Aim What happens when electrons get excited Do







- Slides: 23

Aim: What happens when electrons get excited? Do Now: Define “ground state” and “excited state”.

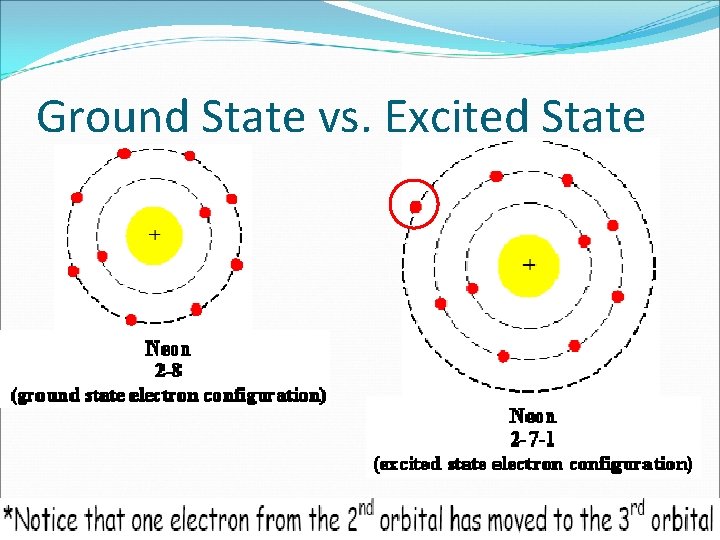
Ground State vs. Excited State

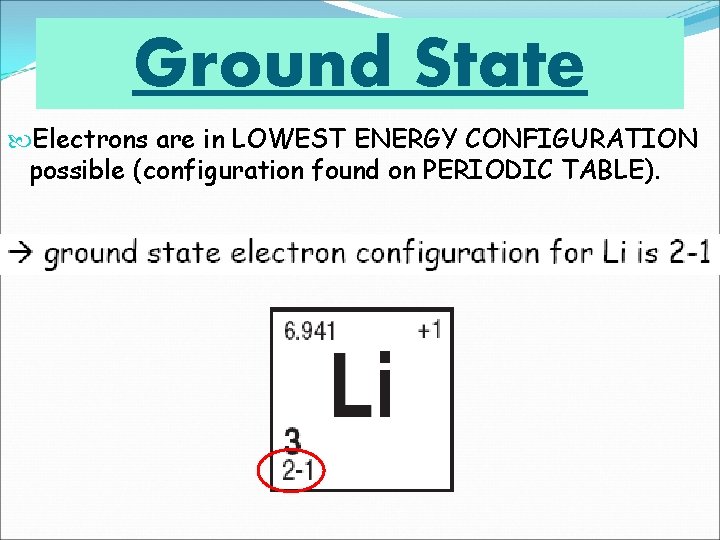
Ground State Electrons are in LOWEST ENERGY CONFIGURATION possible (configuration found on PERIODIC TABLE).

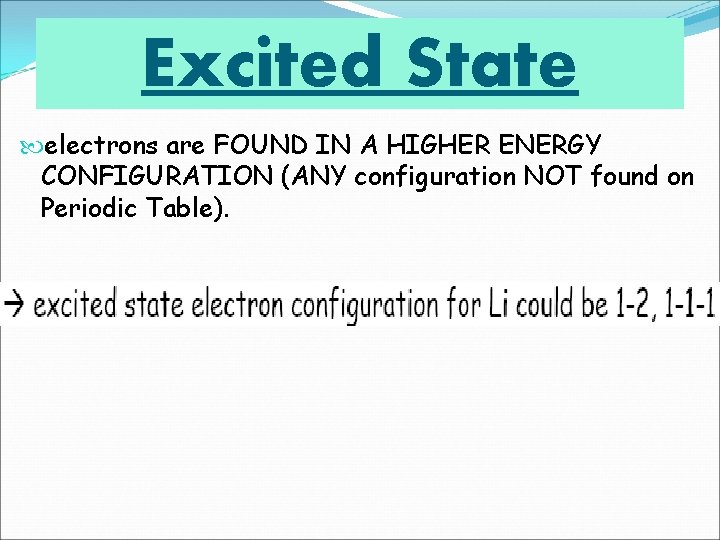
Excited State electrons are FOUND IN A HIGHER ENERGY CONFIGURATION (ANY configuration NOT found on Periodic Table).

