Electron Configuration The arrangement of electrons in an


- Slides: 23

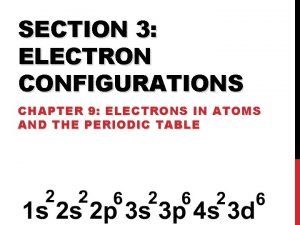
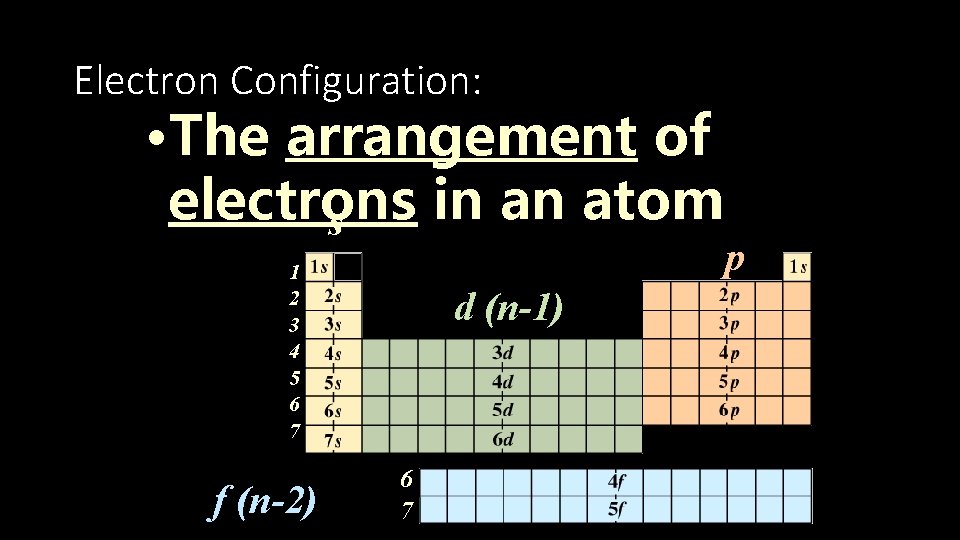
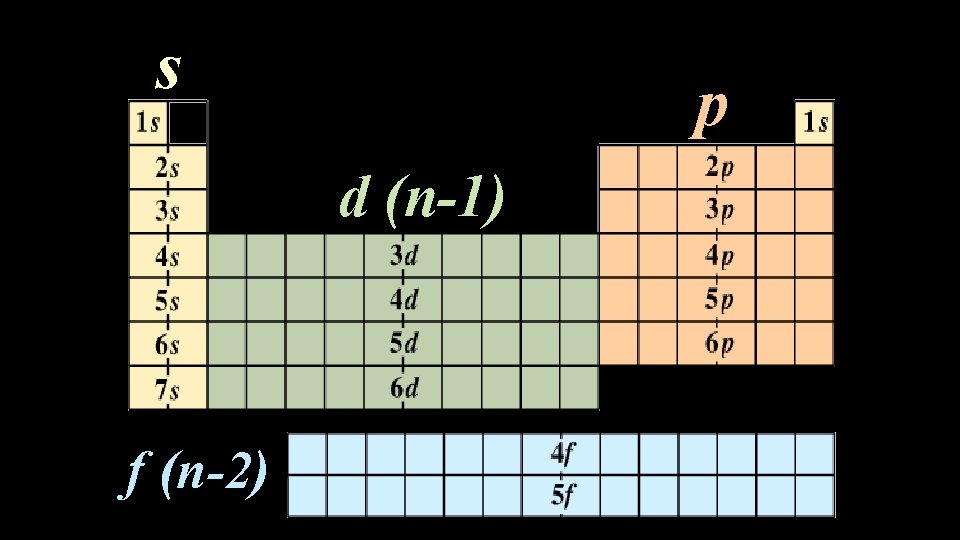
Electron Configuration: • The arrangement of electrons in an atom s 1 2 3 4 5 6 7 f (n-2) p d (n-1) 6 7 © 1998 by Harcourt Brace & Company

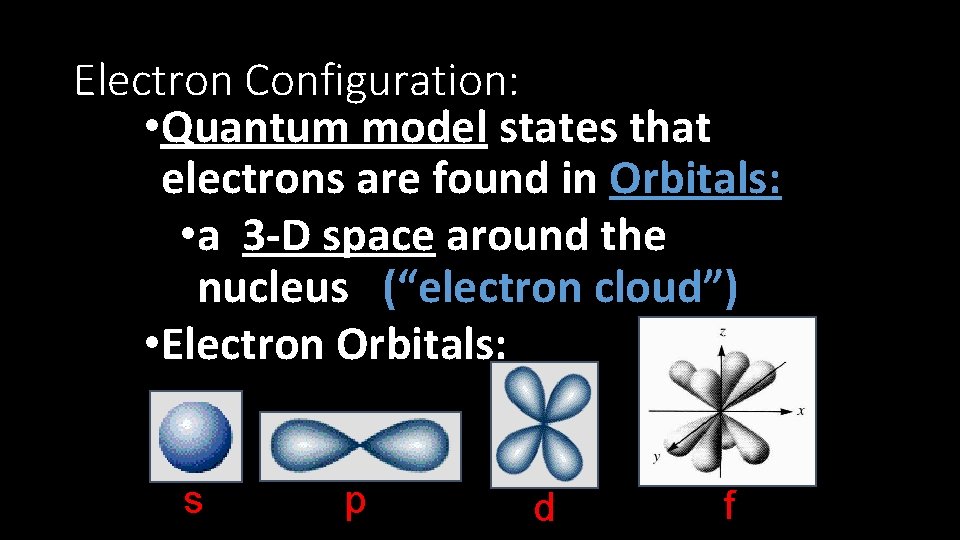
Electron Configuration: • Quantum model states that electrons are found in Orbitals: • a 3 -D space around the nucleus (“electron cloud”) • Electron Orbitals: s p d f

