1 Metal atoms lose electrons 2 Nonmetal atoms

















- Slides: 23

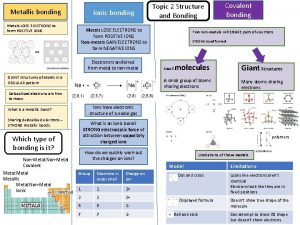
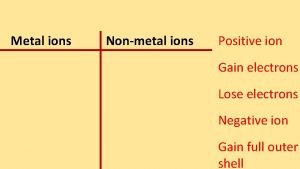
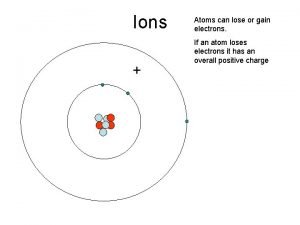
1. Metal atoms lose electrons. 2. Nonmetal atoms can gain electrons. 3. An atom that has gained or lost an electron is an ion. 4. An ionic bond forms between positively and negatively charged ions.

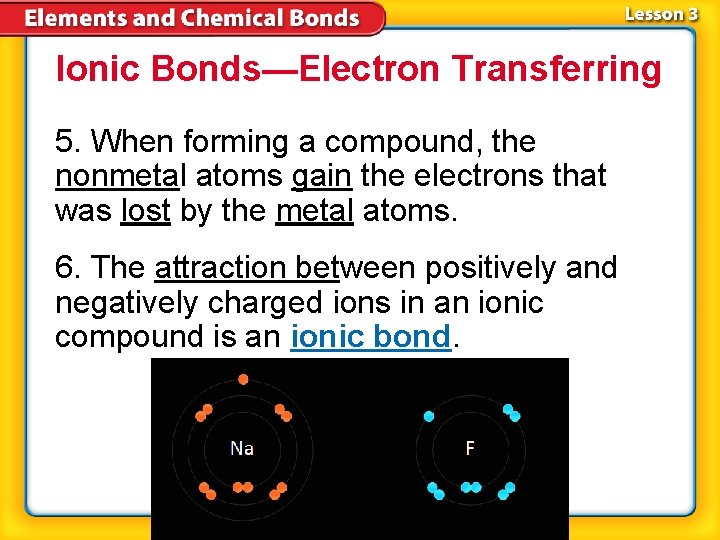
Ionic Bonds—Electron Transferring 5. When forming a compound, the nonmetal atoms gain the electrons that was lost by the metal atoms. 6. The attraction between positively and negatively charged ions in an ionic compound is an ionic bond.

Formation of Cations 7. Metals give away electrons to attain a noble gas configuration. 8. Lose electrons =positive ions (cationsmeow, pawsitive!) 9. Draw a Neon atom

10. 11. 11+

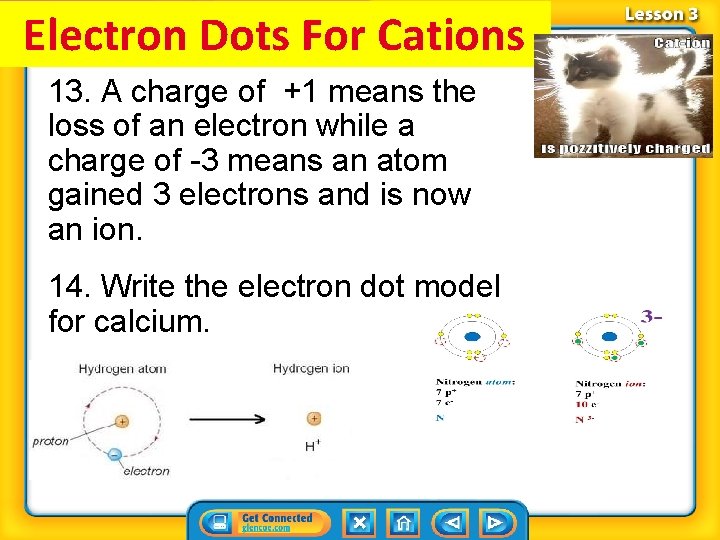
Electron Dots For Cations 13. A charge of +1 means the loss of an electron while a charge of -3 means an atom gained 3 electrons and is now an ion. 14. Write the electron dot model for calcium.

Electron Dots For Cations Did you draw this? Do metals lose or gain electrons? Ca

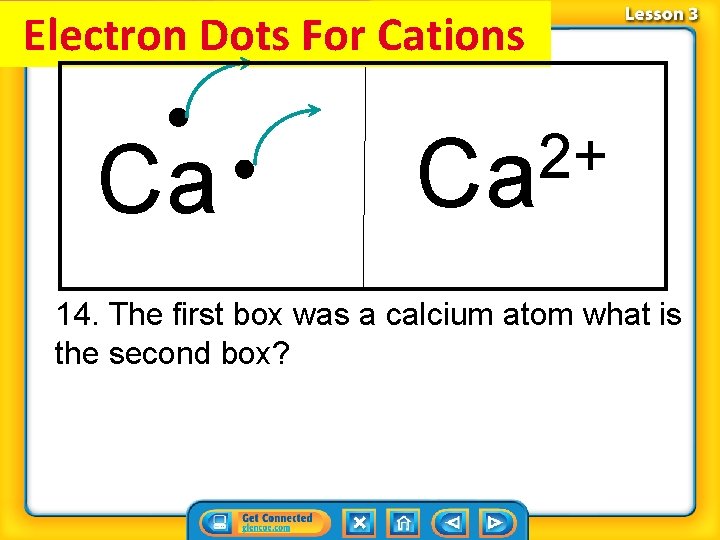
Electron Dots For Cations Ca 2+ Ca 14. The first box was a calcium atom what is the second box?

