How are an atoms electrons configured Section 3
































- Slides: 46

How are an atom’s electrons configured? Section 3. 3 1

Electrons n Most important part of an atom to chemists. n Light emission n Electromagnetic spectrum 2

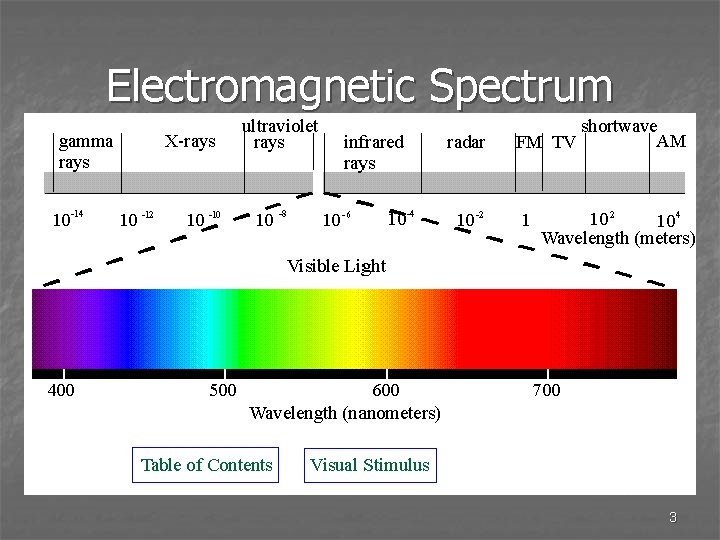
Electromagnetic Spectrum 3

Light is an Electromagnetic Wave n Prism n Waves can be described using speed, wavelength, and frequency. 4

Light Travel n Light travels at a constant speed. n The speed of light equals 8 2. 998 x 10 m/s n 500 seconds to travel 150 million km from sun to Earth 5

Wavelength n Wavelength – distance between 2 consecutive peaks or troughs of a wave (measured in meters) n Range from 10 -13 for gamma rays to over 105 for radio waves 6

Frequency n Frequency – the number of waves that pass thru a stationary point in one second n One wave per second is a hertz, Hz, (the unit for frequency) n Ranges from less than 1000 Hz to more than 1022 Hz 7

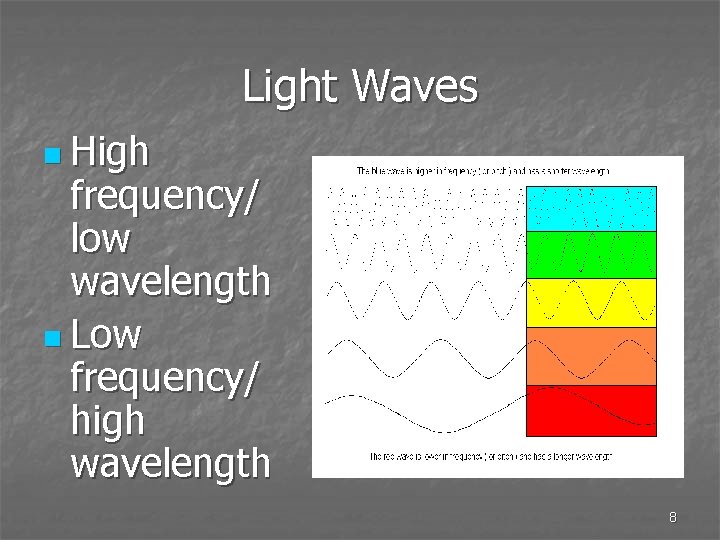
Light Waves n High frequency/ low wavelength n Low frequency/ high wavelength 8