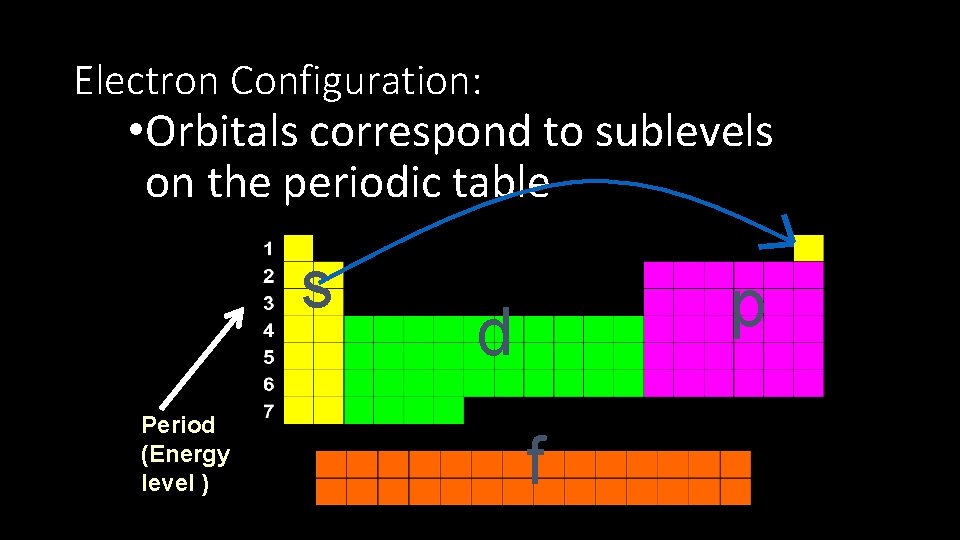
Electron Configuration: • Orbitals correspond to sublevels on the periodic table s Period (Energy level ) p d f

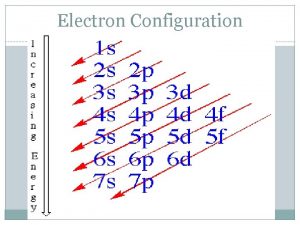
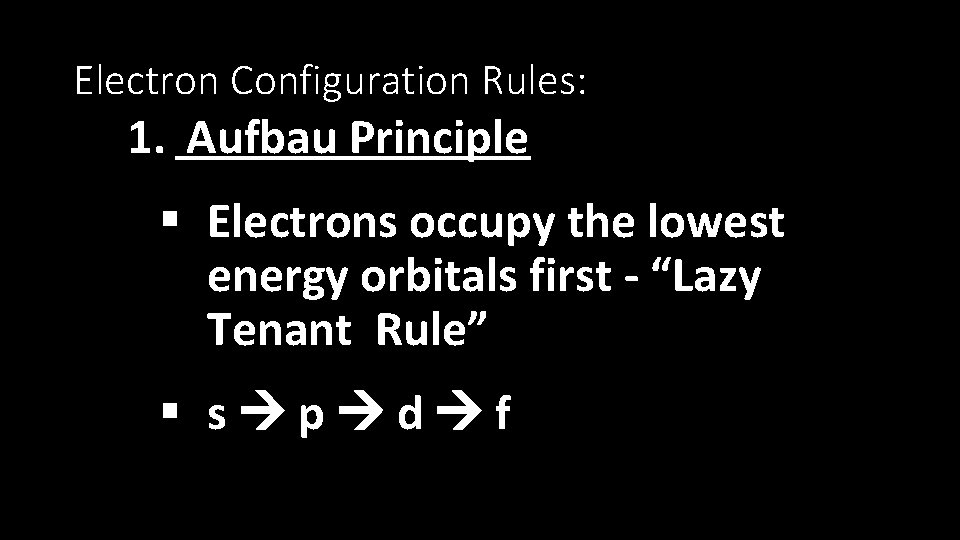
Electron Configuration Rules: 1. Aufbau Principle § Electrons occupy the lowest energy orbitals first - “Lazy Tenant Rule” § s p d f

