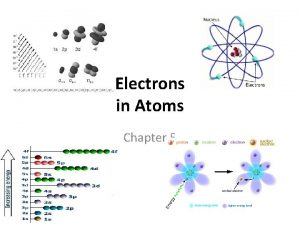
Electrons in Atoms Chapter 5 Chemistry 11 Early















![Exceptions to predicted configurations • Chromium- [Ar] 4 s 13 d 5 • Copper Exceptions to predicted configurations • Chromium- [Ar] 4 s 13 d 5 • Copper](https://slidetodoc.com/presentation_image_h/0bd266f4b4d85e63144d2de199ff40c6/image-34.jpg)









- Slides: 56

Electrons in Atoms Chapter 5

Chemistry 11 Early Models of the Atom

Ancient Greeks were the first to come up with the idea of atoms. Democritus suggested that all matter was made of tiny indivisible particles called atoms. (Greek “atoma”) Democritus

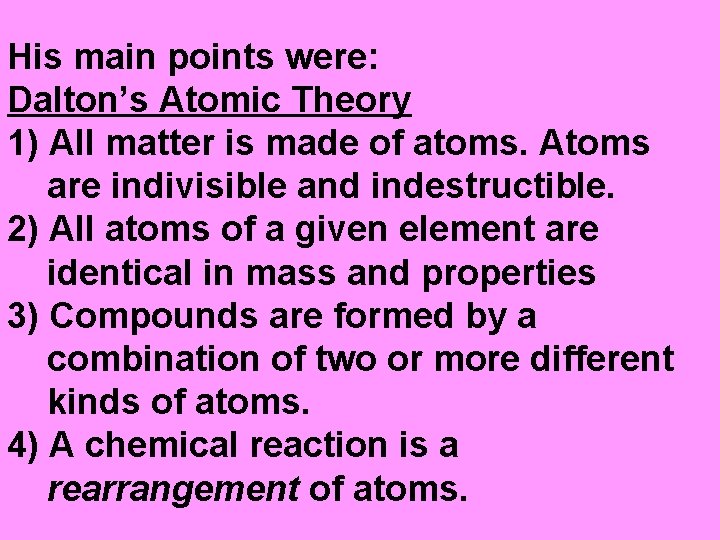
In the early 1800’s, John Dalton came up with the ATOMIC THEORY.

His main points were: Dalton’s Atomic Theory 1) All matter is made of atoms. Atoms are indivisible and indestructible. 2) All atoms of a given element are identical in mass and properties 3) Compounds are formed by a combination of two or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms.

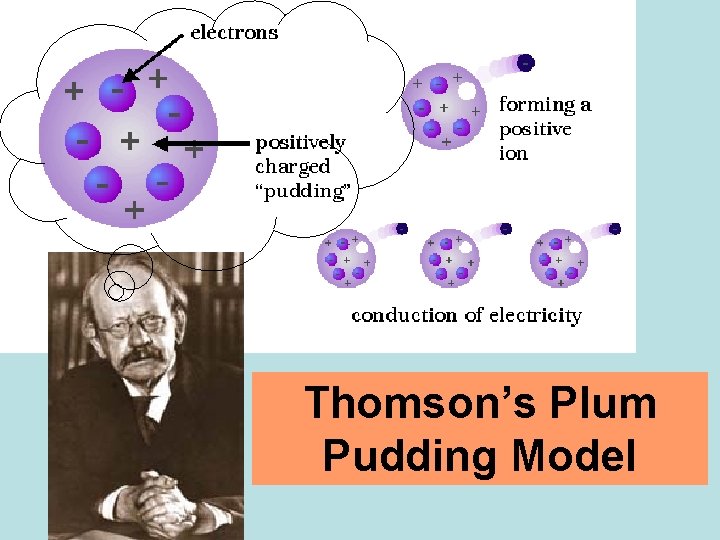
crookes tube J. J. Thomson's Experiments Using Crooke’s tubes and other equipment, J. J. Thomson discovered the electron and measured its e/m (charge to mass) ratio. Later, “e” was found and the mass of an electron was found to be 9. 10938188 × 10 -28 grams (much lighter than H)

Thomson’s Plum Pudding Model

Ernest Rutherford

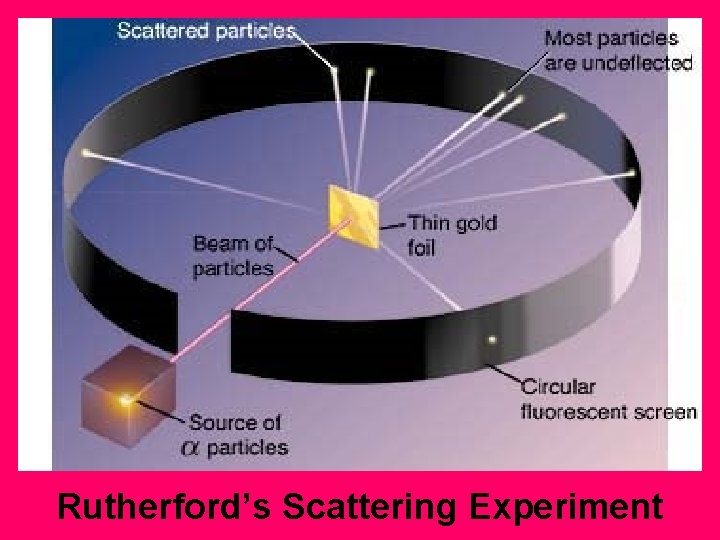
Rutherford’s Scattering Experiment

Rutherford's Experiment

Rutherford could not explain why the electron didn’t fall into the nucleus and destroy the atom.