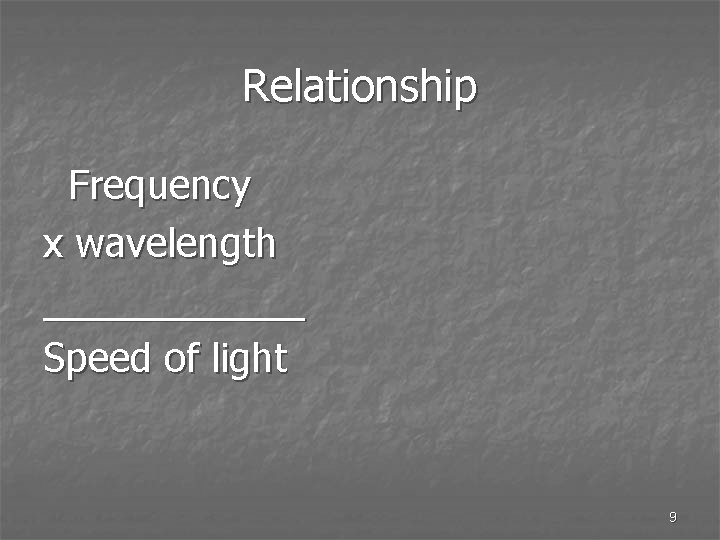
Relationship Frequency x wavelength ______ Speed of light 9

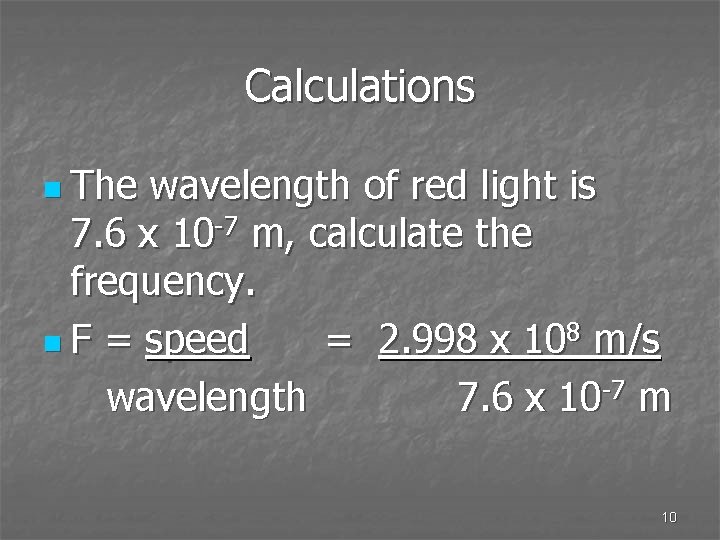
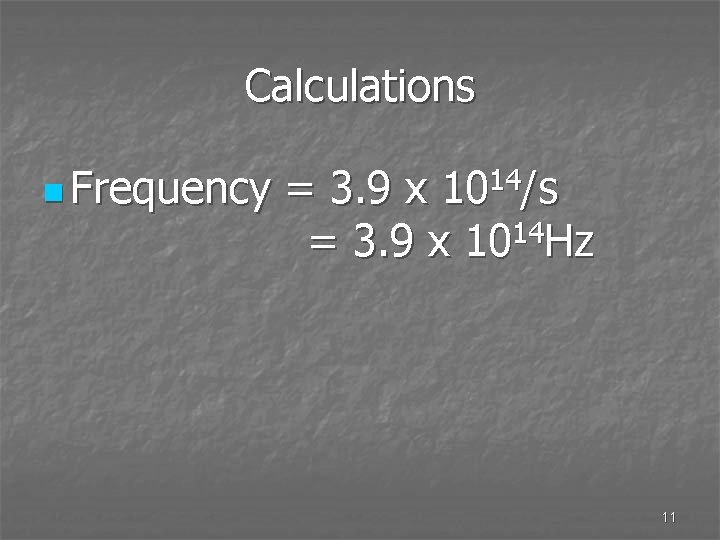
Calculations n The wavelength of red light is 7. 6 x 10 -7 m, calculate the frequency. n F = speed = 2. 998 x 108 m/s wavelength 7. 6 x 10 -7 m 10

Calculations n Frequency = 3. 9 x 1014/s 14 = 3. 9 x 10 Hz 11

Line Emission Spectra n Purple hydrogen gas n Splits and sent to prism n Line-emission spectra n Lines of colored light produced when the light from excited atoms of an element is passed thru a prism 12