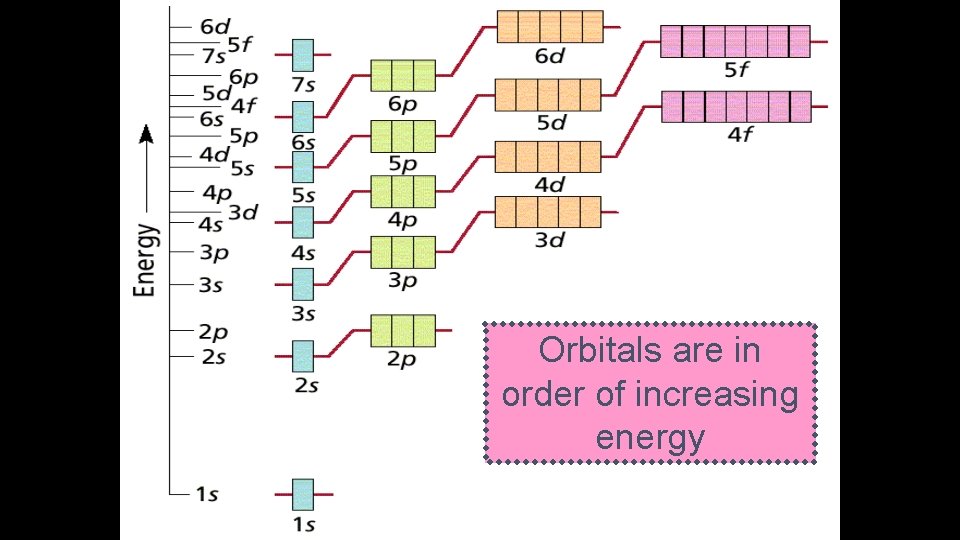
Orbitals are in order of increasing energy

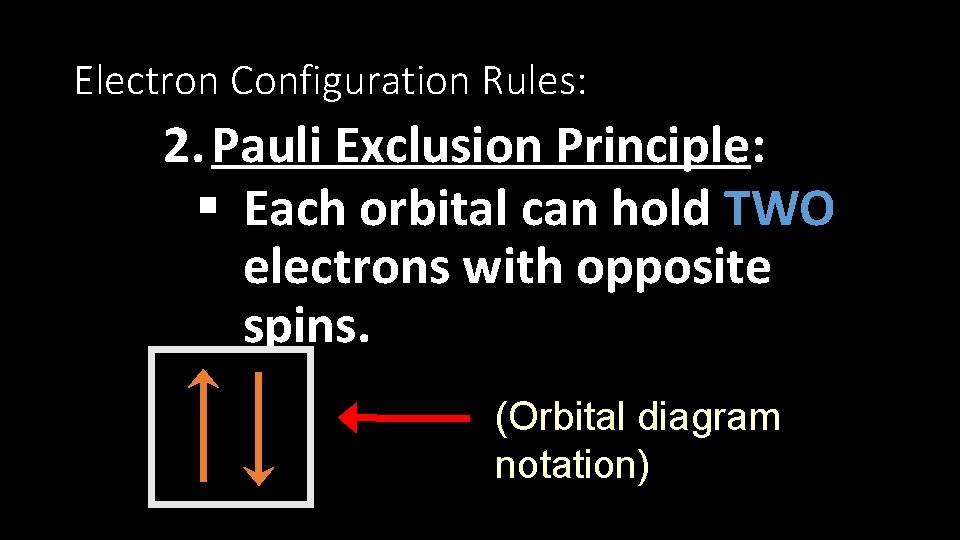
Electron Configuration Rules: 2. Pauli Exclusion Principle: § Each orbital can hold TWO electrons with opposite spins. (Orbital diagram notation)

Electron Configuration Rules: 3. Hund’s Rule § Within a sublevel, place one e per orbital before pairing them. § “Empty Bus Seat Rule” WRONG RIGHT

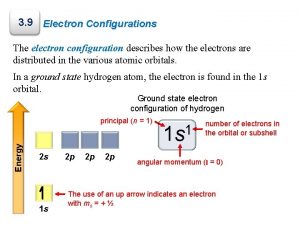
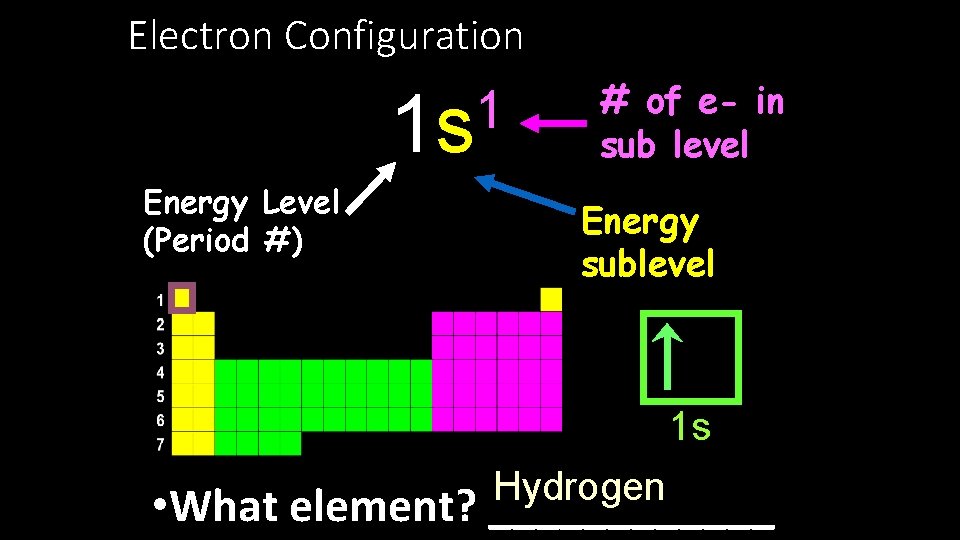
Electron Configuration 1 1 s Energy Level (Period #) # of e- in sub level Energy sublevel 1 s Hydrogen • What element? ______