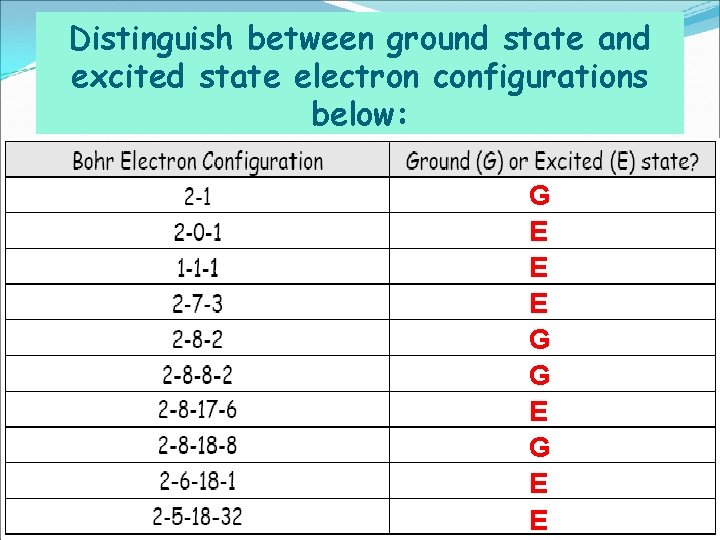
Distinguish between ground state and excited state electron configurations below: G E E E G G E E

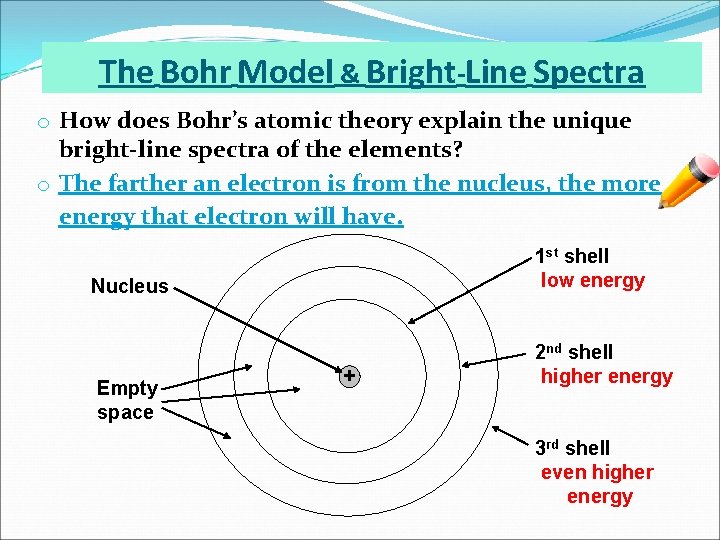
The Bohr Model & Bright-Line Spectra o How does Bohr’s atomic theory explain the unique bright-line spectra of the elements? o The farther an electron is from the nucleus, the more energy that electron will have. 1 st shell low energy Nucleus Empty space + 2 nd shell higher energy 3 rd shell even higher energy

