Reading quiz get out a sheet of paper






- Slides: 21

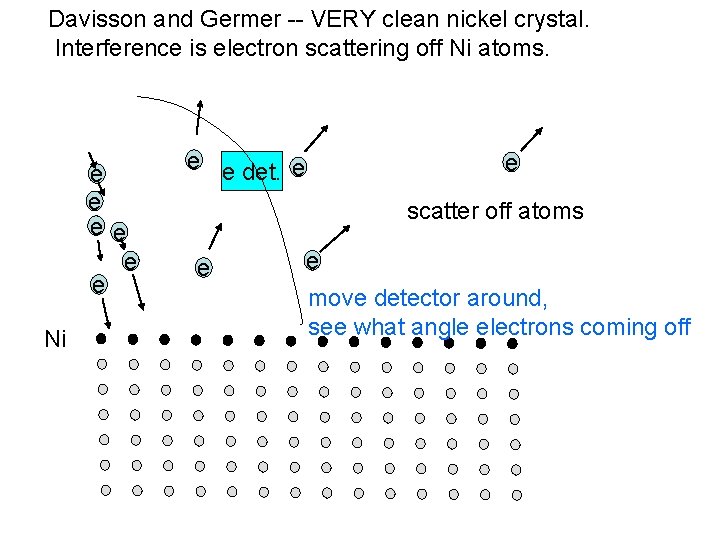
Reading quiz – get out a sheet of paper and a writing utensil. In the Davisson Germer experiment, Davisson and Germer shot a beam of electrons at a lattice of Nickel atoms and found that the electrons were only detected at certain angles. Explain the reason for this result and why it was important.

Review of Bohr and de. Broglie • Background: – Balmer found equation for Hydrogen spectrum but didn’t know what it meant. – Rutherford found that atoms had a nucleus, but didn’t know why electrons didn’t spiral in. • Bohr postulates quantized energy levels for no good reason, and predicts Balmer’s equation. • de. Broglie postulates that electrons are waves, and predicts Bohr’s quantized energy levels. • Note: no experimental difference between Bohr model and de. Broglie model, but de. Broglie is a lot more satisfying.

Models of the Atom • Thomson – Plum Pudding – – – Why? Known that negative charges can be removed from atom. – Problem: just a random guess • Rutherford – Solar System – Why? Scattering showed hard core. – Problem: electrons should spiral into nucleus in ~10 -11 sec. + • Bohr – fixed energy levels – Why? Explains spectral lines. – Problem: No reason for fixed energy levels • de. Broglie – electron standing waves – Why? Explains fixed energy levels – Problem: still only works for Hydrogen. • Schrodinger – will save the day!! + + –

de. Broglie Waves • • This is a great story. But is it true? If so, why no observations of electron waves? What would you need to see to believe that this is actually true? Today: Electron interference! • Why electron waves are hard to see • Designing experiment possible to see with early 1900 s tech. • How done by Clinton Davisson and Lester Germer • Why their technique still used today (LEED) • How to interpret.

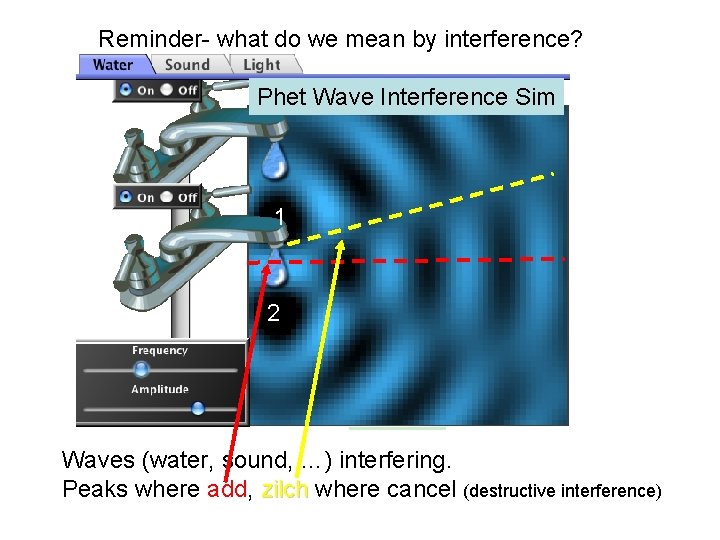
Reminder- what do we mean by interference? Phet Wave Interference Sim 1 2 Waves (water, sound, …) interfering. Peaks where add, zilch where cancel (destructive interference) zilch