Neils Bohr

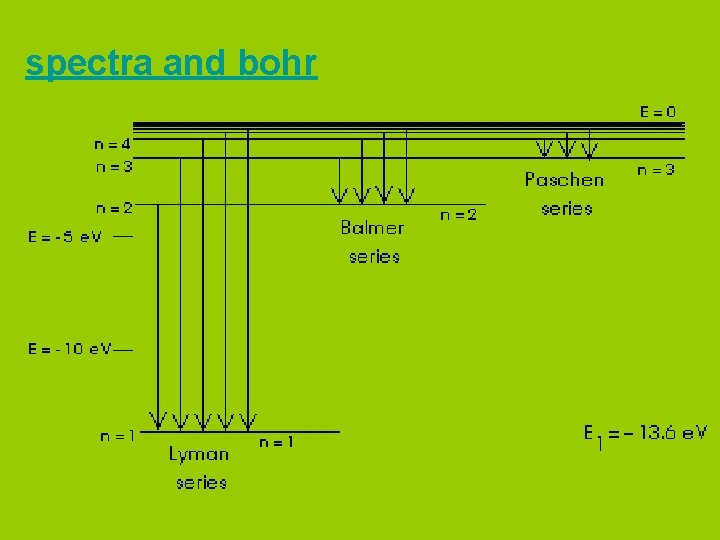
spectra and bohr

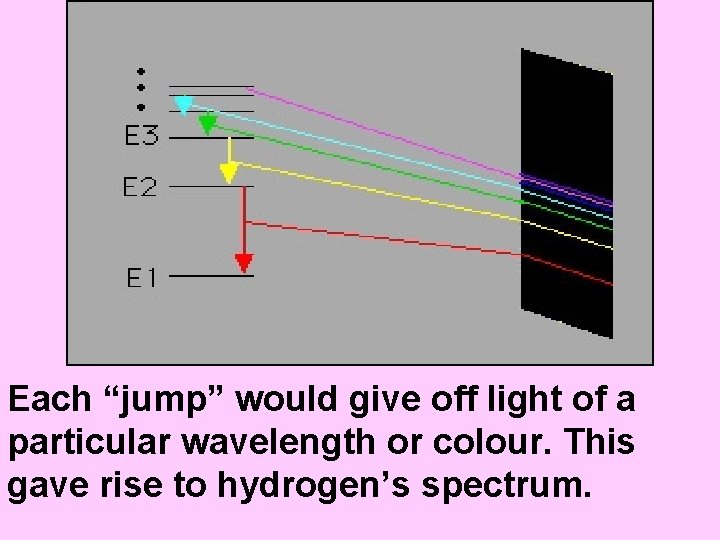
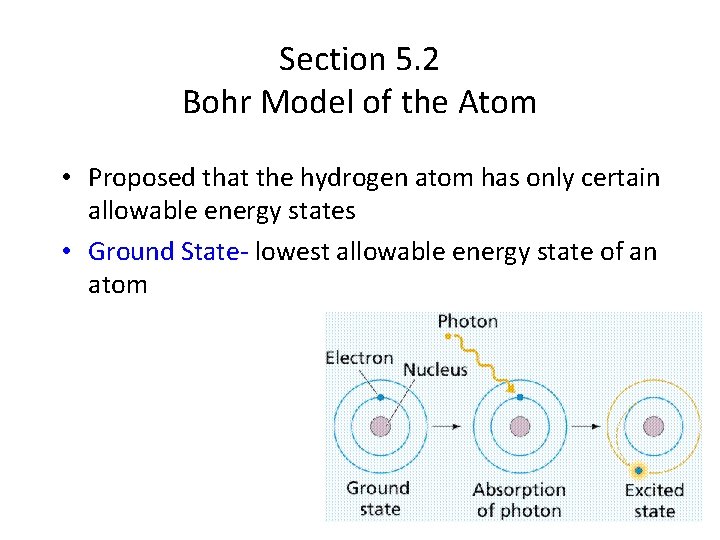
Bohr pictured the hydrogen atom as having discrete energy “levels” which the electron could “inhabit”. In it’s ground state, the electron would be in the lowest level (n=1) When the atom was “excited” the electron could “jump” to a higher level. When the electron came back down, it released energy in the form of light.

Each “jump” would give off light of a particular wavelength or colour. This gave rise to hydrogen’s spectrum.