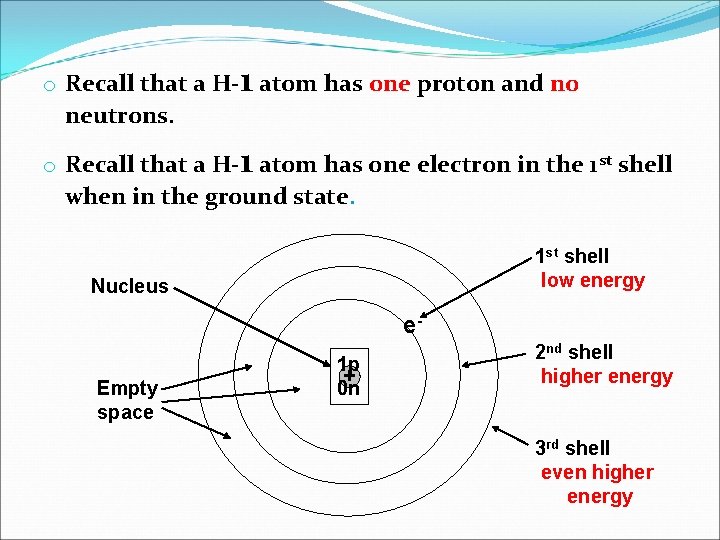
o Recall that a H-1 atom has one proton and no neutrons. o Recall that a H-1 atom has one electron in the 1 st shell when in the ground state. 1 st shell low energy Nucleus e. Empty space 1 p + 0 n 2 nd shell higher energy 3 rd shell even higher energy

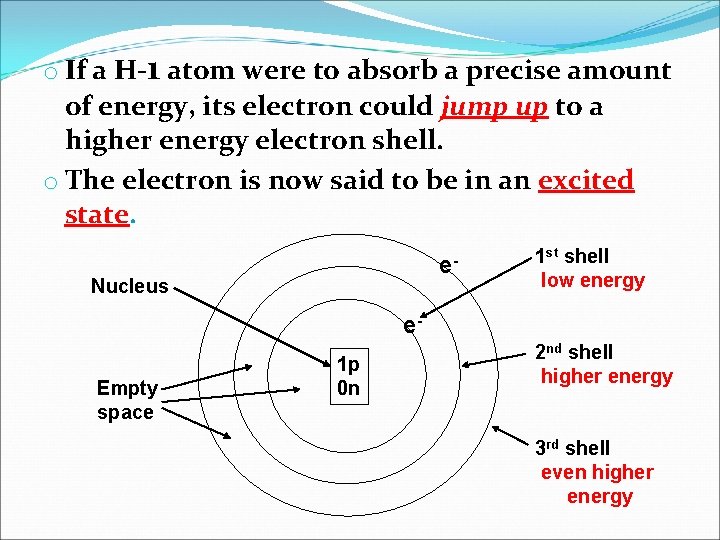
o If a H-1 atom were to absorb a precise amount of energy, its electron could jump up to a higher energy electron shell. o The electron is now said to be in an excited state. e- Nucleus 1 st shell low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy

o Electrons are in the excited state when they are in a shell that is higher than their ground state. o Electrons can only jump up to an excited state by absorbing a precise amount of energy. o All electrons in the excited state will return to their ground state. o Electrons can NEVER jump to a shell that is lower than their ground state.

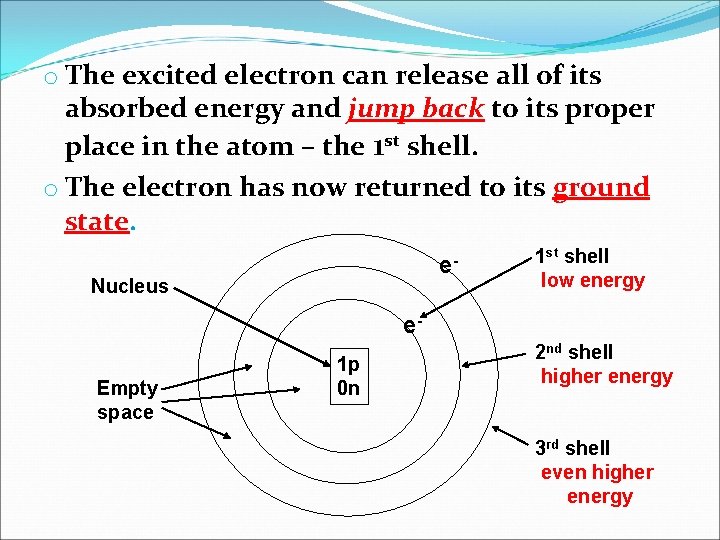
o The excited electron can release all of its absorbed energy and jump back to its proper place in the atom – the 1 st shell. o The electron has now returned to its ground state. e- Nucleus 1 st shell low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy