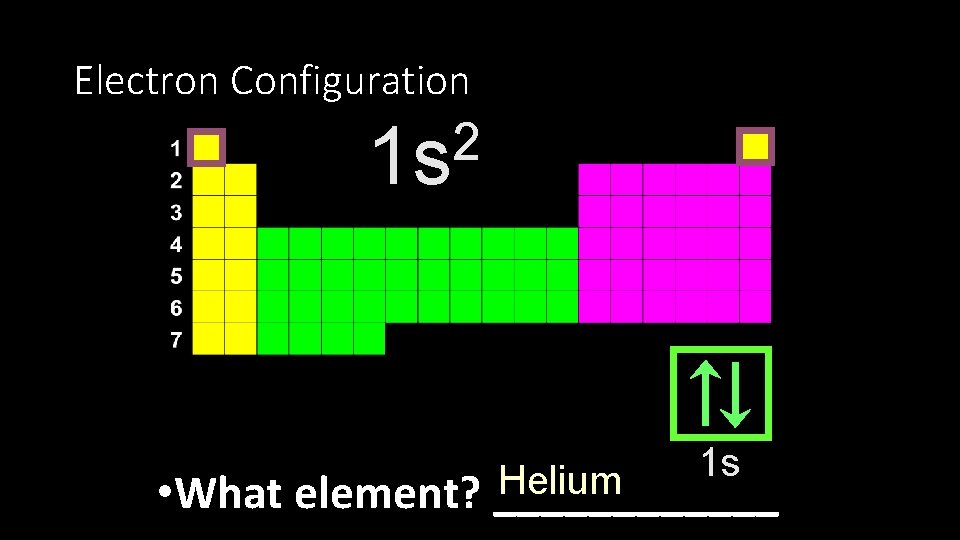
Electron Configuration 2 1 s 1 s Helium • What element? ______

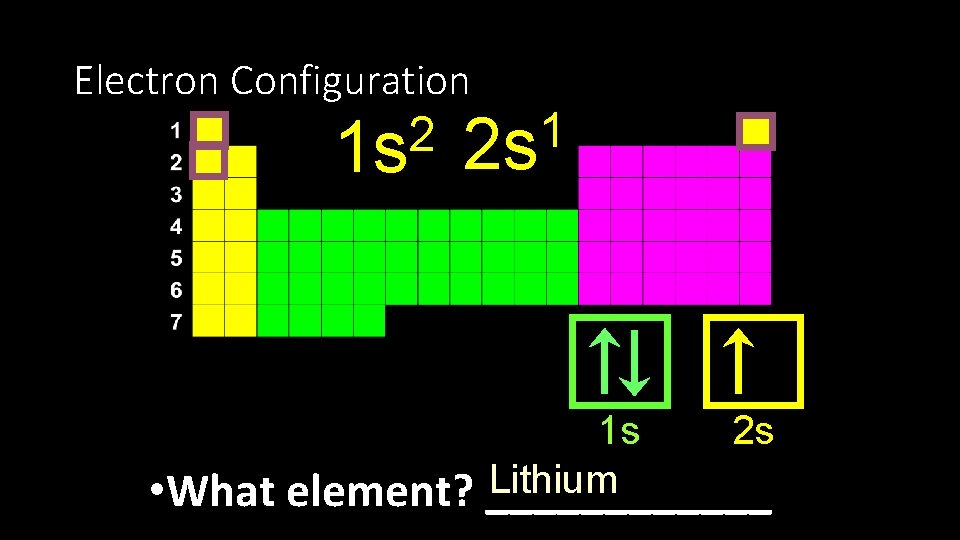
Electron Configuration 2 1 s 1 2 s 2 s 1 s Lithium • What element? ______

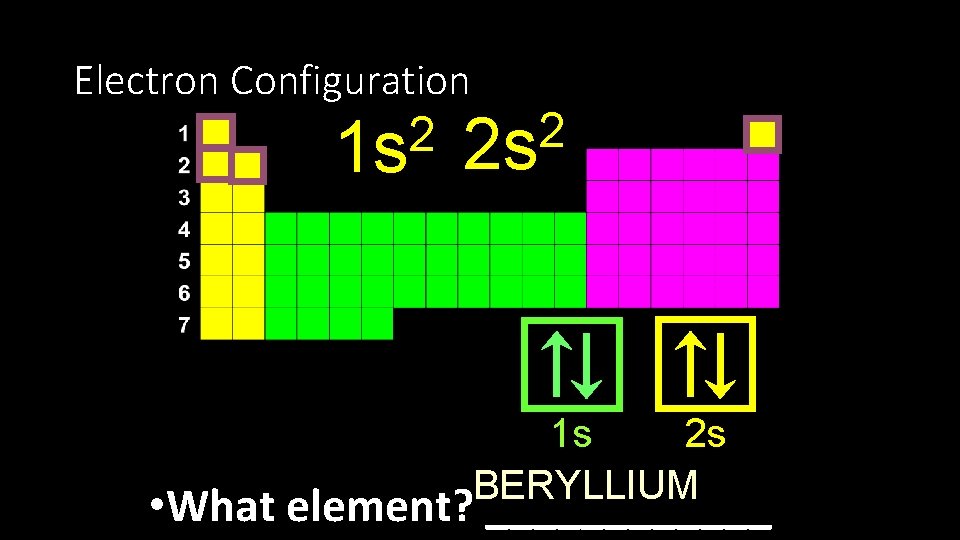
Electron Configuration 2 1 s 2 2 s 2 s 1 s BERYLLIUM • What element? ______