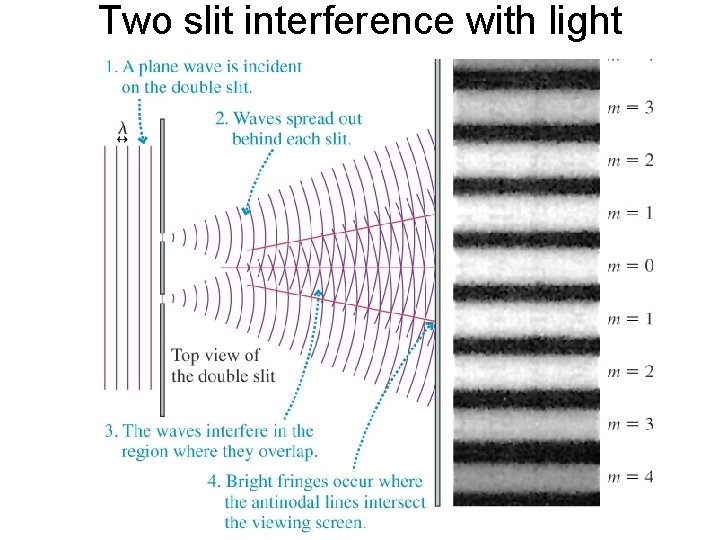
Two slit interference with light

Question in 1920 s So can we just do same experiment but replace beam of light with beam of electrons to check de. Broglie? Let’s work through the design to see what expect to see, what required to do proper experiment. step 1. Go off and play with making beams of electrons. Find can make beams of energies between ~25 -1000 e. V. V step 2. Calculate signal would expect to see from double slit. Typical for light: slits ~0. 5 mm apart

step 1. Go off and play with making beams of electrons. Find can make beams of energies between ~25 -1000 e. V. step 2. Calculate signal would expect to see from double slit. Typical for light: slits ~0. 5 mm apart Can we just repeat light double slit experiment with electrons? a. yes. (if so, precisely what would experimental results would you expect? ) b. no. (if so, precisely why not? )

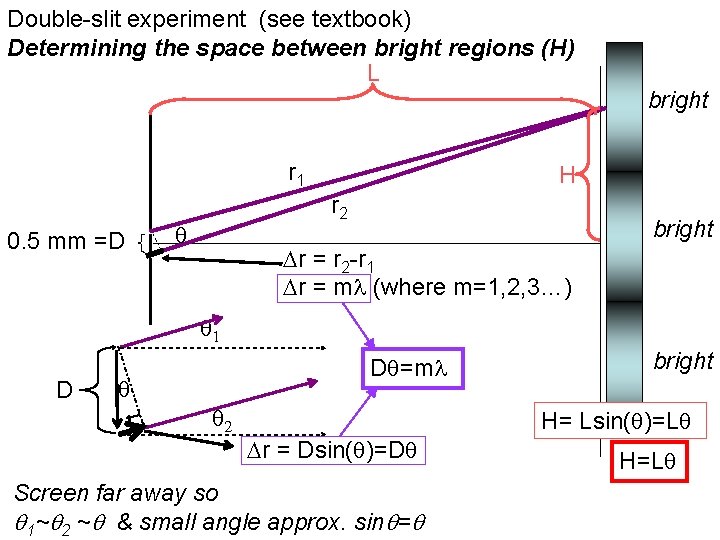
Double-slit experiment (see textbook) Determining the space between bright regions (H) L bright r 1 0. 5 mm =D H r 2 bright r = r 2 -r 1 r = m (where m=1, 2, 3…) 1 D D =m 2 r = Dsin( )=D Screen far away so 1~ 2 ~ & small angle approx. sin = bright H= Lsin( )=L H=L

