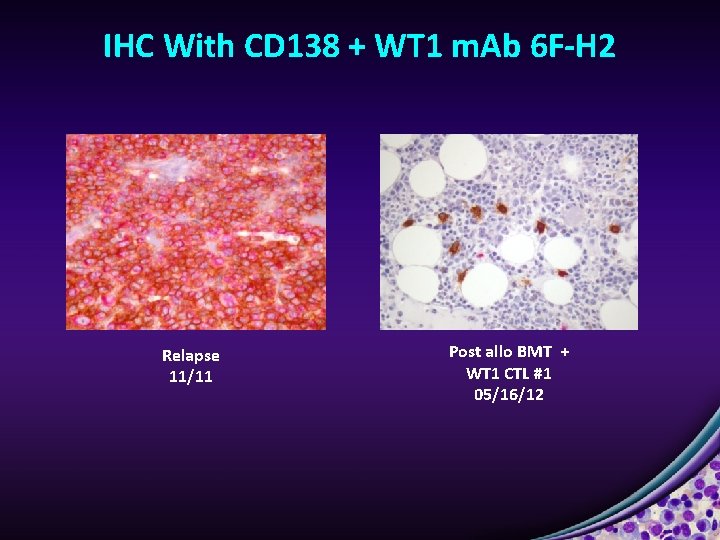
IHC With CD 138 + WT 1 m. Ab 6 F-H 2 Relapse 11/11 Post allo BMT + WT 1 CTL #1 05/16/12

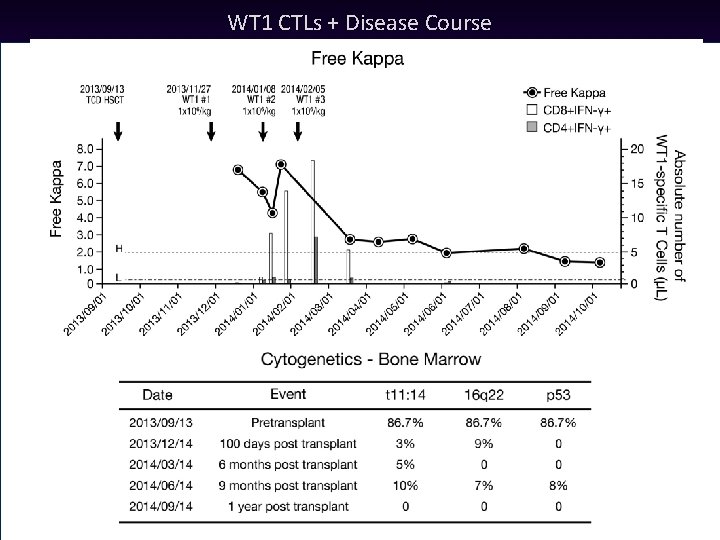
WT 1 CTLs + Disease Course

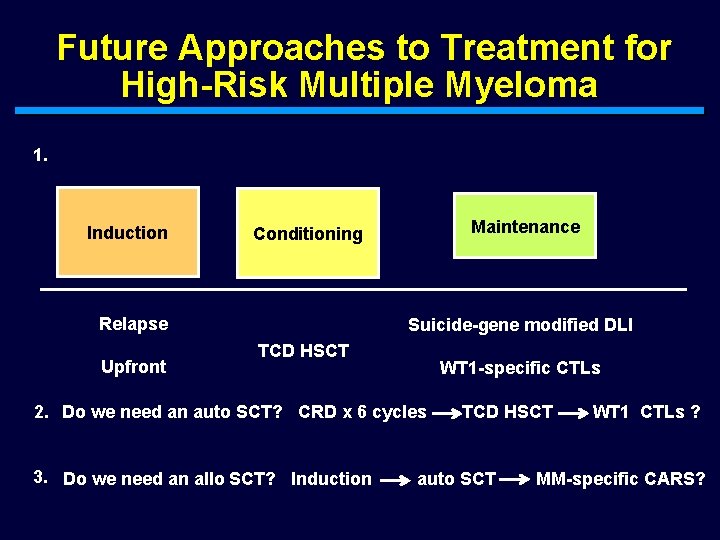
Future Approaches to Treatment for High-Risk Multiple Myeloma 1. Induction Relapse Upfront Maintenance Conditioning Suicide-gene modified DLI TCD HSCT WT 1 -specific CTLs 2. Do we need an auto SCT? CRD x 6 cycles 3. Do we need an allo SCT? Induction TCD HSCT auto SCT WT 1 CTLs ? MM-specific CARS?

Acknowledgement Research Team Myeloma Service Heather Landau MD Hani Hassoun MD Alex Lesokhin MD Nikoletta Lendvai MD Ph. D David Chung MD, Ph. D Sergio Giralt MD Ola Landgren, MD Eleanor Tyler, Ph. D, Cornell Weill College Achim Jungbluth, MD, Pathology, MSKCC Denise Frosina, Senior Research Technician Sean Devlin, Ph. D, Biostatistics, MSKCC Evelyn Orlando, RSA Eric Smith, MD, Ph. D Satya Kosuri, MD Adoptive Immune Cell Therapy Facility (AICT lab) Ekaterina Doubrovina MD Ph. D Richard O’Reilly, MD Otsuka Pharmaceutical Co, Ltd – for generous research support