Hog Hilton You are the manager of a







![Learning Check Use Noble Gas Shorthand write the e- config. 1. Cr [Ar] 4 Learning Check Use Noble Gas Shorthand write the e- config. 1. Cr [Ar] 4](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-28.jpg)



![The trick to f orbitals! Examples: 24 f 115 d 1 [Xe] 6 s The trick to f orbitals! Examples: 24 f 115 d 1 [Xe] 6 s](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-34.jpg)
![Learning Check Use Noble Gas Shorthand write the e- config. 1. Sm [Xe] 6 Learning Check Use Noble Gas Shorthand write the e- config. 1. Sm [Xe] 6](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-35.jpg)
- Slides: 35

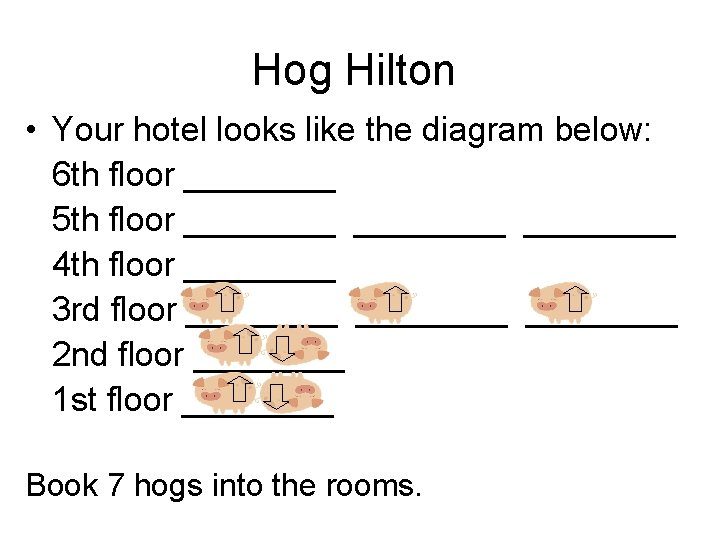
Hog Hilton You are the manager of a prestigious new hotel in downtown Midland—the “Hog Hilton”. It’s just the “snort of the town” and you want to keep its reputation a cut above all the other hotels. Your problem is your clientele. They are hogs in the truest sense. Your major task is to fill rooms in your hotel. The Hog Hilton only has stairs. You must fill up your hotel keeping the following rules in mind: 1) Hogs are lazy, they don’t want to walk up stairs! 2) Hogs want to room by themselves, but they would rather room with another hog than walk up more stairs. 3) If hogs are in the same room they will face in opposite directions. 4) They stink, so you can’t put more than two hogs in each room.

Hog Hilton • Your hotel looks like the diagram below: 6 th floor ____ 5 th floor ________ 4 th floor ____ 3 rd floor ________ 2 nd floor ____ 1 st floor ____ Book 7 hogs into the rooms.

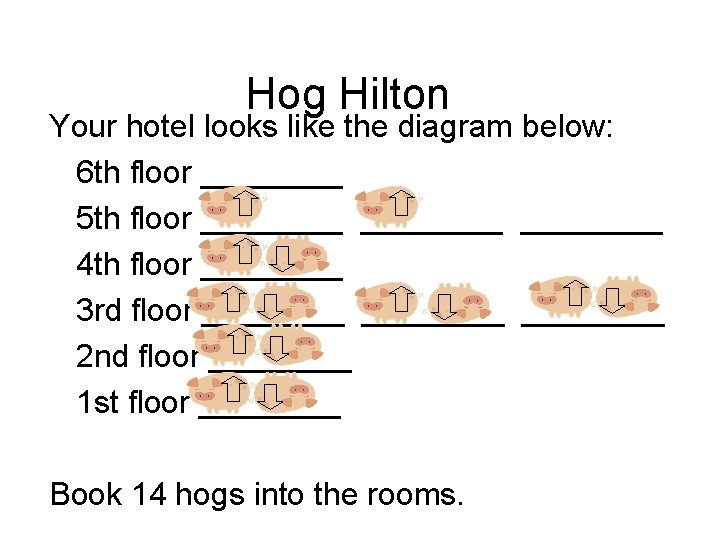
Hog Hilton Your hotel looks like the diagram below: 6 th floor ____ 5 th floor ________ 4 th floor ____ 3 rd floor ________ 2 nd floor ____ 1 st floor ____ Book 14 hogs into the rooms.