Excited electrons emit light n Bohr theory – an electron can exist in different energy states n Electrons can move to different energy levels. n Gain energy n Lose energy 13

States n Ground state – lowest possible energy state an e- can be in n Excited state – e- can move here when it gains energy 14

Excited State n Unstable State n Higher energy than ground state n Electrons can “fall” from excited state to ground state and give off energy in the form of light 15

Excited State n When the electron gives off energy, it is associated with a frequency that reflects the energy released. n We see a color which produces the line emission spectra. 16

Energy of an electron n Energy of an electron = -2. 179 x 10 -18 J n 2 n n is any positive whole number 17

Quantum Number n If n has only certain values it can be, it is called a quantum number n The energy is said to be quantized n The effects are only apparent at the atomic level 18

Concept Check 1. 2. 3. What is the line emission spectra? What does it mean to say that energy is quantized. Look at fig 3 -20. What wavelengths and frequencies define light in the visible spectrum? 19

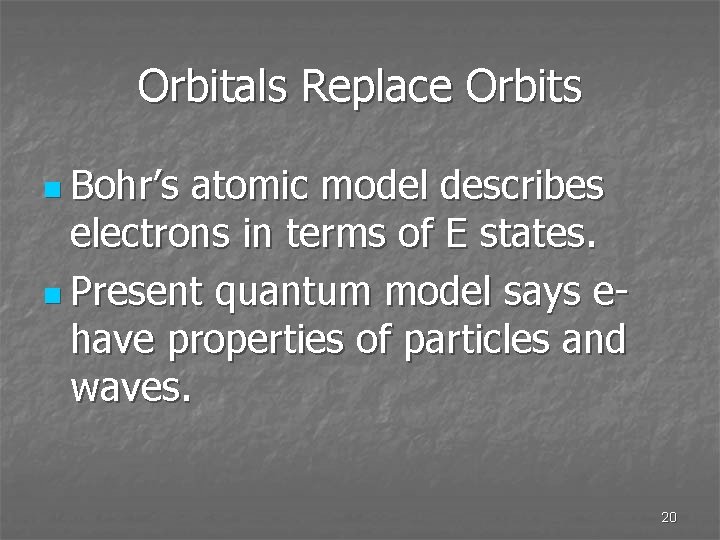
Orbitals Replace Orbits n Bohr’s atomic model describes electrons in terms of E states. n Present quantum model says ehave properties of particles and waves. 20

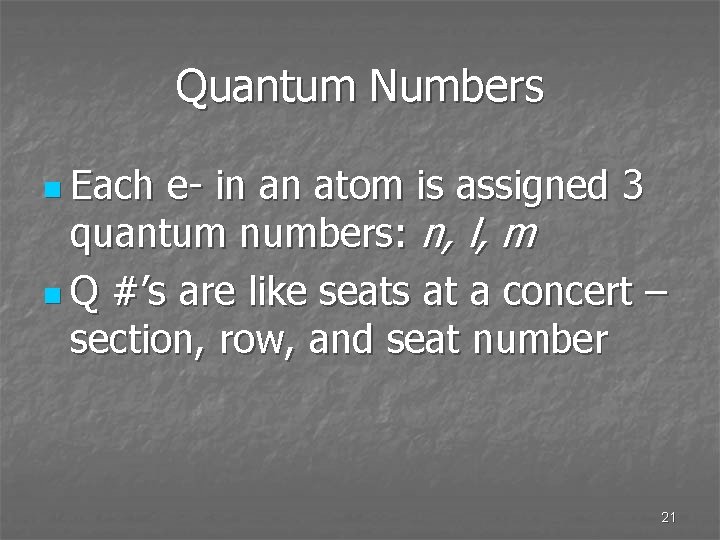
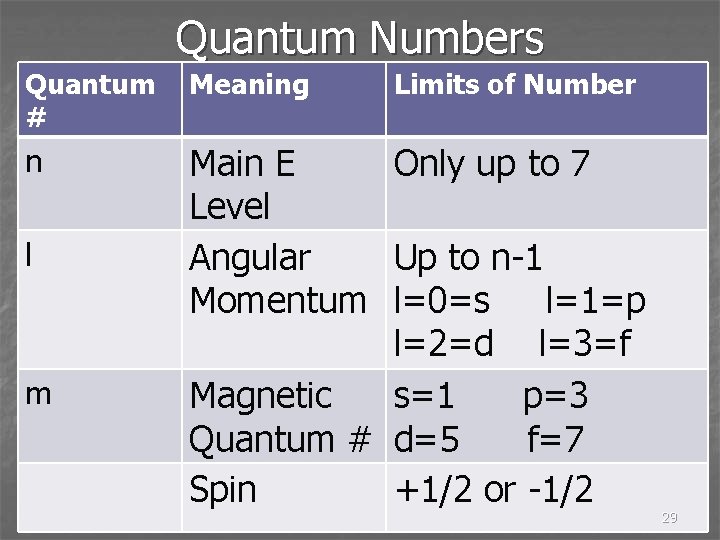
Quantum Numbers n Each e- in an atom is assigned 3 quantum numbers: n, l, m n Q #’s are like seats at a concert – section, row, and seat number 21