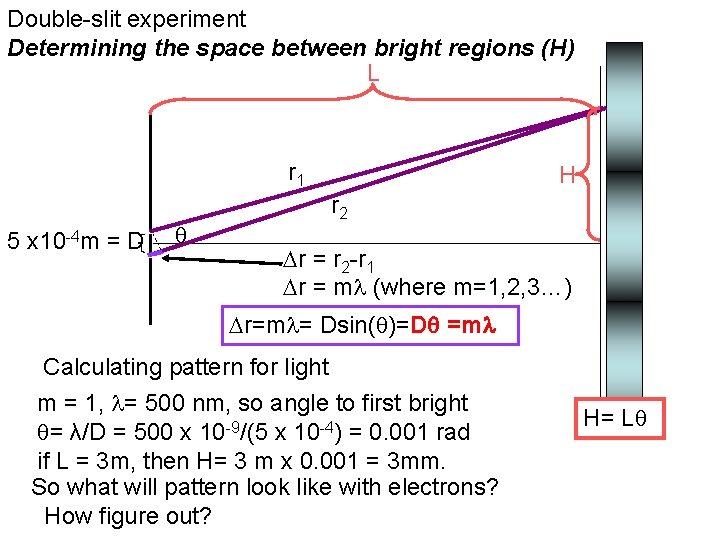
Double-slit experiment Determining the space between bright regions (H) L r 1 5 x 10 -4 m = D H r 2 r = r 2 -r 1 r = m (where m=1, 2, 3…) r=m = Dsin( )=D =m Calculating pattern for light m = 1, = 500 nm, so angle to first bright = λ/D = 500 x 10 -9/(5 x 10 -4) = 0. 001 rad if L = 3 m, then H= 3 m x 0. 001 = 3 mm. So what will pattern look like with electrons? How figure out? H= L

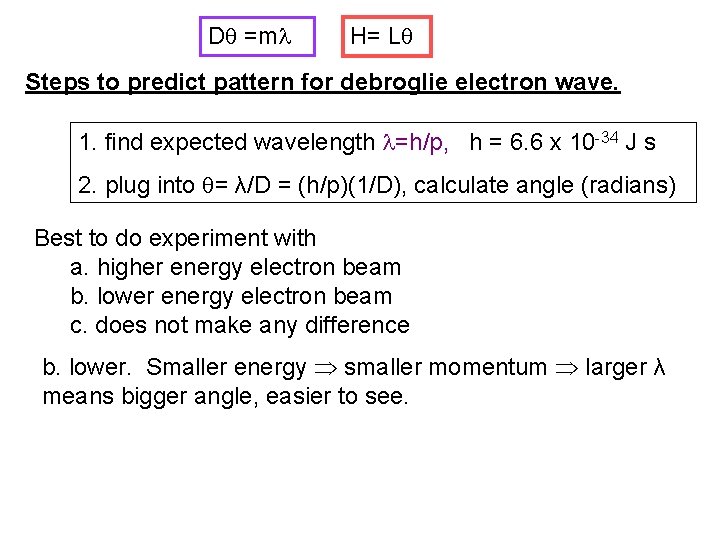
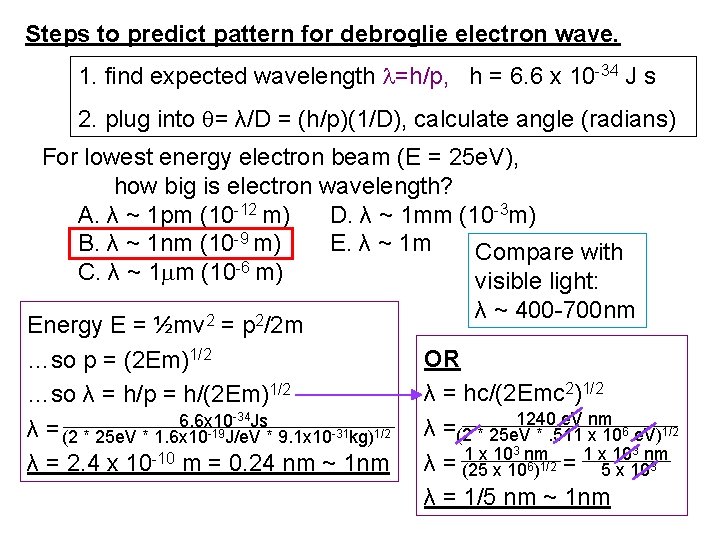
D =m H= L Steps to predict pattern for debroglie electron wave. 1. find expected wavelength =h/p, h = 6. 6 x 10 -34 J s 2. plug into = λ/D = (h/p)(1/D), calculate angle (radians) Best to do experiment with a. higher energy electron beam b. lower energy electron beam c. does not make any difference b. lower. Smaller energy smaller momentum larger λ means bigger angle, easier to see.