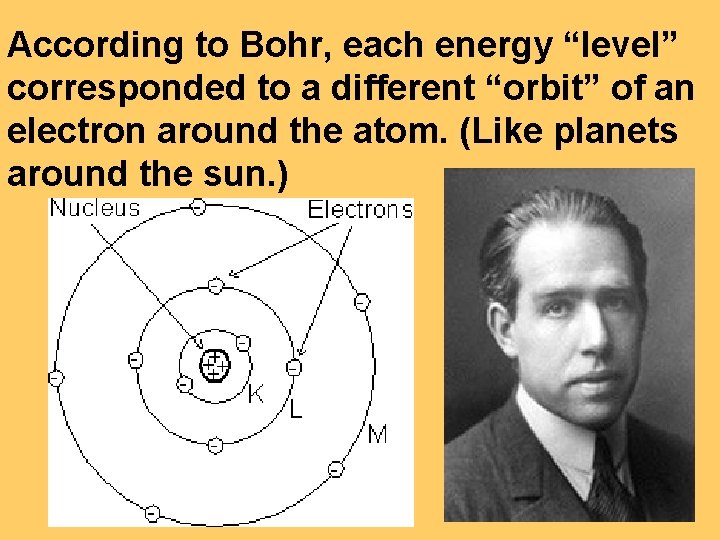
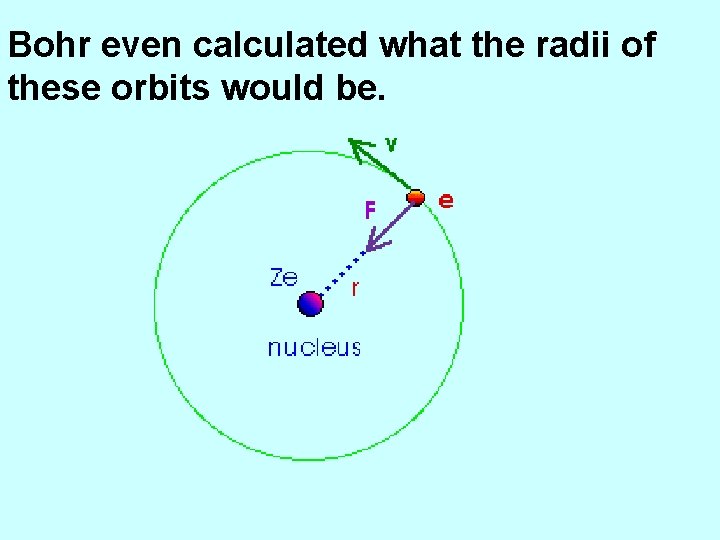
According to Bohr, each energy “level” corresponded to a different “orbit” of an electron around the atom. (Like planets around the sun. )


Bohr even calculated what the radii of these orbits would be.

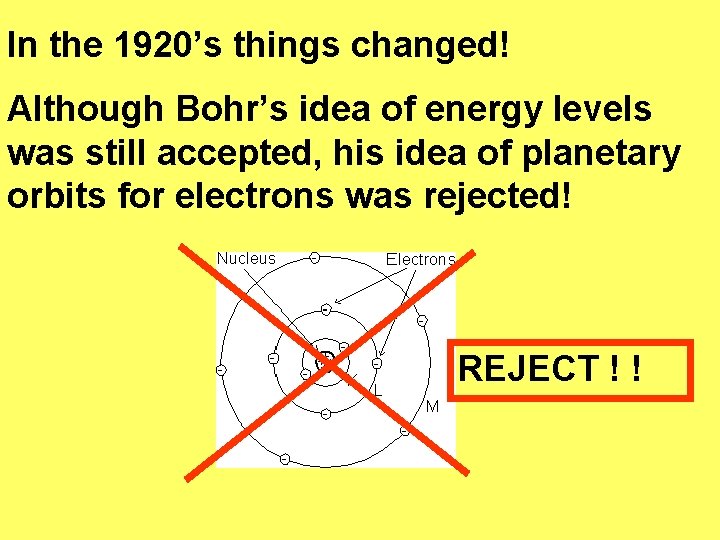
In the 1920’s things changed! Although Bohr’s idea of energy levels was still accepted, his idea of planetary orbits for electrons was rejected! REJECT ! !

The Quantum Mechanical Model (QMM) • 1926 - Austrian physicist Erwin Schrodinger used the results of Rutherford and Bohr to devise and solve a mathematical equation describing the behavior of the electron in a hydrogen atom

• Unlike the Bohr model, the quantum mechanical model does not involve an exact path the electron takes around the nucleus • The quantum mechanical model determines the allowed energies an electron can have and how likely (probability) it is to find the electron in various locations around the nucleus • The cloud is more dense where the probability of finding an electron is high

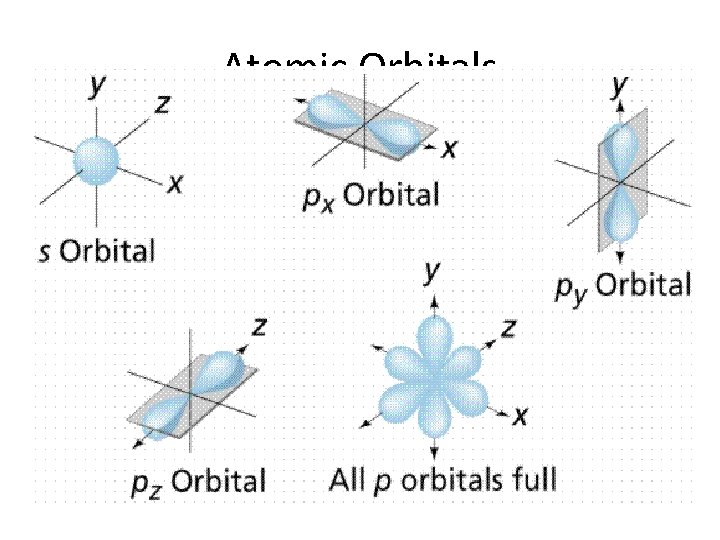
• Atomic Orbital- a 3 D region around the nucleus describing the electron’s probable location