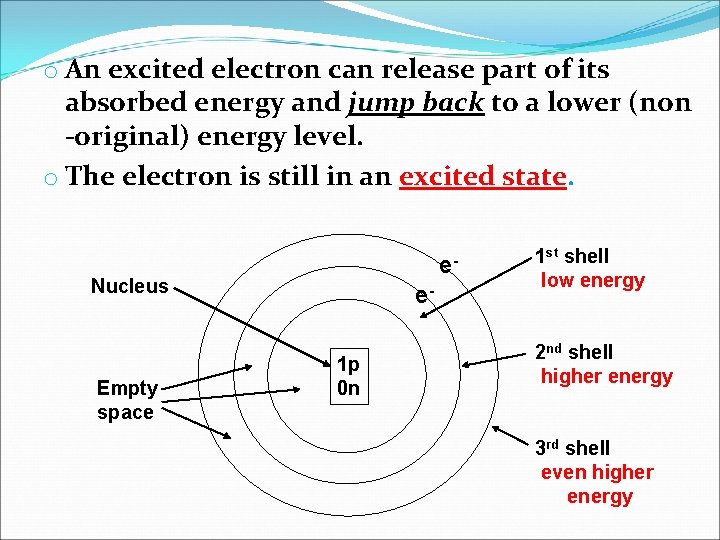
o An excited electron can release part of its absorbed energy and jump back to a lower (non -original) energy level. o The electron is still in an excited state. e- Nucleus Empty space e 1 p 0 n 1 st shell low energy 2 nd shell higher energy 3 rd shell even higher energy

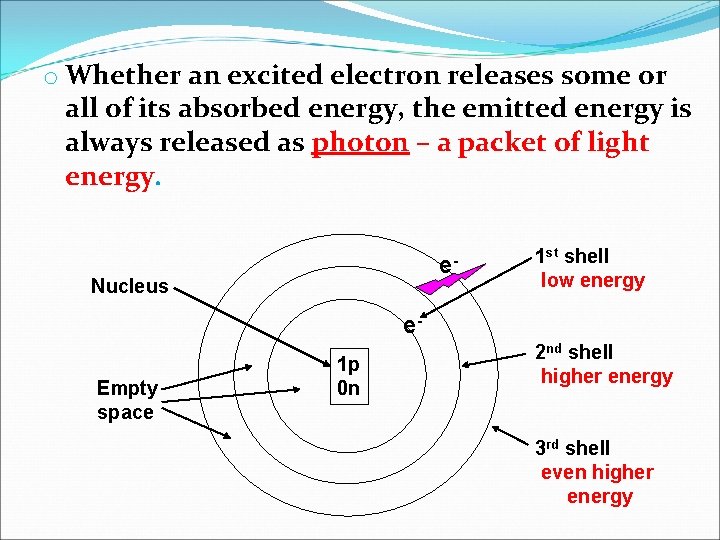
o Whether an excited electron releases some or all of its absorbed energy, the emitted energy is always released as photon – a packet of light energy. e- Nucleus 1 st shell low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy

EThe larger the jump back is, the higher energy the released photon will be. EWhen an electron jumps back to the 1 st shell in a hydrogen atom, the released photon will be in the ultraviolet region of the light spectrum. EWhen an electron jumps back to the 2 nd shell in a hydrogen atom, the released photon will be in the visible region of the light spectrum. EWhen an electron jumps back to the 3 rd shell in a hydrogen atom, the released photon will be in the infrared region of the light spectrum.