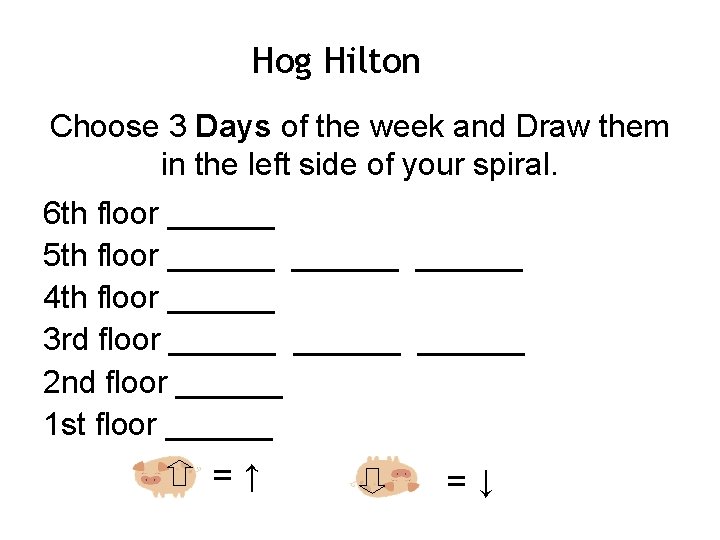
Hog Hilton Choose 3 Days of the week and Draw them in the left side of your spiral. 6 th floor ______ 5 th floor ______ 4 th floor ______ 3 rd floor ______ 2 nd floor ______ 1 st floor ______ =↑ =↓

Let’s play Hog Hilton!!

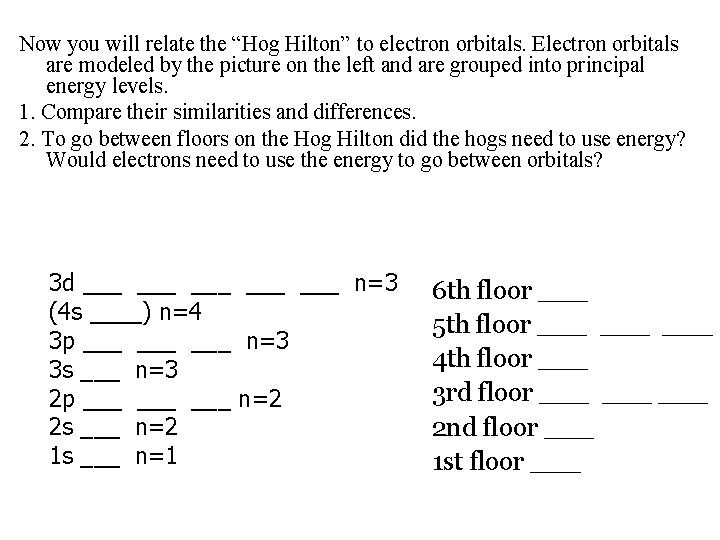
Now you will relate the “Hog Hilton” to electron orbitals. Electron orbitals are modeled by the picture on the left and are grouped into principal energy levels. 1. Compare their similarities and differences. 2. To go between floors on the Hog Hilton did the hogs need to use energy? Would electrons need to use the energy to go between orbitals? 3 d ___ ___ ___ n=3 (4 s ____) n=4 3 p ___ ___ n=3 3 s ___ n=3 2 p ___ ___ n=2 2 s ___ n=2 1 s ___ n=1 6 th floor ___ 5 th floor ___ ___ 4 th floor ___ 3 rd floor ___ ___ 2 nd floor ___ 1 st floor ___

A. Rules for e- configurations 1. Aufbau principle: principle electrons fill the lowest energy orbitals first. (Hogs are lazy, they don’t want to walk up stairs!)

A. Rules for e- configurations 2. Pauli Exclusion principle: principle each orbital can hold TWO electrons with opposite spins (They stink, so you can’t put more than two hogs in each room. & If hogs are in the same room they will face in opposite directions. )

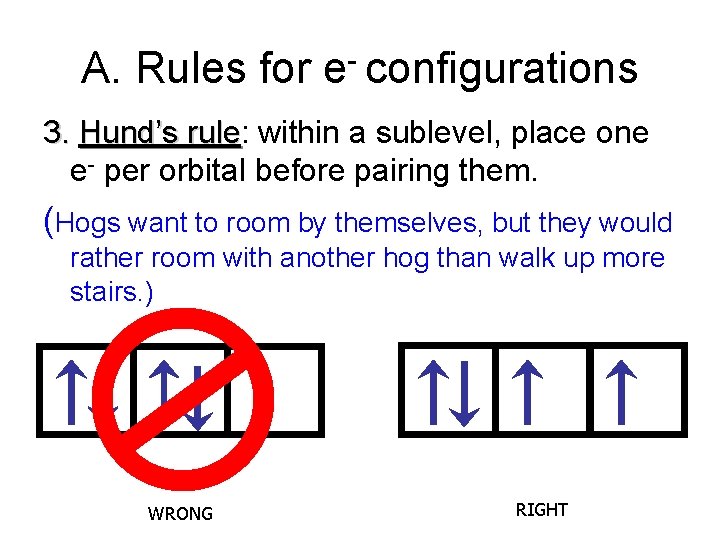
A. Rules for e- configurations 3. Hund’s rule: rule within a sublevel, place one e- per orbital before pairing them. (Hogs want to room by themselves, but they would rather room with another hog than walk up more stairs. ) WRONG RIGHT