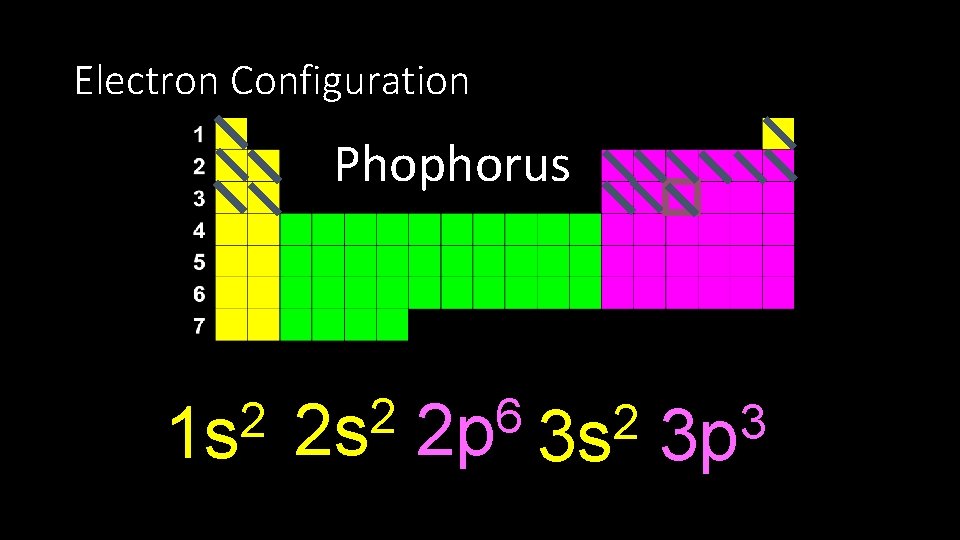
Electron Configuration Phophorus 2 1 s 2 2 s 6 2 p 2 3 s 3 3 p

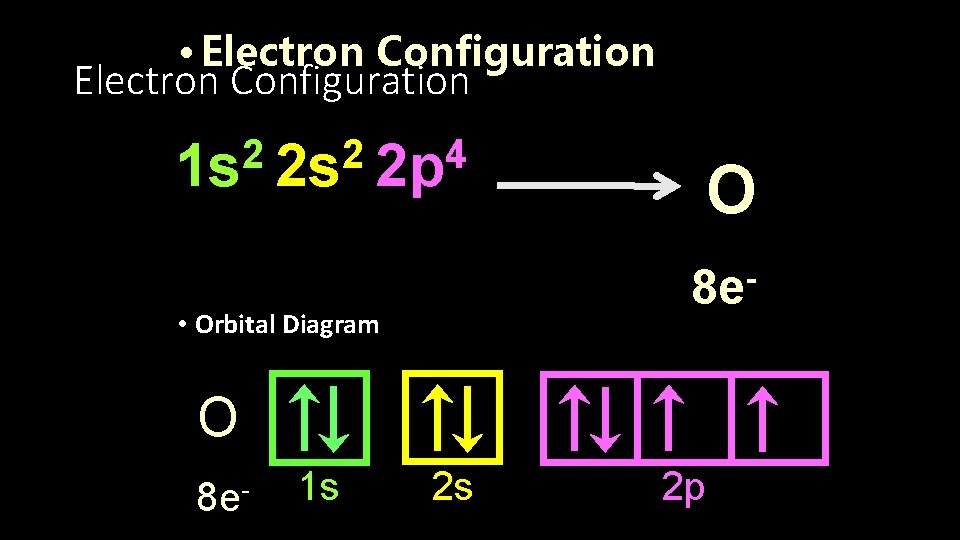
• Electron Configuration 2 2 4 1 s 2 s 2 p O 8 e- • Orbital Diagram O 8 e- 1 s 2 s 2 p