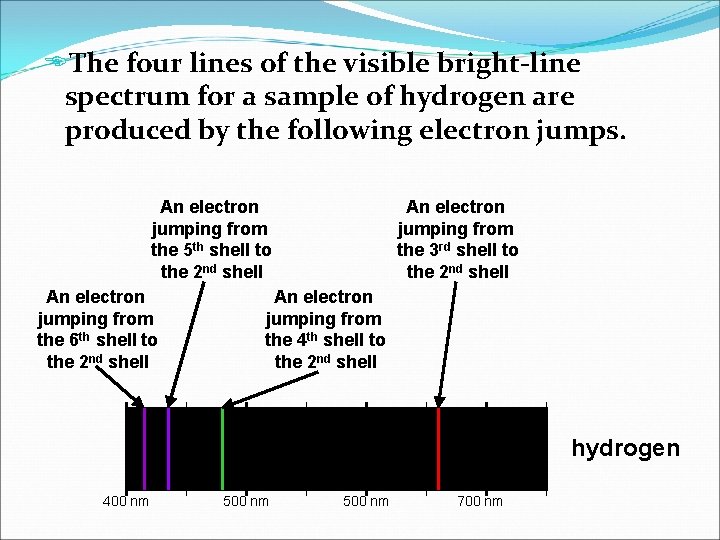
EThe four lines of the visible bright-line spectrum for a sample of hydrogen are produced by the following electron jumps. An electron jumping from the 5 th shell to the 2 nd shell An electron jumping from the 6 th shell to the 2 nd shell An electron jumping from the 3 rd shell to the 2 nd shell An electron jumping from the 4 th shell to the 2 nd shell hydrogen 400 nm 500 nm 700 nm

We are made of star stuff. . . What are stars made of?

o Even though Bohr’s atomic model has some serious flaws, it is still used to explain many chemical phenomena. 1)Which electron shell has the most energy? (1) 1 st shell (2) 2 nd shell 2) (3) 3 rd shell (4) 4 th shell An electron becomes excited by (1) absorbing energy (2) releasing energy (3) producing energy (4) destroying energy

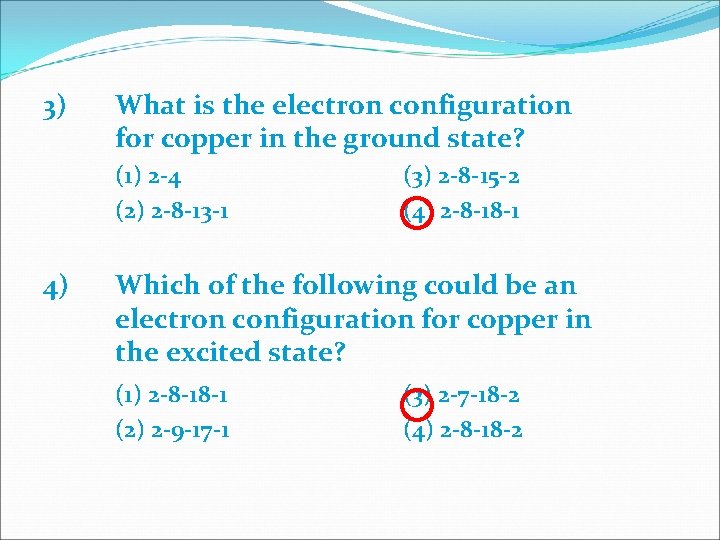
3) What is the electron configuration for copper in the ground state? (1) 2 -4 (2) 2 -8 -13 -1 4) (3) 2 -8 -15 -2 (4) 2 -8 -18 -1 Which of the following could be an electron configuration for copper in the excited state? (1) 2 -8 -18 -1 (2) 2 -9 -17 -1 (3) 2 -7 -18 -2 (4) 2 -8 -18 -2