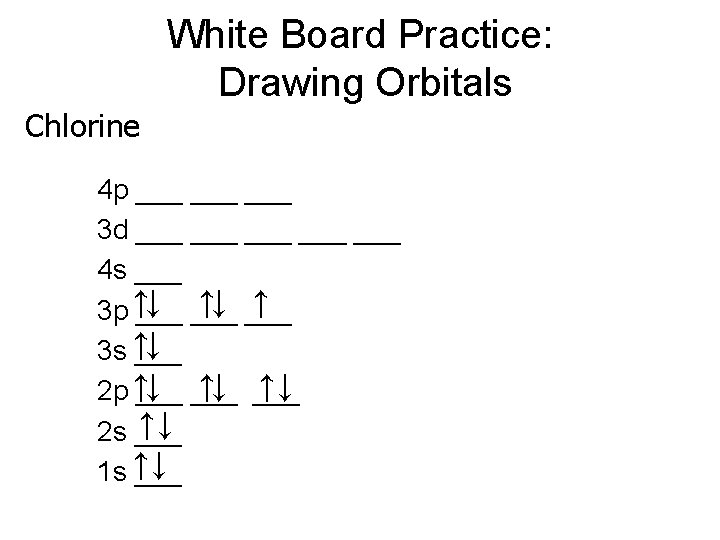
White Board Practice: Drawing Orbitals Chlorine 4 p ___ ___ 3 d ___ ___ ___ 4 s ___ ↓ ___ ↑ 3 p ↑___ 3 s ↑↓ ___ ↑↓ 2 p ↑___ ↑↓ 2 s ___ 1 s ↑↓ ___


C. Writing the Electron Configuration Krypton: atomic number - 36 4 p _ ↑↓ _ 3 d _ ↑↓ _ 4 s _ ↑↓ _ 3 p _ ↑↓ _ 3 s _ ↑↓ _ 2 p _ ↑↓ _ 2 s _ ↑↓ _ 1 s _↑↓_ _ ↑↓ _ _ ↑↓ _ _ ↑↓ _ Exponent is number of e- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 Add the exponents to check your answer

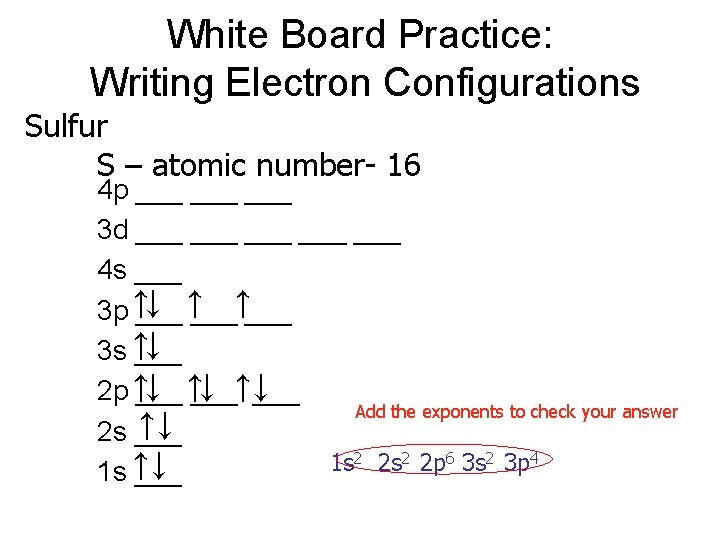
Iron White Board Practice: Writing Electron Configurations Fe – atomic number 26 4 p ___ ___ ↑↓ ___ ↑ ↑___↑ ___ 3 d ___ 4 s ↑↓ ___ ↓ ↑___ ↓ 3 p ↑___ 3 s ↑↓ ___ ↓ ↑ ___ ↓ 2 p ↑___ Add the exponents to check your answer ↑↓ 2 s ___ 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 1 s ↑↓ 1 s ___

White Board Practice: Writing Electron Configurations Sulfur S – atomic number- 16 4 p ___ ___ 3 d ___ ___ ___ 4 s ___ ↓ ↑___ 3 p ↑___ 3 s ↑↓ ___ ↓ ↑ ___ ↓ 2 p ↑___ Add the exponents to check your answer ↑↓ 2 s ___ 2 2 s 2 2 p 6 3 s 2 3 p 4 1 s ↑↓ 1 s ___