Steps to predict pattern for debroglie electron wave. 1. find expected wavelength =h/p, h = 6. 6 x 10 -34 J s 2. plug into = λ/D = (h/p)(1/D), calculate angle (radians) For lowest energy electron beam (E = 25 e. V), how big is electron wavelength? A. λ ~ 1 pm (10 -12 m) D. λ ~ 1 mm (10 -3 m) B. λ ~ 1 nm (10 -9 m) E. λ ~ 1 m Compare with C. λ ~ 1 m (10 -6 m) visible light: Energy E = ½mv 2 = p 2/2 m …so p = (2 Em)1/2 …so λ = h/p = h/(2 Em)1/2 6. 6 x 10 -34 Js λ = (2 * 25 e. V * 1. 6 x 10 -19 J/e. V * 9. 1 x 10 -31 kg)1/2 λ = 2. 4 x 10 -10 m = 0. 24 nm ~ 1 nm λ ~ 400 -700 nm OR λ = hc/(2 Emc 2)1/2 1240 e. V nm 6 e. V)1/2 λ = (2 * 25 e. V *. 511 x 103 nm λ = = (25 x 106)1/2 5 x 103 λ = 1/5 nm ~ 1 nm

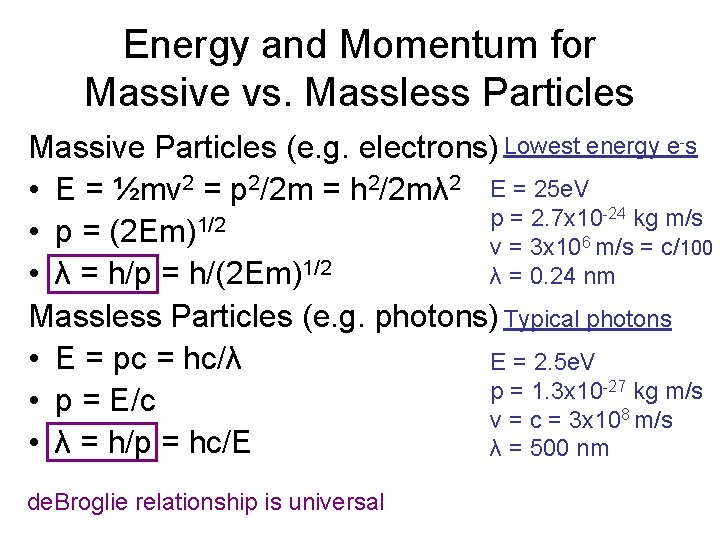
Energy and Momentum for Massive vs. Massless Particles Massive Particles (e. g. electrons) Lowest energy e-s • E = ½mv 2 = p 2/2 m = h 2/2 mλ 2 E = 25 e. V -24 kg m/s p = 2. 7 x 10 • p = (2 Em)1/2 v = 3 x 106 m/s = c/100 λ = 0. 24 nm • λ = h/p = h/(2 Em)1/2 Massless Particles (e. g. photons) Typical photons • E = pc = hc/λ E = 2. 5 e. V p = 1. 3 x 10 -27 kg m/s • p = E/c v = c = 3 x 108 m/s • λ = h/p = hc/E λ = 500 nm de. Broglie relationship is universal