5) In order for an excited state electron to return to the ground state it must (1) absorb energy (2) release energy 6) (3) store energy (4) destroy energy A line in an element’s bright-line spectrum is produced when (1) a ground state electron absorbs energy (2) an excited state electron releases energy (3) an excited state electron absorbs energy (4) a ground state electron releases energy

. 7) Which of the following types of light has the most energy? (1) radio waves (2) infrared light 8) (3) visible light (4) ultraviolet light Which of the following colors in the visible spectrum has the most energy? (1) red (2) violet (3) yellow (4) blue R O Y G B V

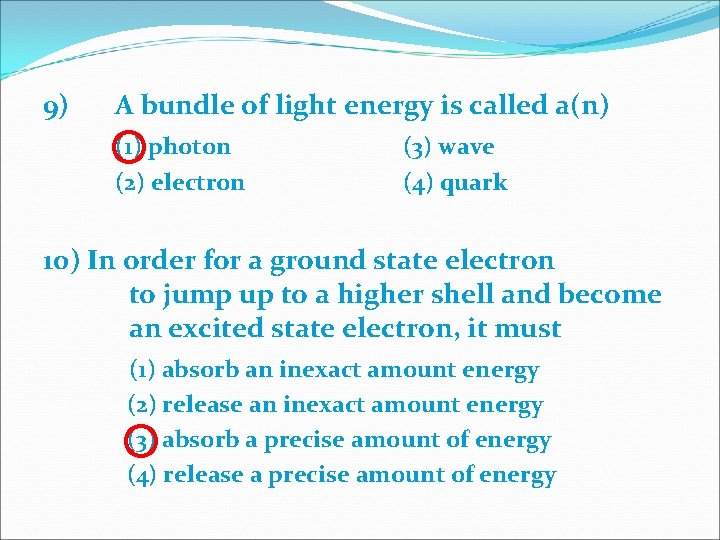
9) A bundle of light energy is called a(n) (1) photon (2) electron (3) wave (4) quark 10) In order for a ground state electron to jump up to a higher shell and become an excited state electron, it must (1) absorb an inexact amount energy (2) release an inexact amount energy (3) absorb a precise amount of energy (4) release a precise amount of energy

11) Which of the following gave evidence that electrons are found in energy shells around the nuclei of atoms? (1) the bright-line spectra of elements (2) elements combine in whole number ratios (3) cathode rays are attracted to a positive plate (4) 99% of alpha particles pass through gold foil

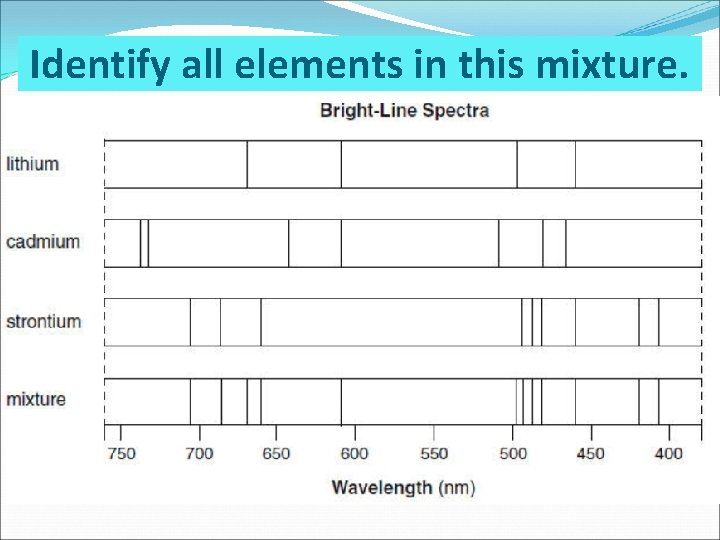
Identify all elements in this mixture.

How Neon Lights Work