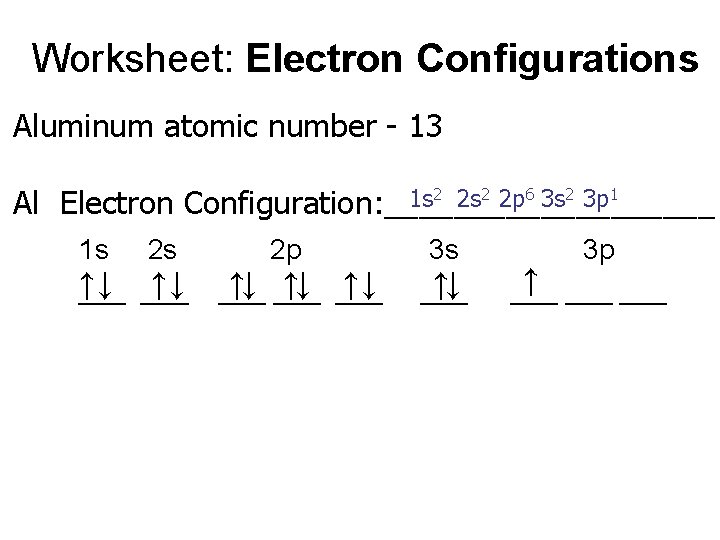
Worksheet: Electron Configurations Aluminum atomic number - 13 1 s 2 2 p 6 3 s 2 3 p 1 Al Electron Configuration: __________ 1 s 2 s ↑↓ ↑↓ ___ 2 p ↑↓ ___ 3 s ↑↓ ___ 3 p ↑ ___ ___

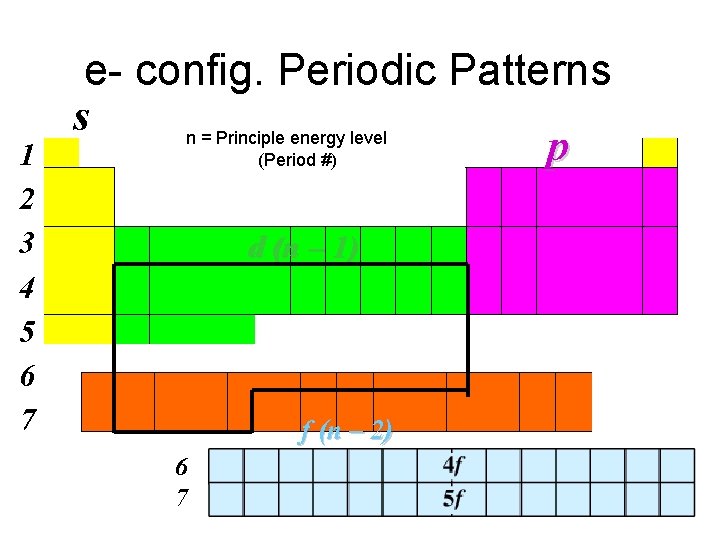
1 2 3 4 5 6 7 e- config. Periodic Patterns s n = Principle energy level p (Period #) d (n – 1) f (n – 2) 6 7

What is the electron configuration for Br? 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 104 p 5 1 2 3 4 5 6 7 Br

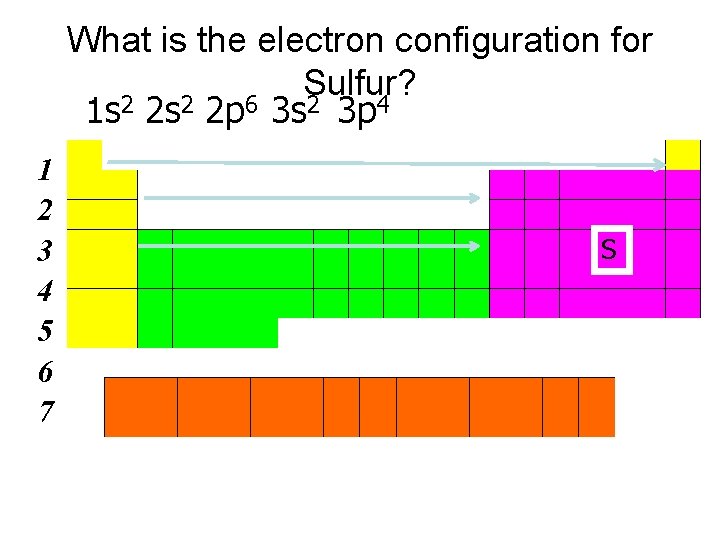
What is the electron configuration for Sulfur? 1 s 2 2 p 6 3 s 2 3 p 4 1 2 3 4 5 6 7 S

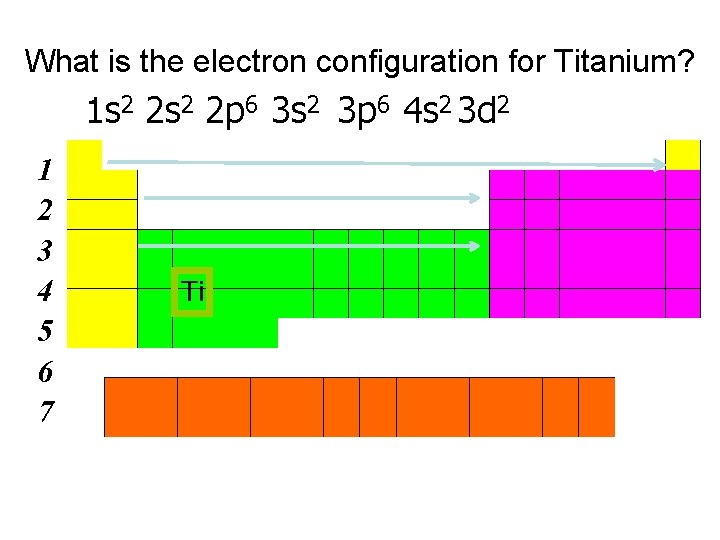
What is the electron configuration for Titanium? 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 2 1 2 3 4 5 6 7 Ti