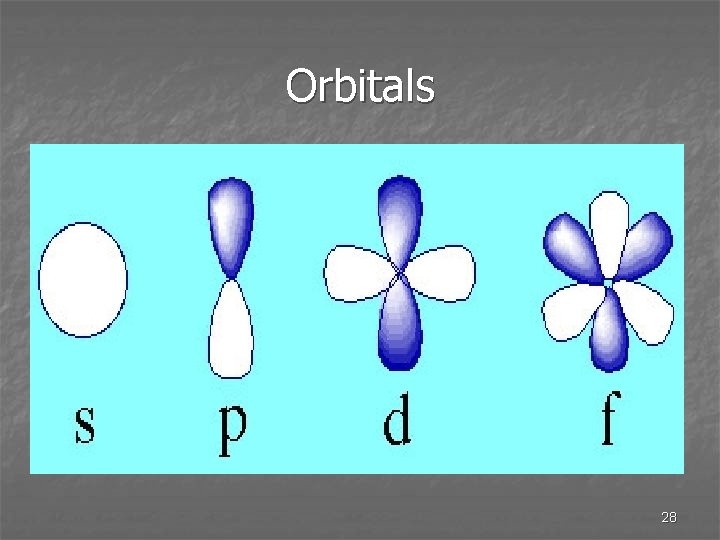
Orbitals n e- are located in orbitals n Orbitals are regions in space that you are likely to find an en They are like e- clouds n Only certain combinations are acceptable. 22

Rules for Assigning Quantum #’s n Principal quantum number – n n n has to be a whole number and typically is not higher than 7 n The larger n is, the further from the nucleus the e- is and the higher the energy is 23

Rules for Quantum Numbers n The l quantum number can be a whole number between 0 and n-1 n If n = 3, l can be 0, 1, or 2 24

l Quantum Number n When l = 0, it is called the s orbital. l = 1, is the p orbital, l =2 is the d orbital, and l=3 is f orbital n If n = 3 and l = 1, it is the 3 p orbital. n 3 p electron 25