s p d (n-1) f (n-2) © 1998 by Harcourt Brace & Company

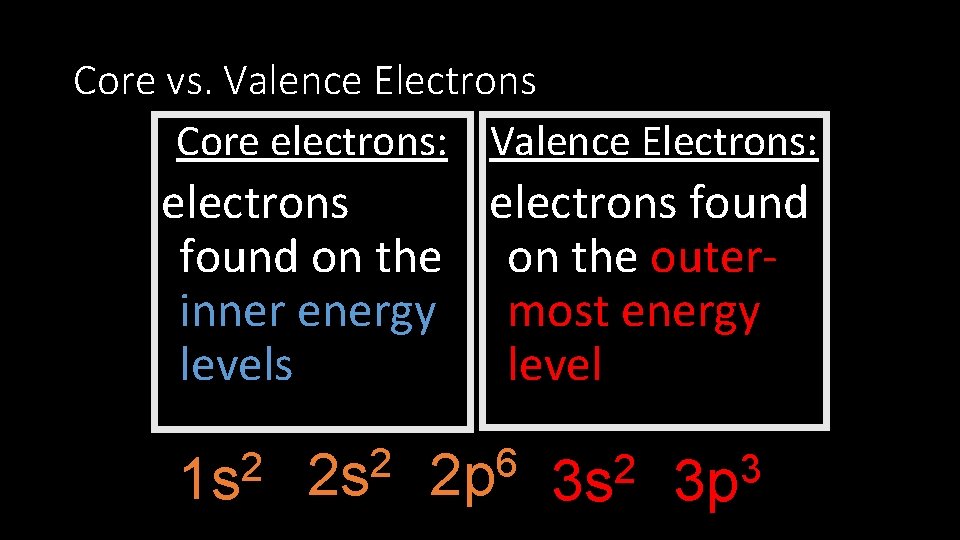
Core vs. Valence Electrons Core electrons: Valence Electrons: electrons found on the outerinner energy most energy levels 2 1 s 2 2 s 6 2 p 2 3 s 3 3 p

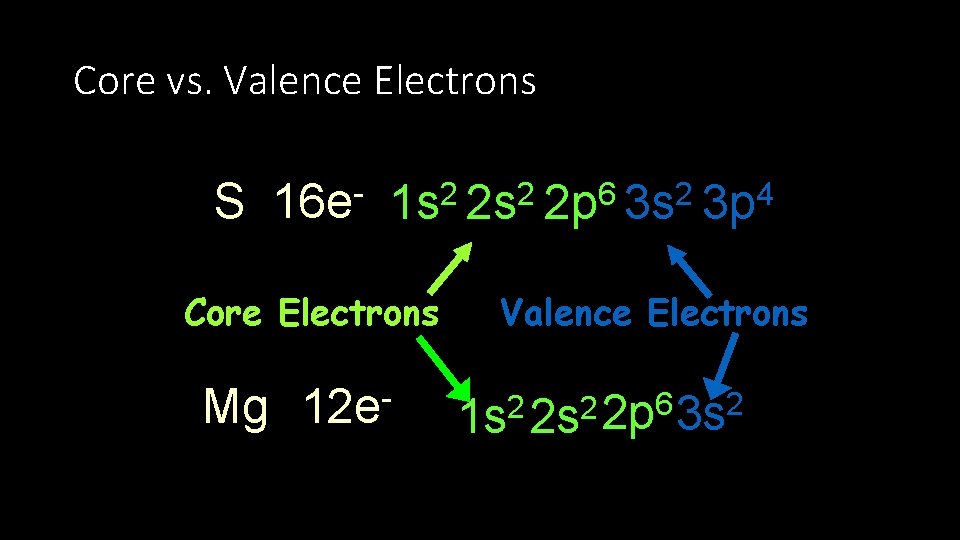
Core vs. Valence Electrons S 16 e 1 s 2 2 p 6 3 s 2 3 p 4 Core Electrons Mg 12 e- Valence Electrons 6 2 2 2 1 s 2 s 2 p 3 s

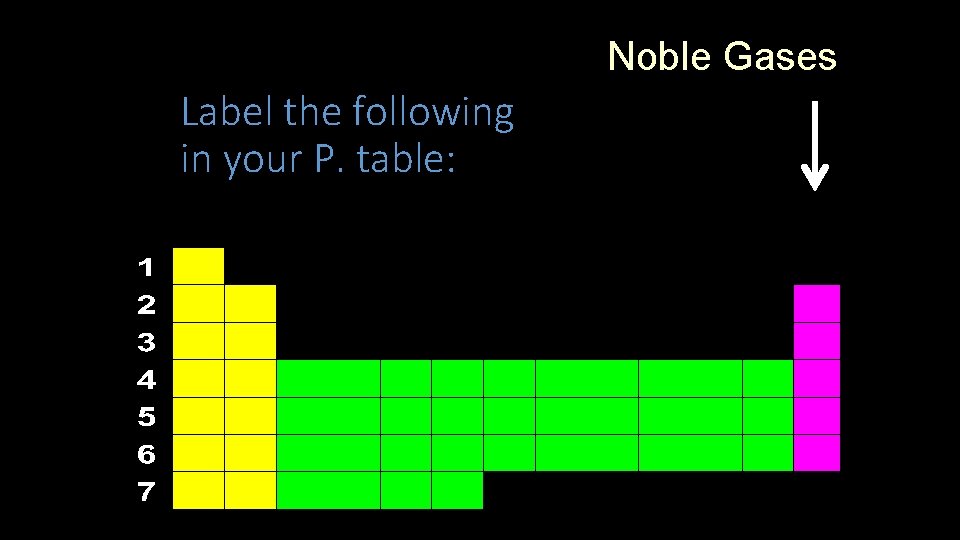
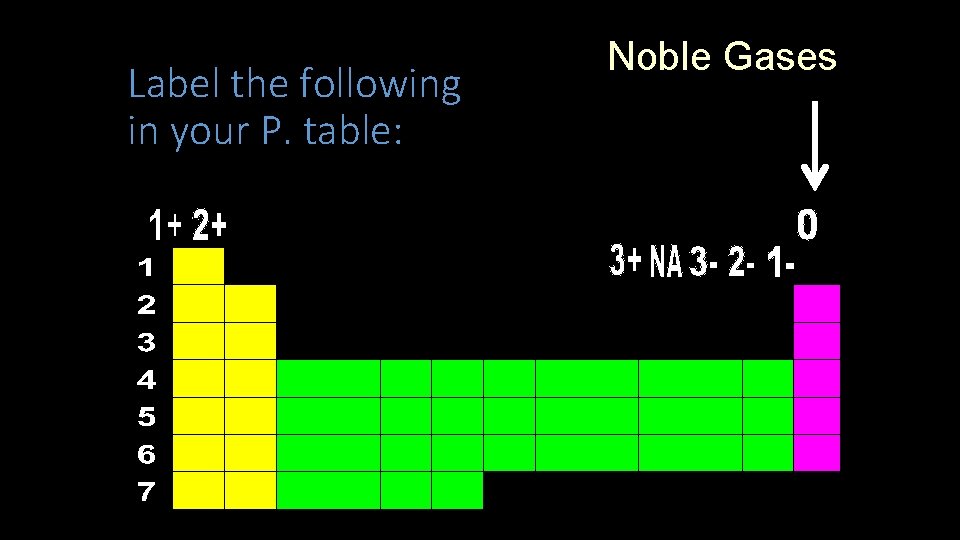
Noble Gases Label the following in your P. table:

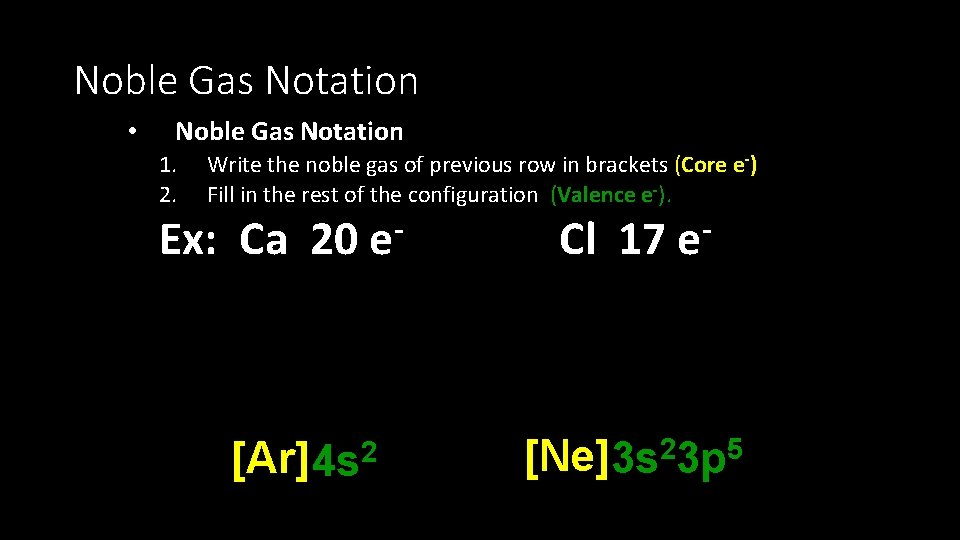
Noble Gas Notation • Noble Gas Notation 1. 2. Write the noble gas of previous row in brackets (Core e-) Fill in the rest of the configuration (Valence e-). Ex: Ca 20 e [Ar] 4 s 2 Cl 17 e [Ne]3 s 23 p 5

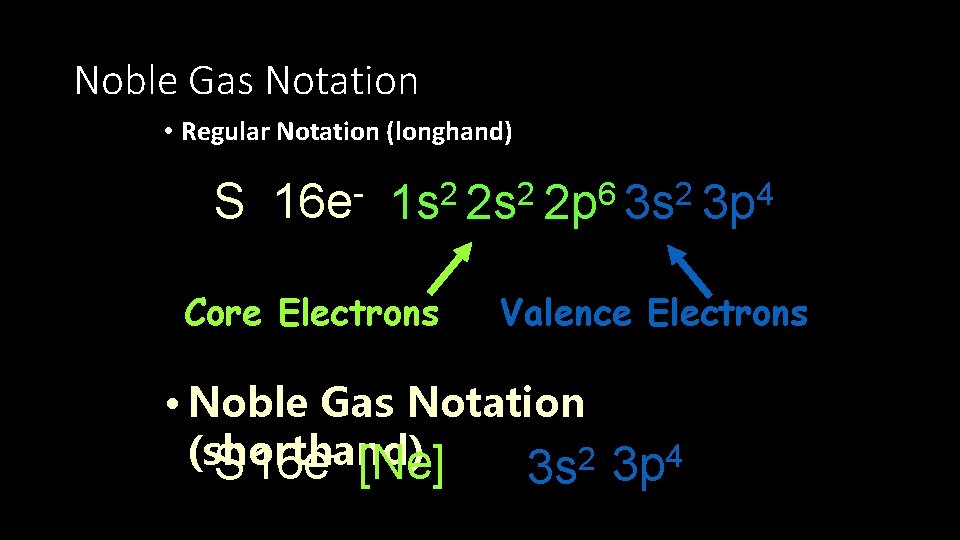
Noble Gas Notation • Regular Notation (longhand) S 16 e 1 s 2 2 p 6 3 s 2 3 p 4 Core Electrons Valence Electrons • Noble Gas Notation (shorthand) 4 2 S 16 e- [Ne] 3 s 3 p