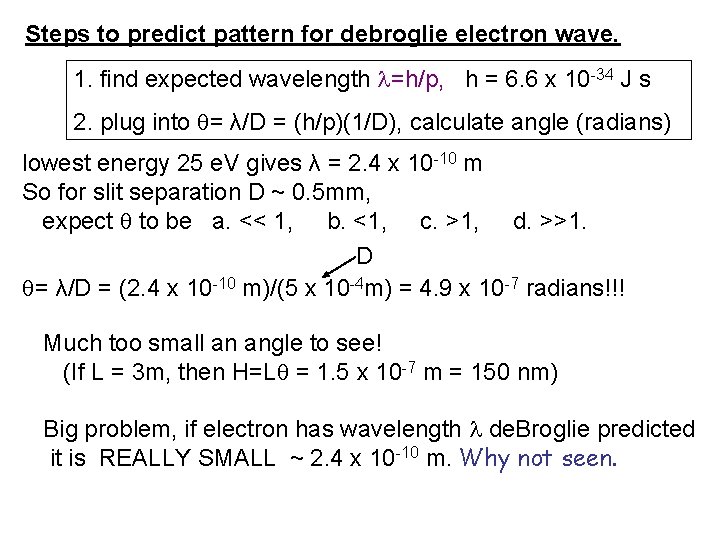
Steps to predict pattern for debroglie electron wave. 1. find expected wavelength =h/p, h = 6. 6 x 10 -34 J s 2. plug into = λ/D = (h/p)(1/D), calculate angle (radians) lowest energy 25 e. V gives λ = 2. 4 x 10 -10 m So for slit separation D ~ 0. 5 mm, expect to be a. << 1, b. <1, c. >1, d. >>1. D = λ/D = (2. 4 x 10 -10 m)/(5 x 10 -4 m) = 4. 9 x 10 -7 radians!!! Much too small an angle to see! (If L = 3 m, then H=L = 1. 5 x 10 -7 m = 150 nm) Big problem, if electron has wavelength de. Broglie predicted it is REALLY SMALL ~ 2. 4 x 10 -10 m. Why not seen.

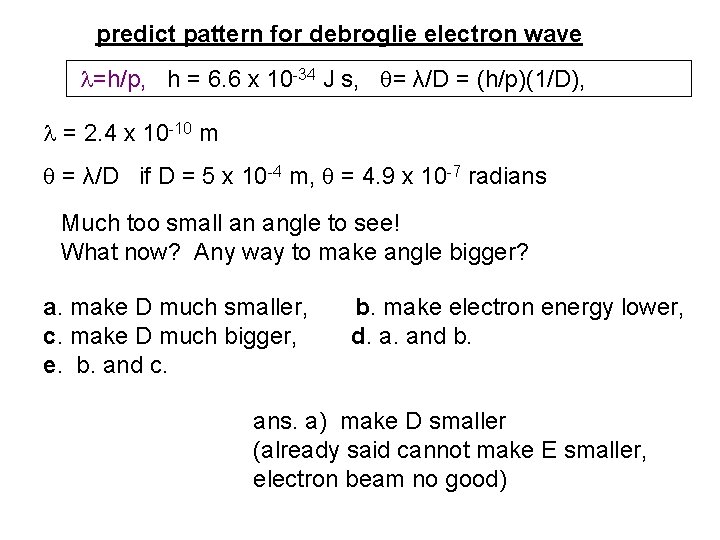
predict pattern for debroglie electron wave =h/p, h = 6. 6 x 10 -34 J s, = λ/D = (h/p)(1/D), = 2. 4 x 10 -10 m = λ/D if D = 5 x 10 -4 m, = 4. 9 x 10 -7 radians Much too small an angle to see! What now? Any way to make angle bigger? a. make D much smaller, b. make electron energy lower, c. make D much bigger, d. a. and b. e. b. and c. ans. a) make D smaller (already said cannot make E smaller, electron beam no good)