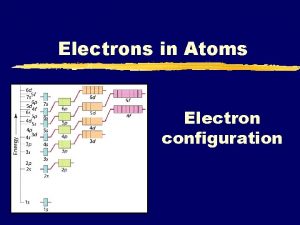
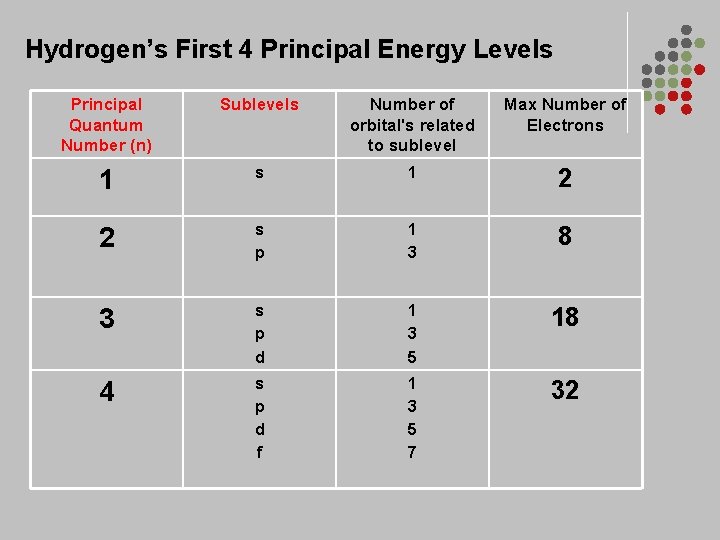
Atomic Orbitals • Energy Levels- are labeled by • Principal Quantum Number (n)- 1, 2, 3, 4… • Within each there are • Energy Sublevels- the energy levels contained with the principal energy level

Atomic Orbitals


Hydrogen’s First 4 Principal Energy Levels Principal Quantum Number (n) Sublevels Number of orbital's related to sublevel Max Number of Electrons 1 s 1 2 2 s p 1 3 8 3 s p d 1 3 5 18 4 s p d f 1 3 5 7 32

Practice read pg 127 -132 Qs 132 on page 1 -7

Electron Arrangement in Atoms • Electrons and the nucleus interact to make the most stable arrangement possible. • Electron Configurations- the ways in which electrons are arranged in various orbitals around the nuclei of atoms

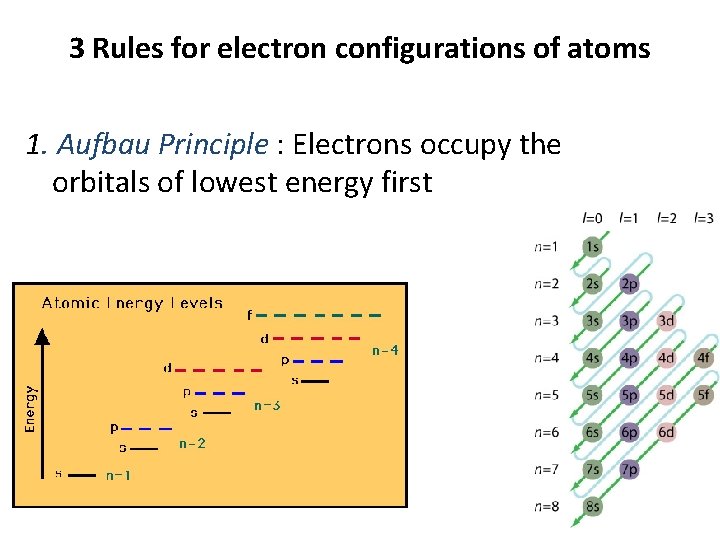
3 Rules for electron configurations of atoms 1. Aufbau Principle : Electrons occupy the orbitals of lowest energy first

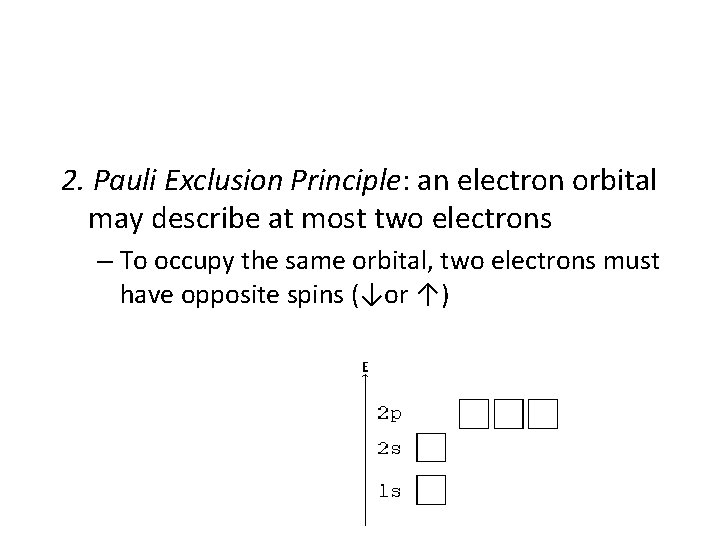
2. Pauli Exclusion Principle: an electron orbital may describe at most two electrons – To occupy the same orbital, two electrons must have opposite spins (↓or ↑)

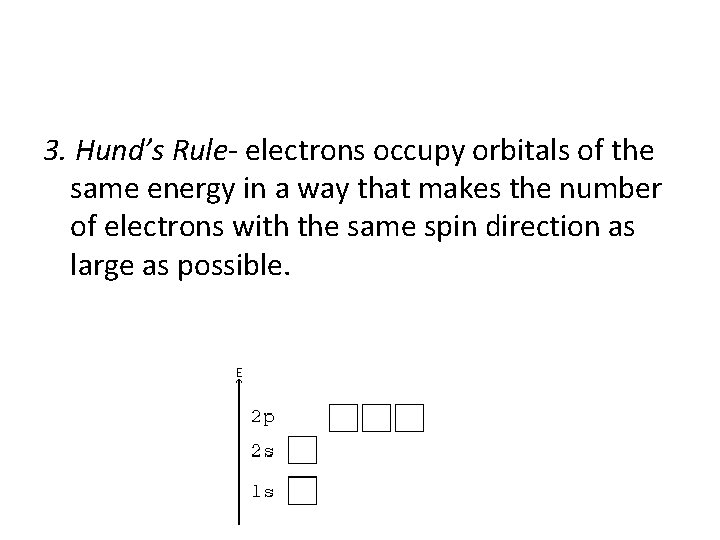
3. Hund’s Rule- electrons occupy orbitals of the same energy in a way that makes the number of electrons with the same spin direction as large as possible.