Formation of Anions 15. Nonmetals gain electrons to look like a noble gas configuration. 16. They “get a minus” or gain electrons so they are negative ions. 17. Draw an Oxygen atom.

Electron Dots For Anions 18. The first box was an oxygen atom what is the second box?


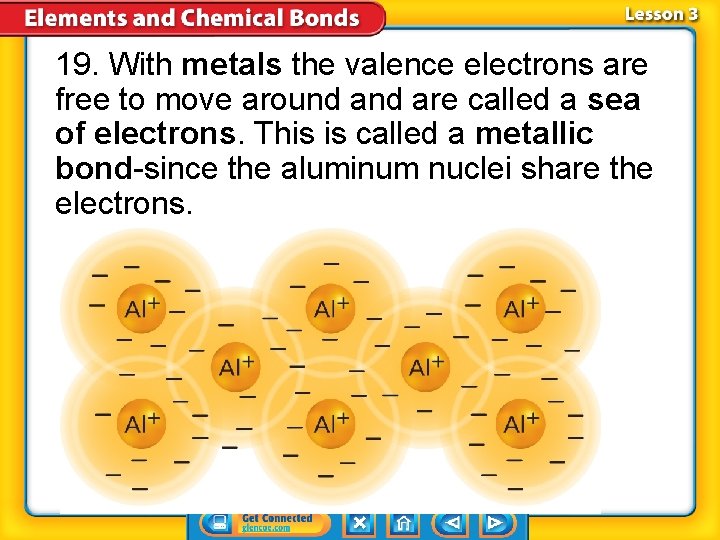
19. With metals the valence electrons are free to move around are called a sea of electrons. This is called a metallic bond-since the aluminum nuclei share the electrons.

21. Which of these describes an atom that is no longer electrically neutral because it has lost or gained valence electrons ? A. covalent compound B. proton C. ion D. molecule

22. What is a bond formed when many metal atoms share their pooled valence electrons? A. ionic bond B. metallic bond C. covalent compound D. ionic compound

23. Which term refers to c? A. valence electron B. positive charge C. chemical bond D. negative charge

24. Which of these is the outermost electron of an atom that participates in chemical bonding? A. valence electron B. nucleus C. negative electrons D. proton

25. Which of these is a group of atoms held together by covalent bonding that acts as an independent unit? A. electron B. proton C. molecule D. valence electrons

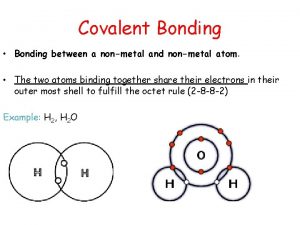
Which of these describes a chemical bond formed when two atoms share one or more pairs of valence electrons? A. polar molecule B. covalent bond C. chemical compound D. none of these

A metallic bond is a bond formed when many metal atoms share what? A. their pooled valence electrons B. ions C. charged ions D. molecules

Which of these is a model that represents valence electrons in an atom as dots around the element’s chemical symbol? A. periodic table B. energy level C. atomic number D. electron dot diagram

Which term describes atoms with unpaired dots in their electron dot diagrams? A. reactive B. chemically unstable C. chemically stable D. A and B

Which of these describes a group of chemical symbols and numbers that represent the elements and the number of atoms of each element that make up a compound? A. chemical bond B. chemical formula C. covalent bond D. molecule

Once an atom gains or loses electrons, it becomes which of these? A. charged ion B. covalent compound C. molecule D. proton

Which of these refers to the attraction between positively and negatively charged ions in an ionic compound? A. metallic bond B. ionic bond C. covalent compound D. none of these
 Win win situacija
Win win situacija Lose-win the doormat
Lose-win the doormat Win win win lose lose lose
Win win win lose lose lose When a metal reacts with a nonmetal the metal will
When a metal reacts with a nonmetal the metal will What is the oxidation number of lithium
What is the oxidation number of lithium Metals lose electrons to form
Metals lose electrons to form How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Metallic bond formula
Metallic bond formula Is sucrose a metal or nonmetal
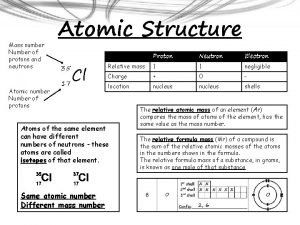
Is sucrose a metal or nonmetal Neutrons
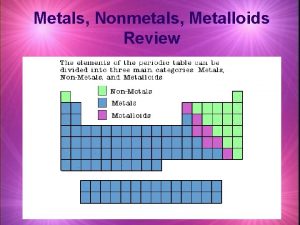
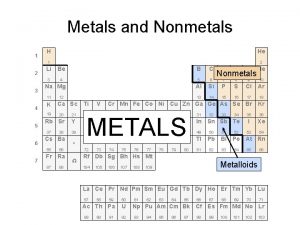
Neutrons Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Substances
Substances Metals vs metalloids and nonmetals
Metals vs metalloids and nonmetals Metal and non metal example
Metal and non metal example Metals are used
Metals are used Metals nonmetals and metalloids chart
Metals nonmetals and metalloids chart Carbon nonmetal
Carbon nonmetal Is co a metal or nonmetal
Is co a metal or nonmetal Metal or nonmetal periodic table
Metal or nonmetal periodic table Metals vs nonmetals vs metalloids
Metals vs nonmetals vs metalloids Example of a lose lose situation
Example of a lose lose situation Win win situation examples
Win win situation examples Metals lose electrons
Metals lose electrons Does carbon gain or lose electrons
Does carbon gain or lose electrons