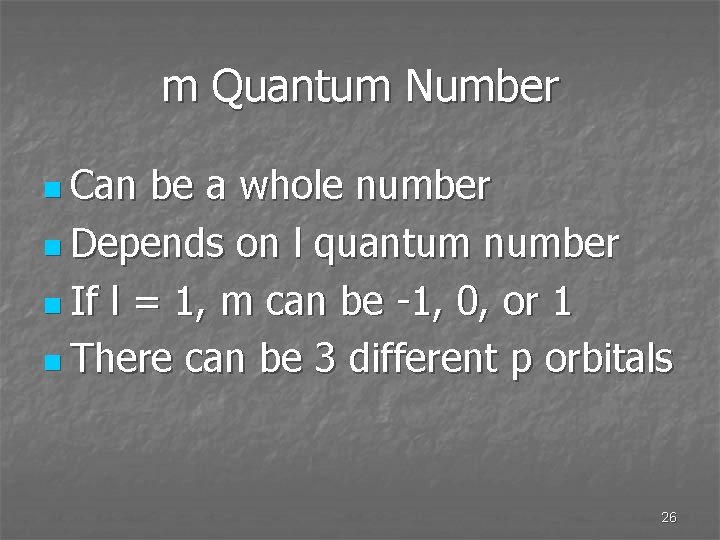
m Quantum Number n Can be a whole number n Depends on l quantum number n If l = 1, m can be -1, 0, or 1 n There can be 3 different p orbitals 26

l and m quantum numbers n They indicate shapes and orientations of the orbitals. n Quantum theory tells us the exact energy, but not the exact location of the e-. It only tells the probability of the location of the e 27

Orbitals 28

Quantum Numbers Quantum # Meaning n Main E Only up to 7 Level Angular Up to n-1 Momentum l=0=s l=1=p l=2=d l=3=f Magnetic s=1 p=3 Quantum # d=5 f=7 Spin +1/2 or -1/2 l m Limits of Number 29

Pauli Exclusion Principle n No more than two e- can occupy a single orbital at a time n e- spin in opposite directions n Spin quantum number, m n m can be +½ or -½ 30

Electron Configuration n. A description of the electron orbitals in an atom n Aufbau principle – electrons in an atom will occupy the lowest energy orbitals available. 31

Electron Configurations n Remember, the smaller the principal quantum number, the lower the energy, and the smaller the l quantum number, the lower the energy 32

Electron Configurations n The order in which energy levels fill is … 1 s<2 s<2 p<3 s<3 p 33

Electron Configurations n After this, the energy levels are less straightforward n The E levels of the 3 d orbitals are slightly higher than those of the 4 s orbitals. 1 s<2 s<2 p<3 s<3 p<4 s≈3 d 34

Electron Configurations n The next irregularity is 5 s and 4 d are close in energy 1 s<2 s<2 p<3 s<3 p<4 s≈3 d<4 p<5 s≈4 d n Still more irregularities exist with higher energy orbitals. 35

Electron Configurations n Tells us how the 16 e- of S are configured n The electron configuration for S is n S=1 s 22 p 63 s 23 p 4 n Each s orbital has 2 e-, each p orbital can have 6 e- (2 per orbital) 36

Electron Configurations n To save space, some configurations are written like n S = [Ne]3 s 23 p 4 n This means take neon’s configuration and add 3 s 23 p 4 to the end of it. 37

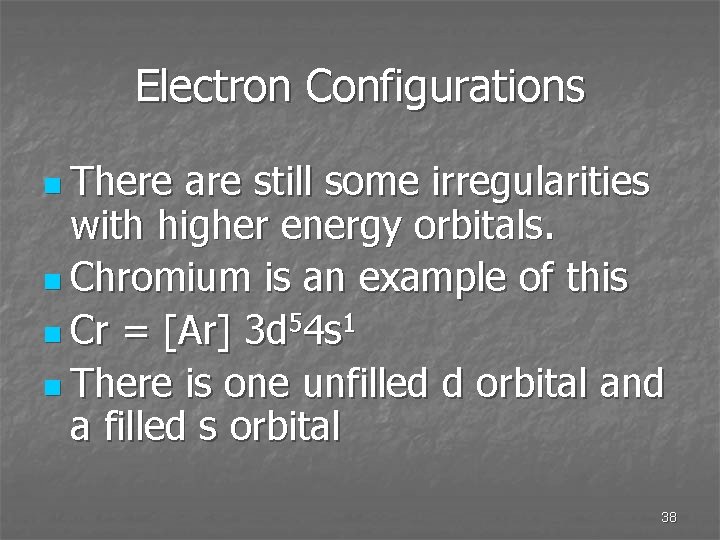
Electron Configurations n There are still some irregularities with higher energy orbitals. n Chromium is an example of this n Cr = [Ar] 3 d 54 s 1 n There is one unfilled d orbital and a filled s orbital 38