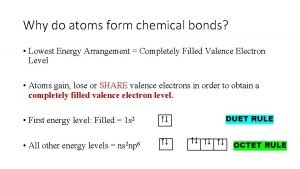
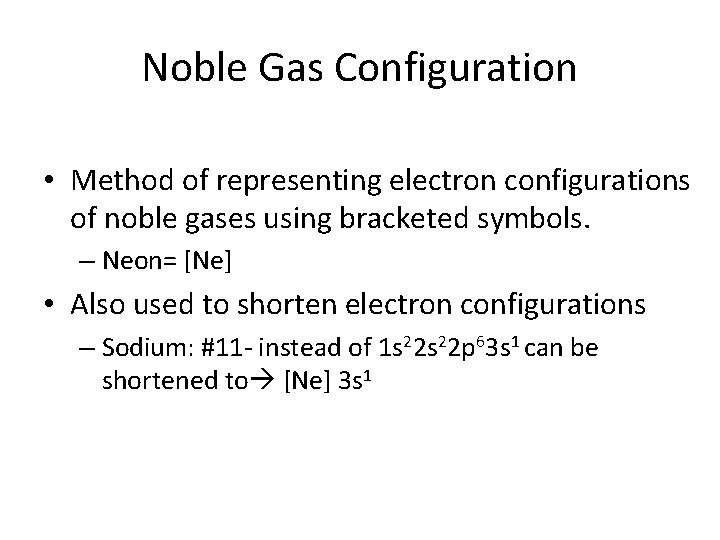
Noble Gas Configuration • Method of representing electron configurations of noble gases using bracketed symbols. – Neon= [Ne] • Also used to shorten electron configurations – Sodium: #11 - instead of 1 s 22 p 63 s 1 can be shortened to [Ne] 3 s 1
![Exceptions to predicted configurations Chromium Ar 4 s 13 d 5 Copper Exceptions to predicted configurations • Chromium- [Ar] 4 s 13 d 5 • Copper](https://slidetodoc.com/presentation_image_h/0bd266f4b4d85e63144d2de199ff40c6/image-34.jpg)
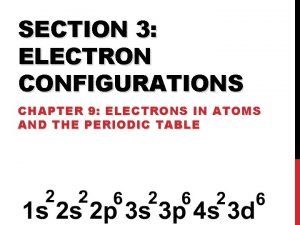
Exceptions to predicted configurations • Chromium- [Ar] 4 s 13 d 5 • Copper - [Ar] 4 s 13 d 10 • Illustrates the increased stability of half-filled and filled sets of s and d orbital's

Valence Electrons (V. E. ) • Electrons in the atom’s outermost orbital's • Determine the chemical properties of an element • V. E. are used in forming chemical bonds


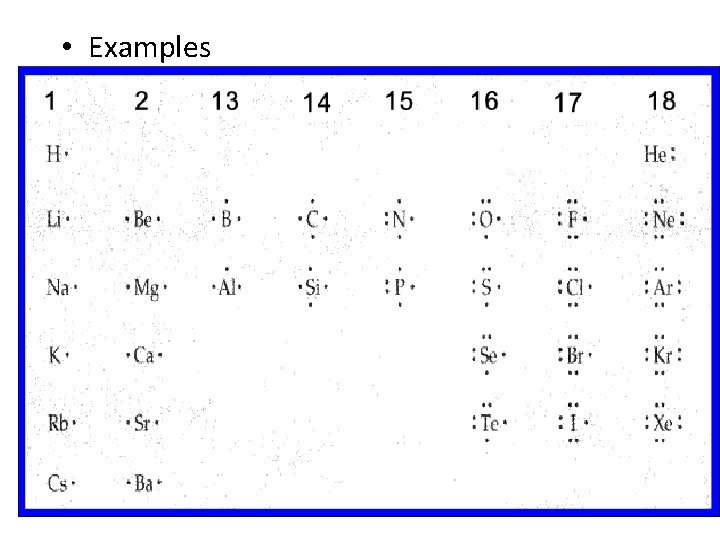
Electron Dot Structures • Consists of the element’s symbol and inner-level electrons surrounded by dots representing the atom’s valence electrons • V. E. are placed one at a time on the four sides of the symbol and then paired up until all are used

• Examples


• Chemists found Rutherford’s nuclear model to be lacking because it did not begin to account for the differences in chemical behavior among various elements • Early 1900’s- scientists observed that certain elements emit visible light when heated in a flame chemical behavior

Wave Nature of Light • Electromagnetic Radiation- form of energy that exhibits wavelike behavior as it travels through space