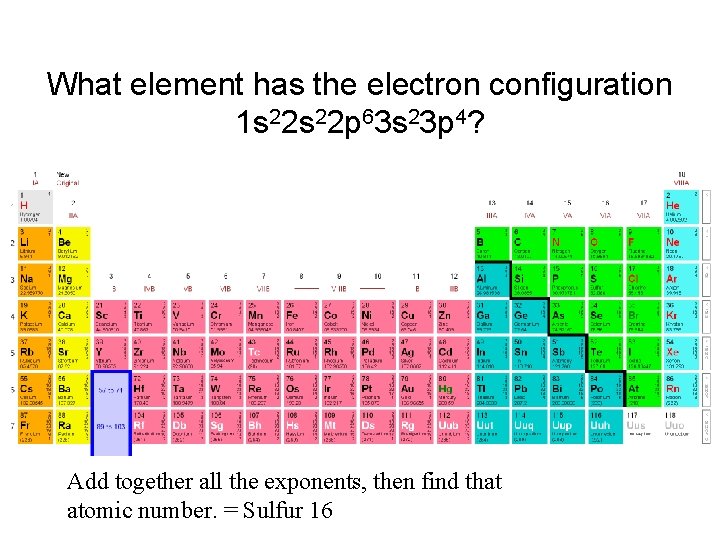
What element has the electron configuration 1 s 22 p 63 s 23 p 4? Add together all the exponents, then find that atomic number. = Sulfur 16

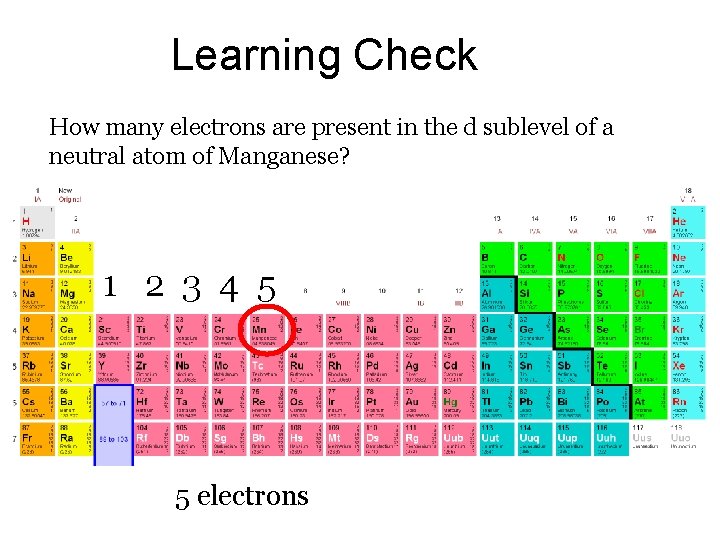
Learning Check How many electrons are present in the d sublevel of a neutral atom of Manganese? 1 2 3 4 5 5 electrons

D. Noble Gases Shorthand • Use the noble gas in the previous row. • Write noble gas symbol in brackets then rest of the e-configuration.

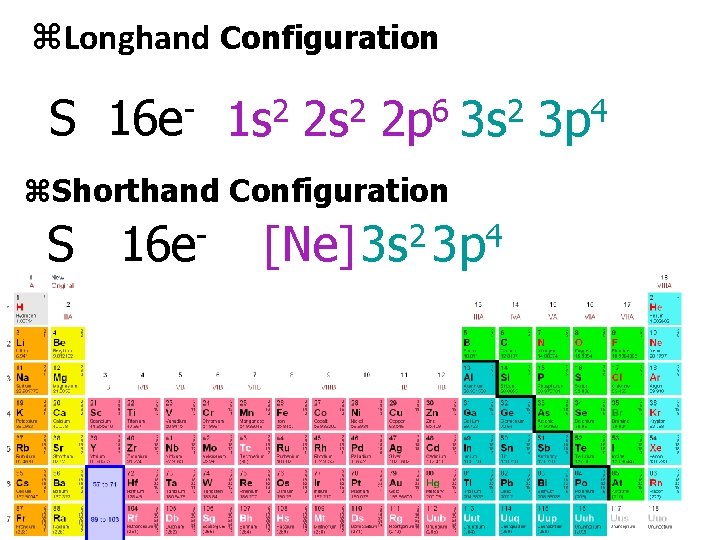
z. Longhand Configuration S 16 e- 1 s 2 2 p 6 3 s 2 3 p 4 z. Shorthand Configuration 2 4 S 16 e [Ne]3 s 3 p