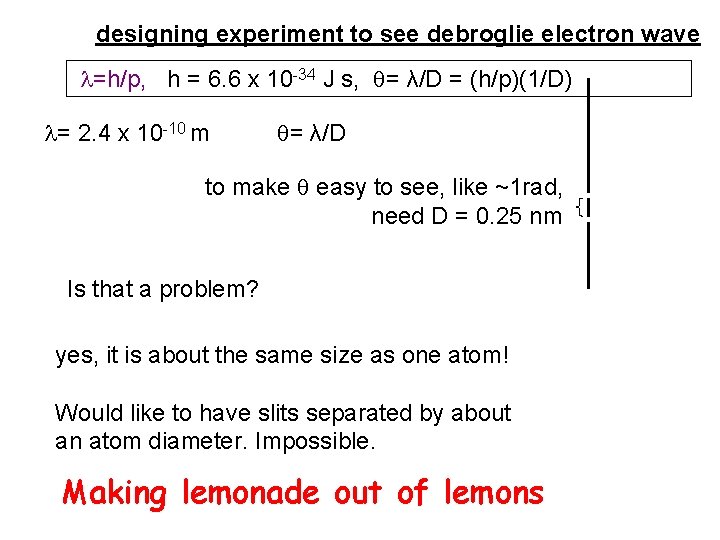
designing experiment to see debroglie electron wave =h/p, h = 6. 6 x 10 -34 J s, = λ/D = (h/p)(1/D) = 2. 4 x 10 -10 m = λ/D to make easy to see, like ~1 rad, need D = 0. 25 nm Is that a problem? yes, it is about the same size as one atom! Would like to have slits separated by about an atom diameter. Impossible. Making lemonade out of lemons

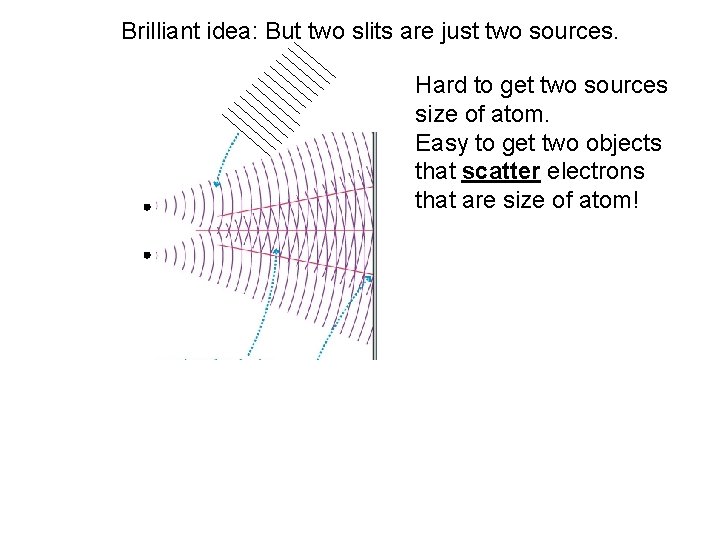
Brilliant idea: But two slits are just two sources. Hard to get two sources size of atom. Easy to get two objects that scatter electrons that are size of atom!

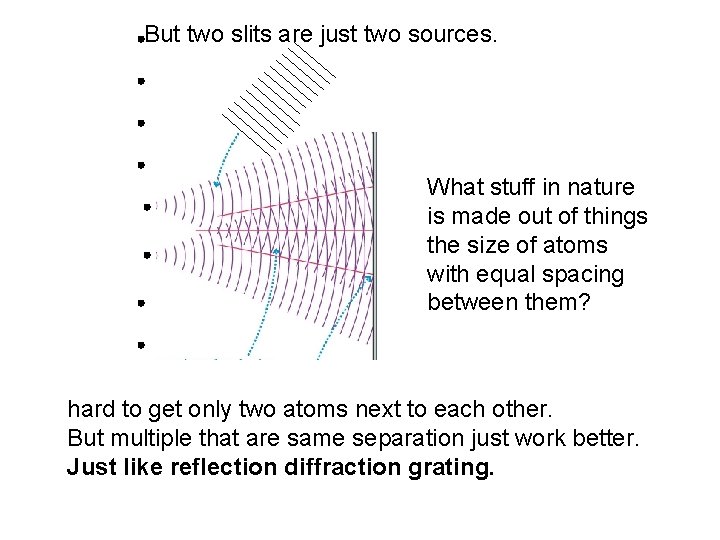
But two slits are just two sources. What stuff in nature is made out of things the size of atoms with equal spacing between them? hard to get only two atoms next to each other. But multiple that are same separation just work better. Just like reflection diffraction grating.