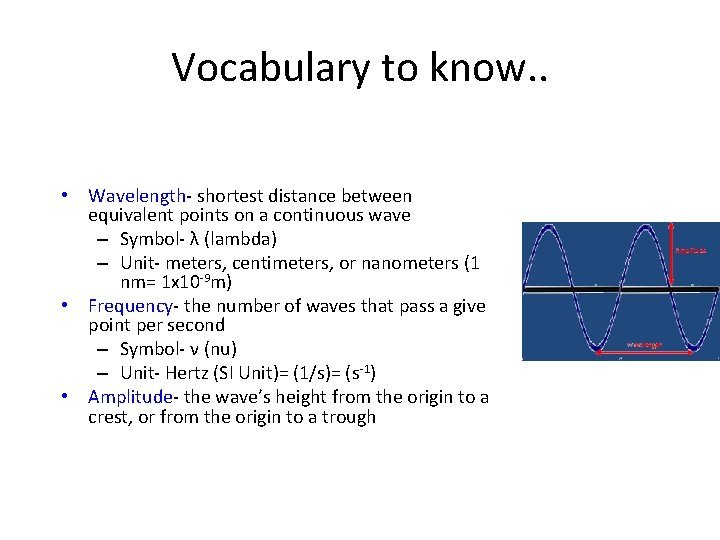
Vocabulary to know. . • Wavelength- shortest distance between equivalent points on a continuous wave – Symbol- λ (lambda) – Unit- meters, centimeters, or nanometers (1 nm= 1 x 10 -9 m) • Frequency- the number of waves that pass a give point per second – Symbol- ν (nu) – Unit- Hertz (SI Unit)= (1/s)= (s-1) • Amplitude- the wave’s height from the origin to a crest, or from the origin to a trough

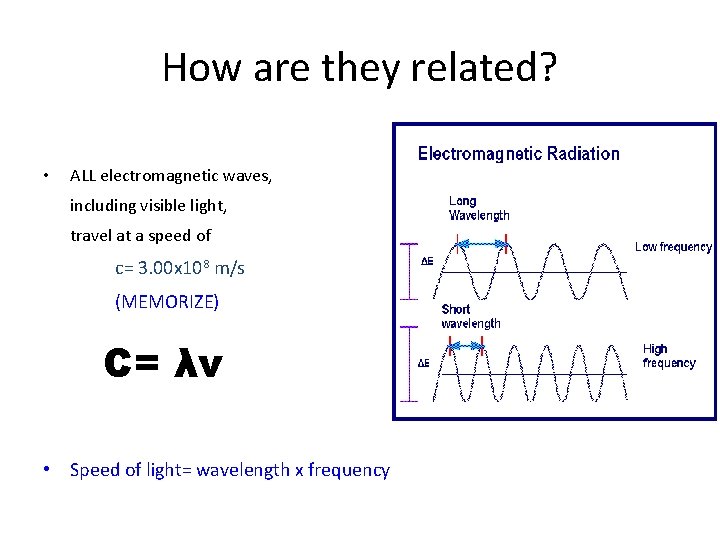
How are they related? • ALL electromagnetic waves, including visible light, travel at a speed of c= 3. 00 x 108 m/s (MEMORIZE) C= λν • Speed of light= wavelength x frequency

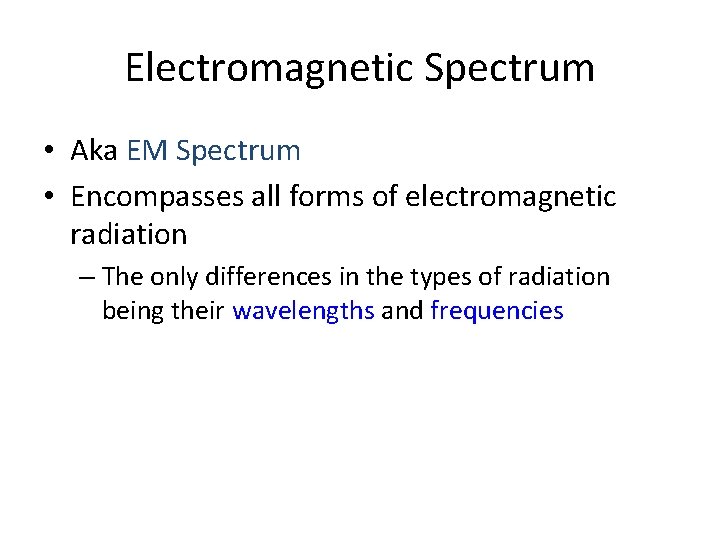
Electromagnetic Spectrum • Aka EM Spectrum • Encompasses all forms of electromagnetic radiation – The only differences in the types of radiation being their wavelengths and frequencies

ROYGBIV


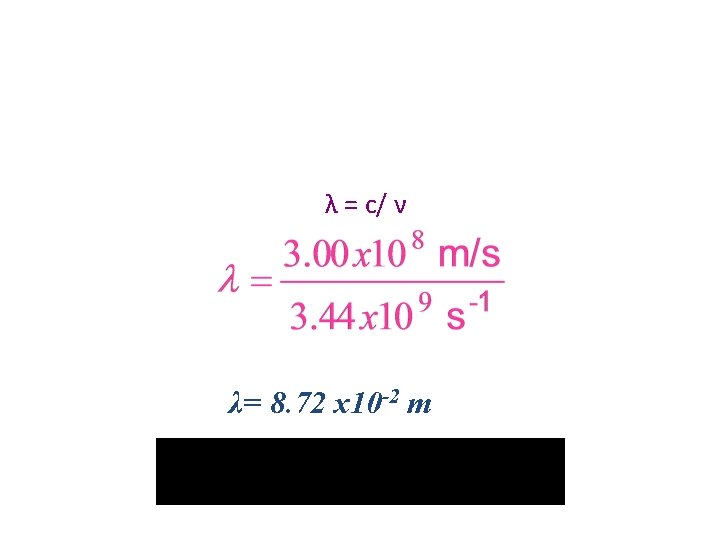
Calculations • Microwaves are used to transmit information. What is the wavelength of a microwave having a frequency of 3. 44 x 109 Hz? – Know: C= λν • C= 3. 00 x 108 m/s • ν = 3. 44 x 109 Hz • λ = ? ? ?