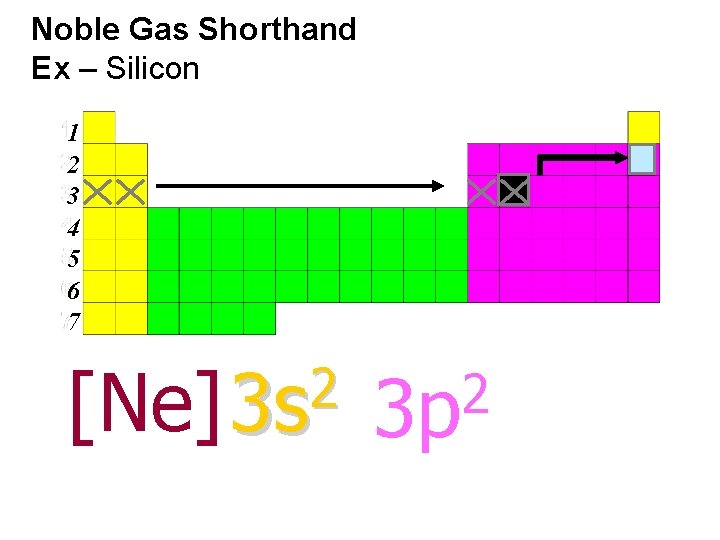
Noble Gas Shorthand Ex – Silicon 1 2 3 4 5 6 7 2 [Ne] 3 s 2 3 p

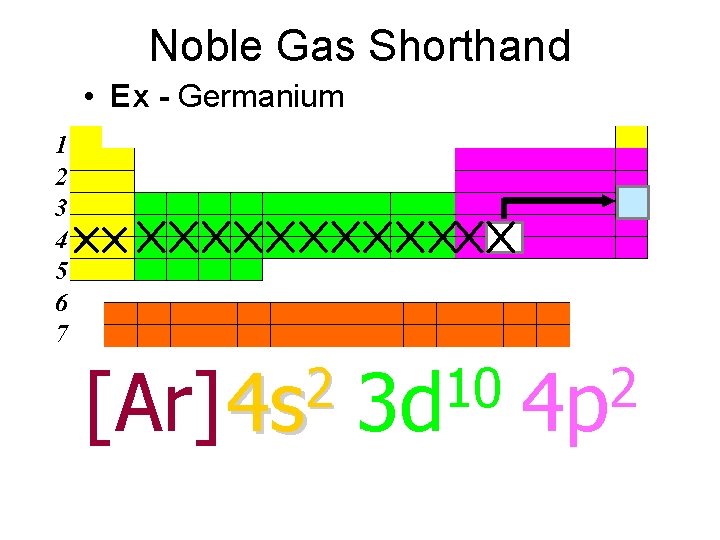
Noble Gas Shorthand • Ex - Germanium 1 2 3 4 5 6 7 2 [Ar] 4 s 10 3 d 2 4 p

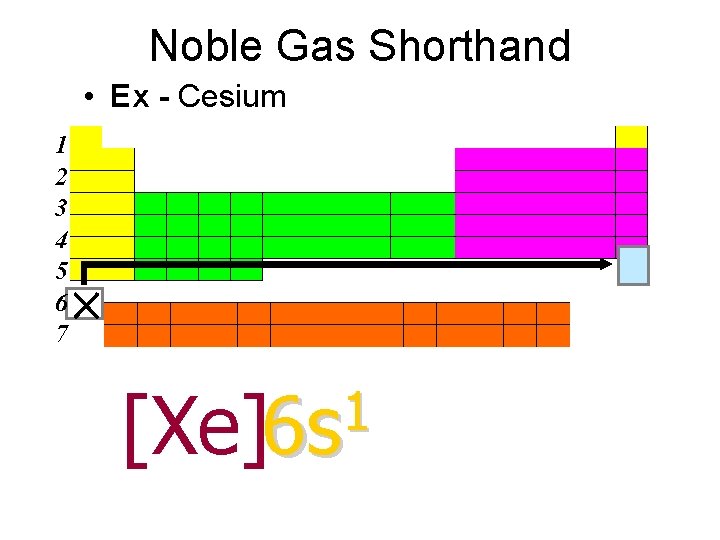
Noble Gas Shorthand • Ex - Cesium 1 2 3 4 5 6 7 1 [Xe]6 s
![Learning Check Use Noble Gas Shorthand write the e config 1 Cr Ar 4 Learning Check Use Noble Gas Shorthand write the e- config. 1. Cr [Ar] 4](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-28.jpg)
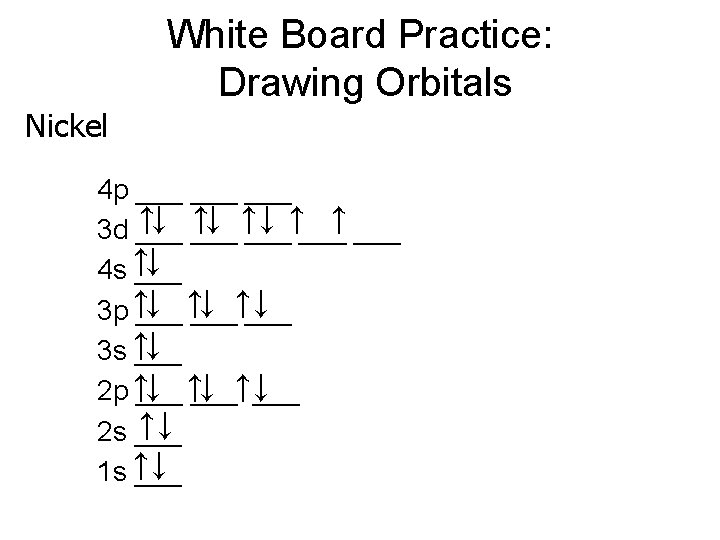
Learning Check Use Noble Gas Shorthand write the e- config. 1. Cr [Ar] 4 s 2 3 d 4 2. Br [Ar] 4 s 2 3 d 10 4 p 5 3. Sn [Kr] 5 s 2 4 d 10 5 p 2 4. Ba [Xe] 6 s 2