Ion Notation • Ion- atom with a charge because of a gain or lose valence electrons to become more stable. • Noble Gases are already stable • Other atoms will try to be like the Noble Gases

Label the following in your P. table: Noble Gases

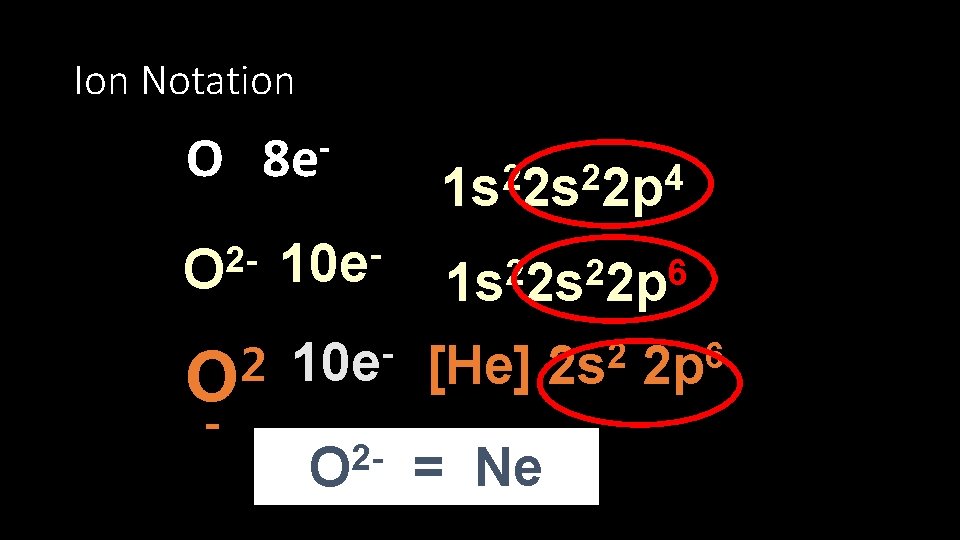
Ion Notation O 8 e 2 O 2 O - 10 e 2 2 4 1 s 2 s 2 p 2 2 6 1 s 2 s 2 p 10 e [He] 2 O = Ne 2 2 s 6 2 p

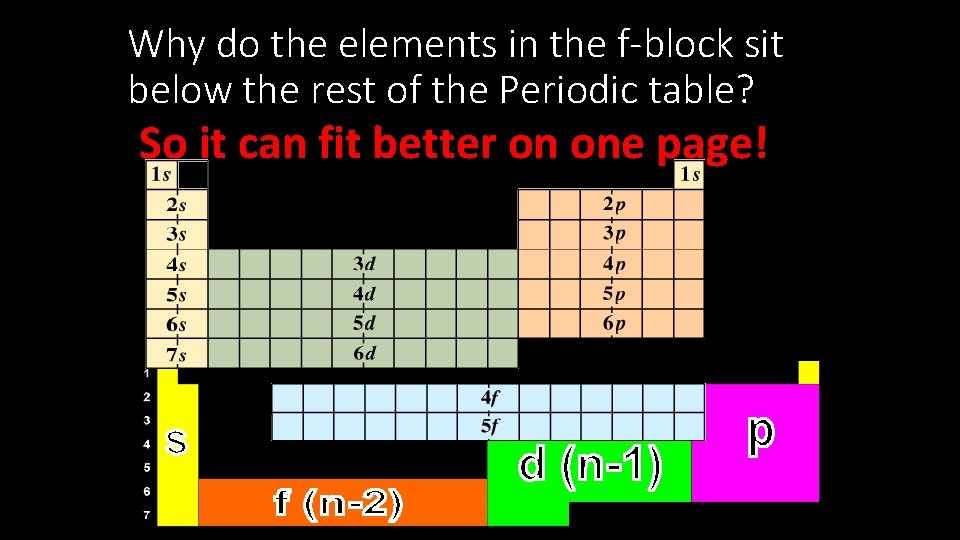
Why do the elements in the f-block sit below the rest of the Periodic table? So it can fit better on one page!
 Arrangement of electrons
Arrangement of electrons Electron configuration for oxygen
Electron configuration for oxygen Unpaired electrons in electron configuration
Unpaired electrons in electron configuration Br valence electrons
Br valence electrons Electrons configurations
Electrons configurations Electron configuration vs noble gas configuration
Electron configuration vs noble gas configuration Electronic configuration of elements
Electronic configuration of elements Orbital diagram for ca
Orbital diagram for ca Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Chapter 4 arrangement of electrons in atoms test
Chapter 4 arrangement of electrons in atoms test Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons What is relative configuration
What is relative configuration What is absolute configuration
What is absolute configuration Chiral or achiral
Chiral or achiral Electron configuration song
Electron configuration song Excited state electron configuration
Excited state electron configuration Boron orbital filling diagram
Boron orbital filling diagram E=h x v
E=h x v Sc electron configuration
Sc electron configuration What happens when electrons get excited
What happens when electrons get excited What does electron configuration notation eliminate?
What does electron configuration notation eliminate? Periodic table electron configuration
Periodic table electron configuration Electron hotel worksheet answers
Electron hotel worksheet answers