λ = c/ ν λ= 8. 72 x 10 -2 m DON’T FORGET YOUR SIG FIG RULES!!!

Particle Nature of Light • Quantum Concept – Explained why colors of heated matter correspond to different frequencies and wavelengths – Max Plank- “matter can gain or lose only in small, specific amounts called quanta” • Quantum- the minimum amount of energy that can be gained or lost by an atom

• Energy of a quantum is related to the frequency of the emitted radiation by the equation Equantum= hv – E= energy – h = Plank’s Constant (6. 626 x 10 -34 J) – v= frequency – Joule (J)= SI unit for energy

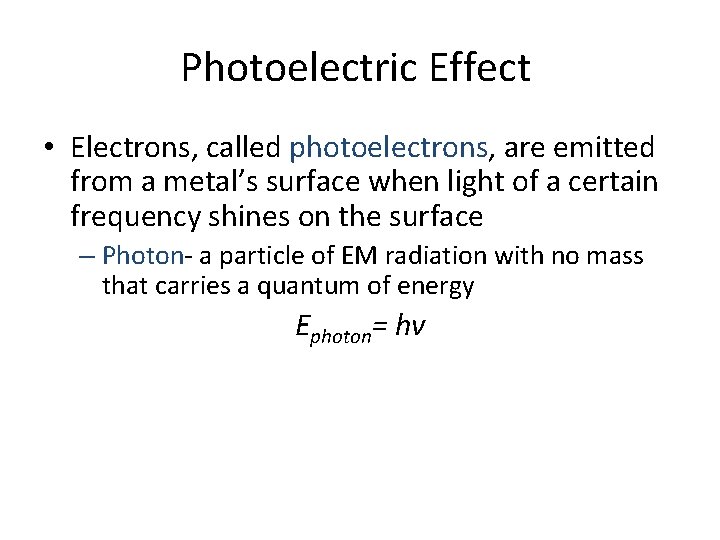
Photoelectric Effect • Electrons, called photoelectrons, are emitted from a metal’s surface when light of a certain frequency shines on the surface – Photon- a particle of EM radiation with no mass that carries a quantum of energy Ephoton= hv

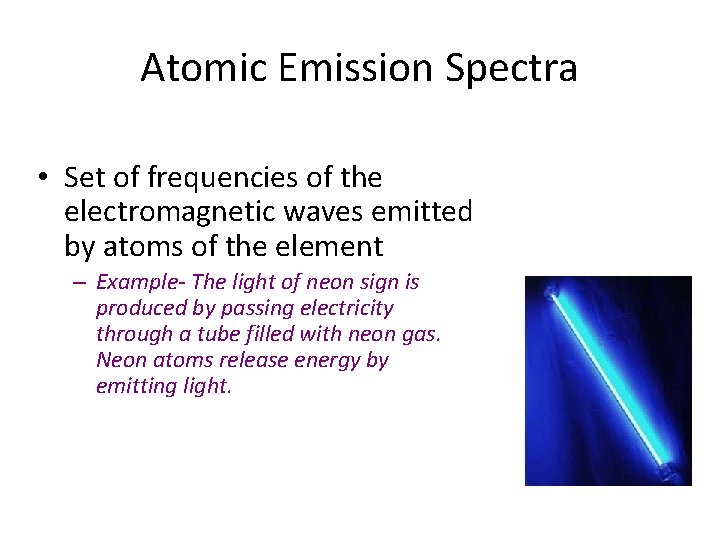
Atomic Emission Spectra • Set of frequencies of the electromagnetic waves emitted by atoms of the element – Example- The light of neon sign is produced by passing electricity through a tube filled with neon gas. Neon atoms release energy by emitting light.

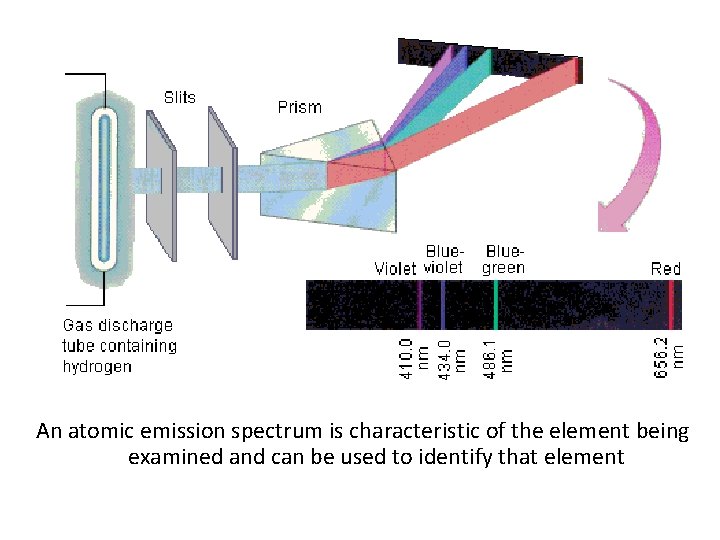
An atomic emission spectrum is characteristic of the element being examined and can be used to identify that element