Learning Check 1. Which orbital quantum number combination is not possible? A. 2 s B. 2 d C. 4 d D. 3 p

Learning Check 2. How many electrons are required to fill the 1 st energy level? A. 2 B. 4 C. 8 D. 10

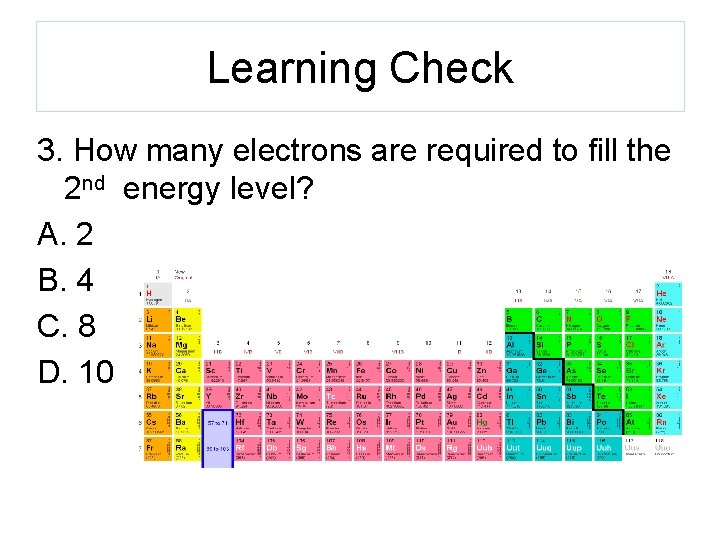
Learning Check 3. How many electrons are required to fill the 2 nd energy level? A. 2 B. 4 C. 8 D. 10

Learning Check 4. How many electrons are required to fill the 3 rd energy level? A. 4 B. 8 C. 10 D. 18