Electron Configurations n There are e- configs listed in the p. t. in the back of your book. n These are the ground state configs of the isolated atoms in the gas phase. n Under other conditions, the configs could be different 39

Rules for Writing e- Configs 1. Determine the # of e- the atom has (atomic #) Fluorine as an example has 9 electrons 40

Rules for Writing e- Configs Fill orbitals in order of increasing energy S orbital – 2 e. P orbitals – 6 e. D orbitals – 10 e. F orbitals – 14 e 2. 41

Rules for Writing e- Configs 42

Rules for Writing e- Configs n 3. The configuration for F is 1 s 22 p 5 Make sure the total number of e- in the config match the atomic number 43

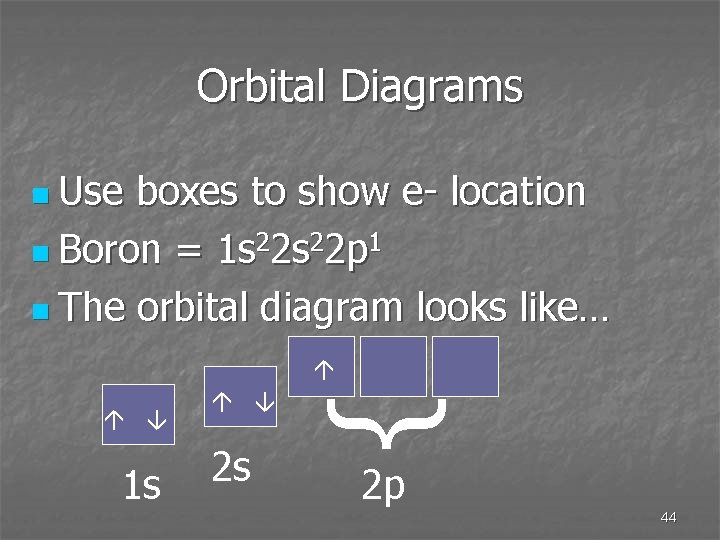
Orbital Diagrams boxes to show e- location n Boron = 1 s 22 p 1 n The orbital diagram looks like… 1 s { 2 s n Use 2 p 44

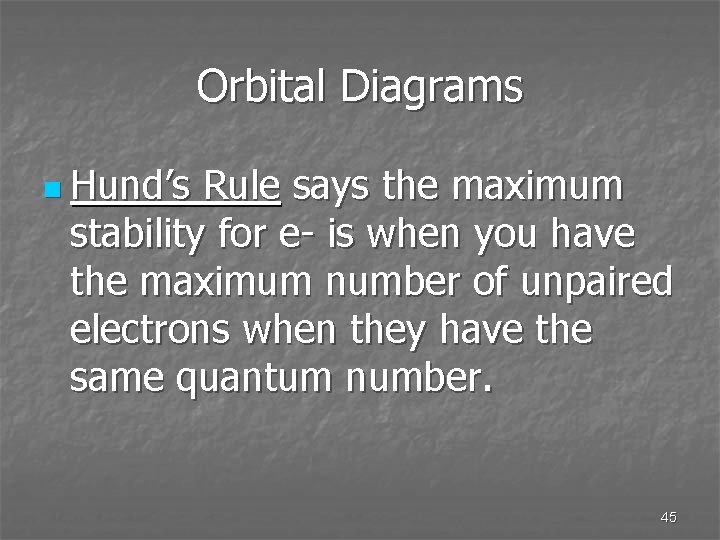
Orbital Diagrams n Hund’s Rule says the maximum stability for e- is when you have the maximum number of unpaired electrons when they have the same quantum number. 45

Orbital Diagrams n What is the Carbon orbital diagram. n The config is 1 s 22 p 2 46