Section 5. 2 Bohr Model of the Atom • Proposed that the hydrogen atom has only certain allowable energy states • Ground State- lowest allowable energy state of an atom

• Bohr’s model worked well to explain Hydrogen - however it did not explain other elements • Substantial evidence indicates that electrons do not move around the nucleus in circular orbits

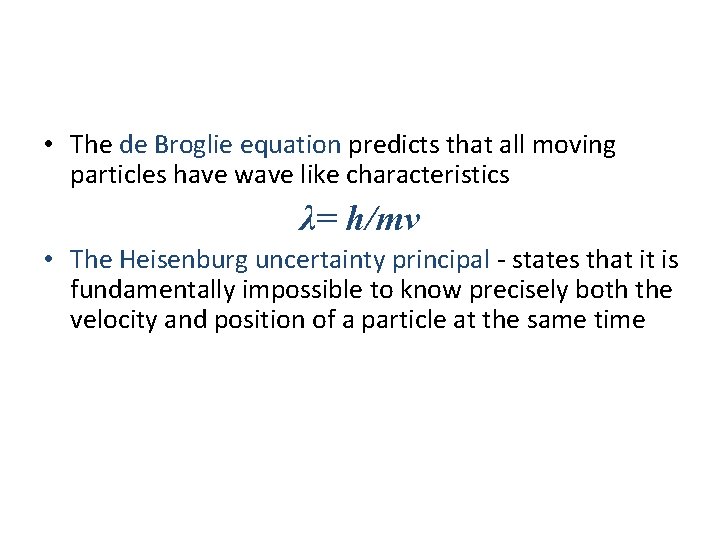
• The de Broglie equation predicts that all moving particles have wave like characteristics λ= h/mv • The Heisenburg uncertainty principal - states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time
 Chapter 4 arrangement of electrons in atoms
Chapter 4 arrangement of electrons in atoms Ccechs
Ccechs Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Atoms with 4 valence electrons
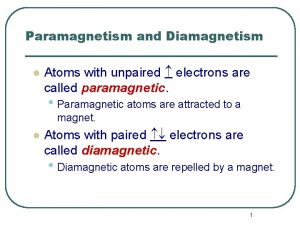
Atoms with 4 valence electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. How to find neutrons on periodic table
How to find neutrons on periodic table Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Quantum mechanical model
Quantum mechanical model Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Atoms tend to gain lose or share electrons
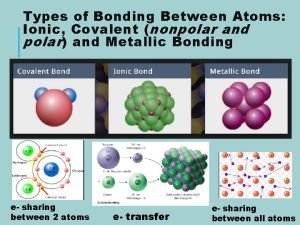
Atoms tend to gain lose or share electrons How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Ionic and metallic bonding chapter 7 practice problems
Ionic and metallic bonding chapter 7 practice problems S orbital
S orbital 5 electrons in atoms
5 electrons in atoms Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Which of the d orbitals most resembles a pz orbital?
Which of the d orbitals most resembles a pz orbital? Chapter 3 atoms the building blocks of matter
Chapter 3 atoms the building blocks of matter Which subatomic particle has the least mass
Which subatomic particle has the least mass Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Types of early childhood programs chapter 2
Types of early childhood programs chapter 2 Ancient india lesson 1 early civilizations
Ancient india lesson 1 early civilizations Chapter 7 early childhood ages 3 through 5
Chapter 7 early childhood ages 3 through 5 Ancient rome and early christianity
Ancient rome and early christianity Lesson 2 early challenges
Lesson 2 early challenges Early childhood is ____ for language learning
Early childhood is ____ for language learning Chapter 11 section 1 early civilizations of africa
Chapter 11 section 1 early civilizations of africa Chapter 14 pre columbian america answers
Chapter 14 pre columbian america answers Chapter 11 political developments in the early republic
Chapter 11 political developments in the early republic Chapter 1 early learning
Chapter 1 early learning Chapter 4 oklahoma in early america
Chapter 4 oklahoma in early america Early humans and the agricultural revolution
Early humans and the agricultural revolution Early humans chapter 1 section 1
Early humans chapter 1 section 1 Chapter 2 early river valley civilizations
Chapter 2 early river valley civilizations Chapter 1 section 3 early british colonies
Chapter 1 section 3 early british colonies Chapter 1 section 3 early british colonies
Chapter 1 section 3 early british colonies Chapter 4 from territory to early statehood vocabulary
Chapter 4 from territory to early statehood vocabulary Chapter 2 early river valley civilizations
Chapter 2 early river valley civilizations Chapter 6 ancient rome and early christianity
Chapter 6 ancient rome and early christianity C3h5 name
C3h5 name Dot ans
Dot ans Relationship between atoms and molecules
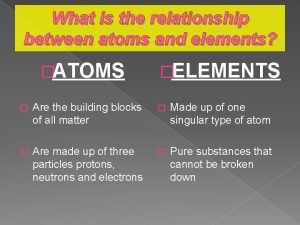
Relationship between atoms and molecules What are atoms?
What are atoms? Keslerscience
Keslerscience Matterville answer key
Matterville answer key Electronegativity trend
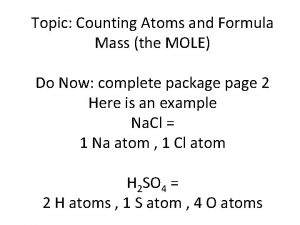
Electronegativity trend Mass formula
Mass formula