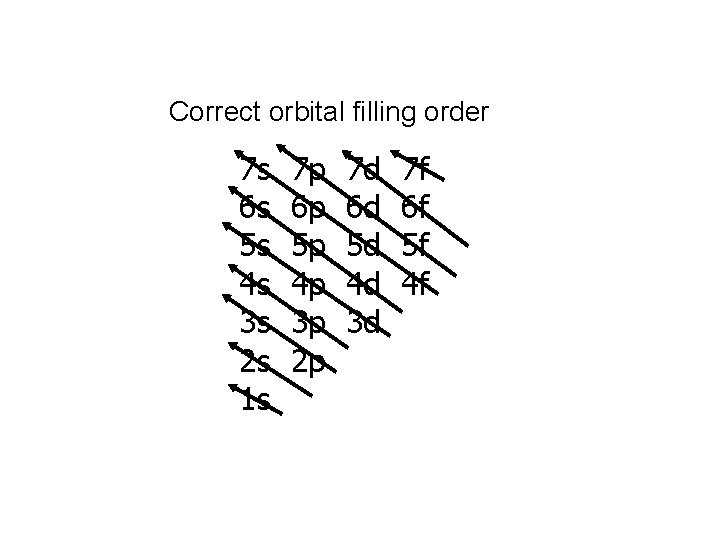
Correct orbital filling order 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 5 p 4 p 3 p 2 p 7 d 6 d 5 d 4 d 3 d 7 f 6 f 5 f 4 f
![The trick to f orbitals Examples 24 f 115 d 1 Xe 6 s The trick to f orbitals! Examples: 24 f 115 d 1 [Xe] 6 s](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-34.jpg)
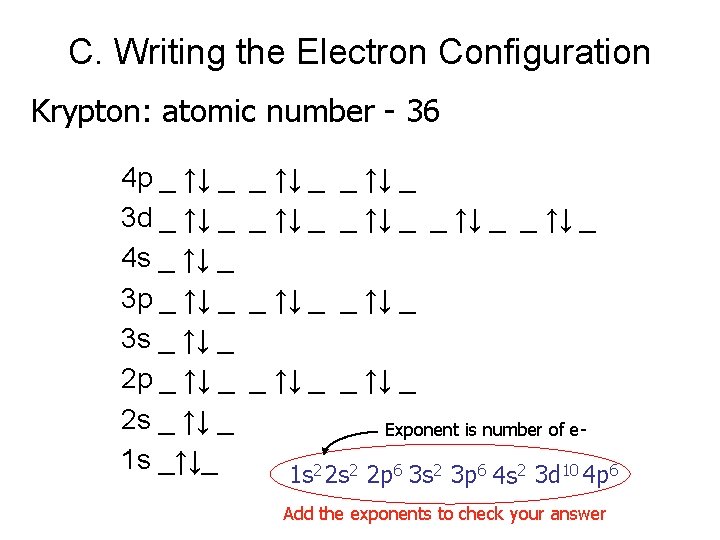
The trick to f orbitals! Examples: 24 f 115 d 1 [Xe] 6 s Erbium- Er 68 Hassium- Hs [Rn] 7 s 25 f 146 d 6
![Learning Check Use Noble Gas Shorthand write the e config 1 Sm Xe 6 Learning Check Use Noble Gas Shorthand write the e- config. 1. Sm [Xe] 6](https://slidetodoc.com/presentation_image/506e06194a026c91464dd9c9b6ec8bb7/image-35.jpg)
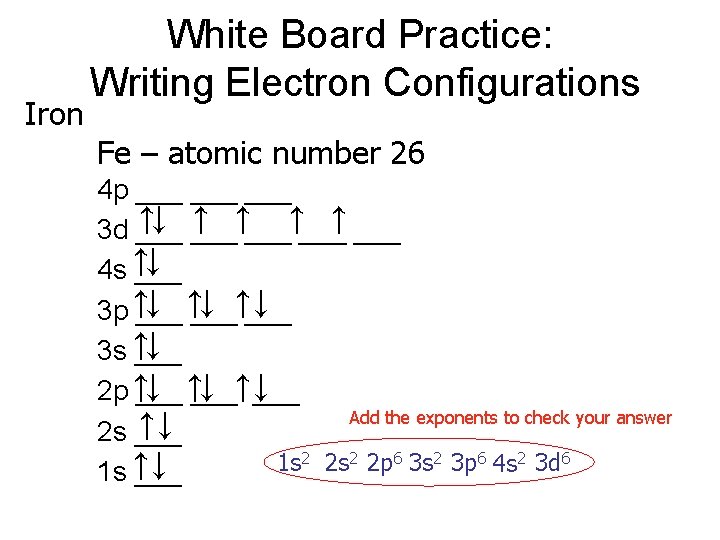
Learning Check Use Noble Gas Shorthand write the e- config. 1. Sm [Xe] 6 s 2 4 f 5 5 d 1 2. Db [Rn] 7 s 2 5 f 14 6 d 3
 Hog hilton activity answer key
Hog hilton activity answer key Insidan region jh
Insidan region jh Senior manager vs general manager
Senior manager vs general manager Portfolio manager synergy manager parental developer
Portfolio manager synergy manager parental developer Hog tech university
Hog tech university Hog jahrmarkt
Hog jahrmarkt Sound of waves onomatopoeia
Sound of waves onomatopoeia Smog hog electrostatic precipitators nyc
Smog hog electrostatic precipitators nyc Ciacco the hog
Ciacco the hog Htu ntu
Htu ntu Grunt grunt goes the hog figure of speech
Grunt grunt goes the hog figure of speech Porcine myology
Porcine myology Hog back structure
Hog back structure The harley owners group (hog) is an example of a ______.
The harley owners group (hog) is an example of a ______. Pseudolisteners
Pseudolisteners Hog busters
Hog busters Banater schwaben crailsheim
Banater schwaben crailsheim Hög ämbetsman under ming-dynastin
Hög ämbetsman under ming-dynastin Hog middles
Hog middles Hyatt regency walkway disaster
Hyatt regency walkway disaster Hilton gardin inn
Hilton gardin inn Hilton hotel podgorica
Hilton hotel podgorica Ruffini corpuscle
Ruffini corpuscle Michael bradford hilton
Michael bradford hilton Hilton's law
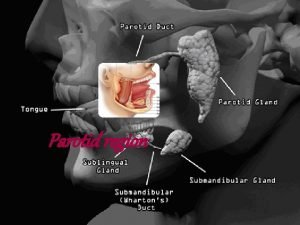
Hilton's law Unyielding nature of parotid fascia
Unyielding nature of parotid fascia Hilton's law
Hilton's law Hilton's law
Hilton's law Hilton's law
Hilton's law Hilton's law
Hilton's law Hilton honors military program
Hilton honors military program Golgi tendon reflex
Golgi tendon reflex Hilton garden inn atlanta aquarium
Hilton garden inn atlanta aquarium Hilton head gis
Hilton head gis Dr roger hilton
Dr roger hilton Julia hilton oxford
Julia hilton oxford