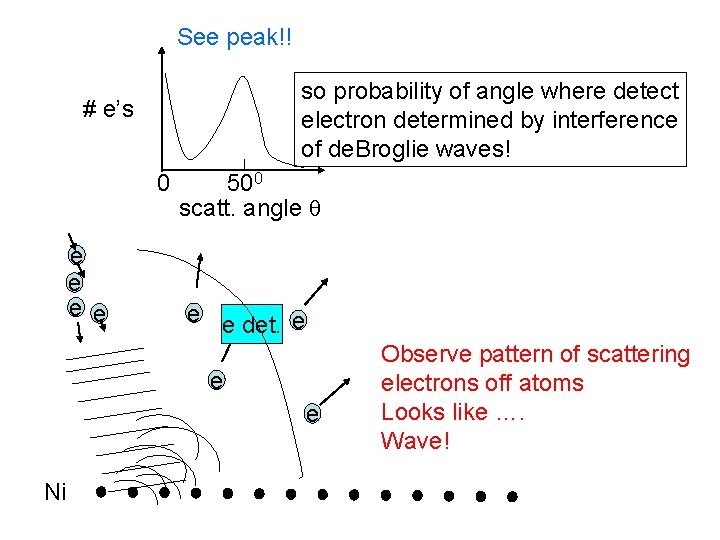
Davisson and Germer -- VERY clean nickel crystal. Interference is electron scattering off Ni atoms. e e e Ni e e det. e e scatter off atoms e e move detector around, see what angle electrons coming off

See peak!! so probability of angle where detect electron determined by interference of de. Broglie waves! # e’s 0 e e 500 scatt. angle e e det. e e e Ni Observe pattern of scattering electrons off atoms Looks like …. Wave!

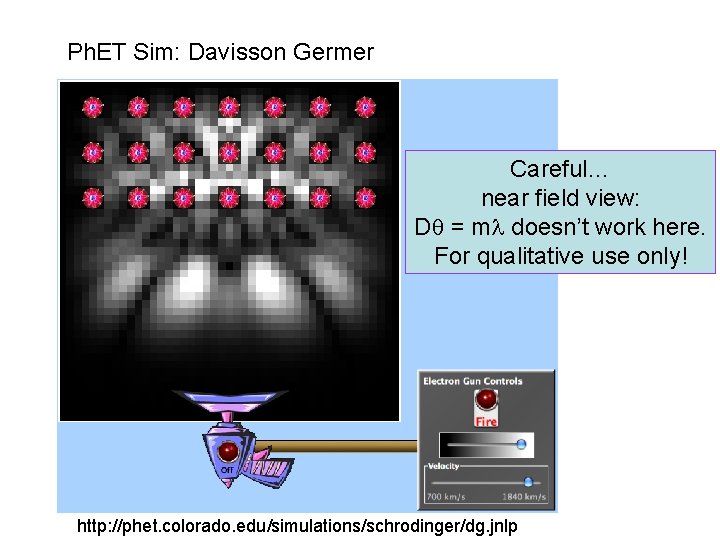
Ph. ET Sim: Davisson Germer Careful… near field view: D = m doesn’t work here. For qualitative use only! http: //phet. colorado. edu/simulations/schrodinger/dg. jnlp
 One direction songs with alliteration
One direction songs with alliteration Get on get off
Get on get off Get up get moving quiz
Get up get moving quiz Get up get moving quiz
Get up get moving quiz Get up get moving quiz
Get up get moving quiz Get up get moving quiz
Get up get moving quiz A piece get out of paper
A piece get out of paper Take out a sheet of paper
Take out a sheet of paper Take out a sheet of paper
Take out a sheet of paper Pre reading while reading and post reading activities
Pre reading while reading and post reading activities Sequence pseudocode example
Sequence pseudocode example Get focused get results
Get focused get results Place value gcse foundation questions
Place value gcse foundation questions Error intervals worksheet
Error intervals worksheet Cvpr buzz
Cvpr buzz Do you get a formula sheet on the ap physics 1 test
Do you get a formula sheet on the ap physics 1 test How to get a radical out of the denominator
How to get a radical out of the denominator Get out of jail free card
Get out of jail free card Get out of my school
Get out of my school What options did an accused witch have in salem
What options did an accused witch have in salem Get the word out we back up
Get the word out we back up Do words have power
Do words have power