Contents www trilondon ac uk New anticoagulants new










- Slides: 121

Contents www. tri-london. ac. uk

New anticoagulants, new guidelines, old problems: Why do we need a global AF registry? The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

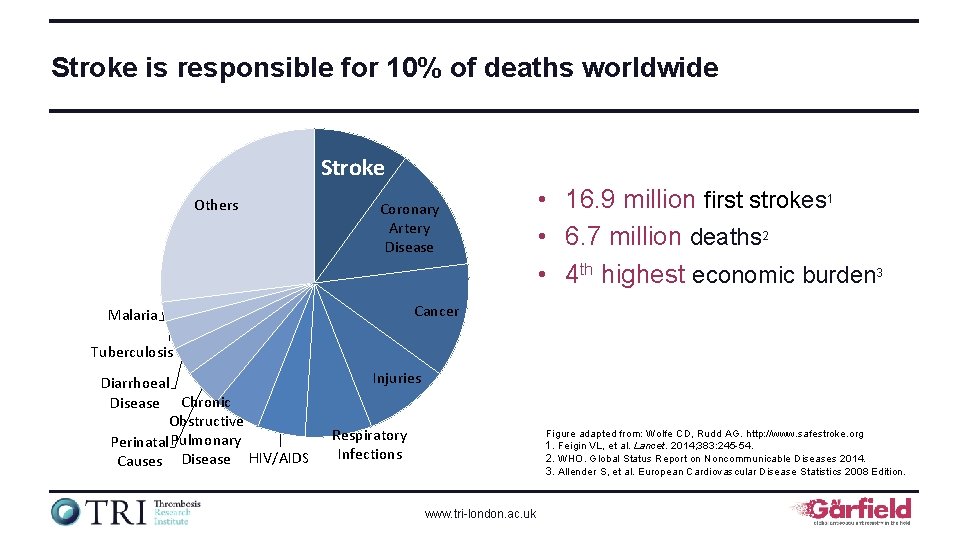
Stroke is responsible for 10% of deaths worldwide Stroke Others Coronary Artery Disease • 16. 9 million first strokes 1 • 6. 7 million deaths 2 • 4 th highest economic burden 3 Cancer Malaria Tuberculosis Diarrhoeal Disease Chronic Obstructive Perinatal Pulmonary Causes Disease HIV/AIDS Injuries Respiratory Infections Figure adapted from: Wolfe CD, Rudd AG. http: //www. safestroke. org 1. Feigin VL, et al. Lancet. 2014; 383: 245 -54. 2. WHO. Global Status Report on Noncommunicable Diseases 2014. 3. Allender S, et al. European Cardiovascular Disease Statistics 2008 Edition. www. tri-london. ac. uk

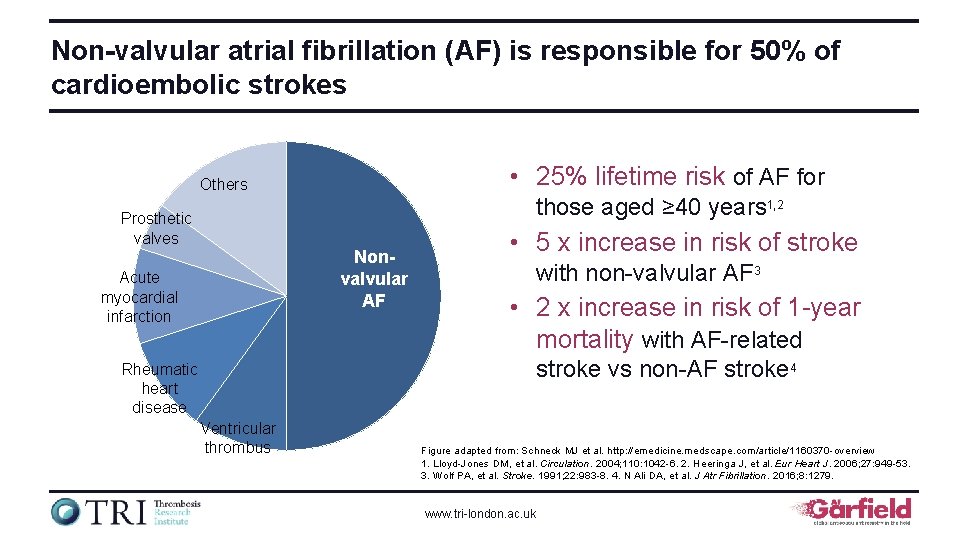
Non-valvular atrial fibrillation (AF) is responsible for 50% of cardioembolic strokes • 25% lifetime risk of AF for Others those aged ≥ 40 years 1, 2 Prosthetic valves Nonvalvular AF Acute myocardial infarction • 5 x increase in risk of stroke with non-valvular AF 3 • 2 x increase in risk of 1 -year mortality with AF-related stroke vs non-AF stroke 4 Rheumatic heart disease Ventricular thrombus Figure adapted from: Schneck MJ et al. http: //emedicine. medscape. com/article/1160370 -overview 1. Lloyd-Jones DM, et al. Circulation. 2004; 110: 1042 -6. 2. Heeringa J, et al. Eur Heart J. 2006; 27: 949 -53. 3. Wolf PA, et al. Stroke. 1991; 22: 983 -8. 4. N Ali DA, et al. J Atr Fibrillation. 2016; 8: 1279. www. tri-london. ac. uk

Atrial fibrillation • AF is the most common heart rhythm disturbance 1 • It is estimated that 1 in 4 individuals aged 40 years and older will develop AF 2, 3 • In 2010, the global prevalence of AF was estimated at 33. 5 million 4 • Owing to the aging population, this number is expected to double within 30 years 5 1. 2. 3. 4. 5. www. tri-london. ac. uk Nattel S, et al. Circ Arrhythm Electrophysiol. 2008; 1: 62 -73. Lloyd-Jones DM, et al. Circulation 2004; 110: 1042 -6. Heeringa J, et al. Eur Heart J. 2006; 27: 949 -53. Mozaffarian D, et al. Circulation. 2016; 133: e 38 -360. Go AS, et al. JAMA. 2001; 285: 2370 -5.

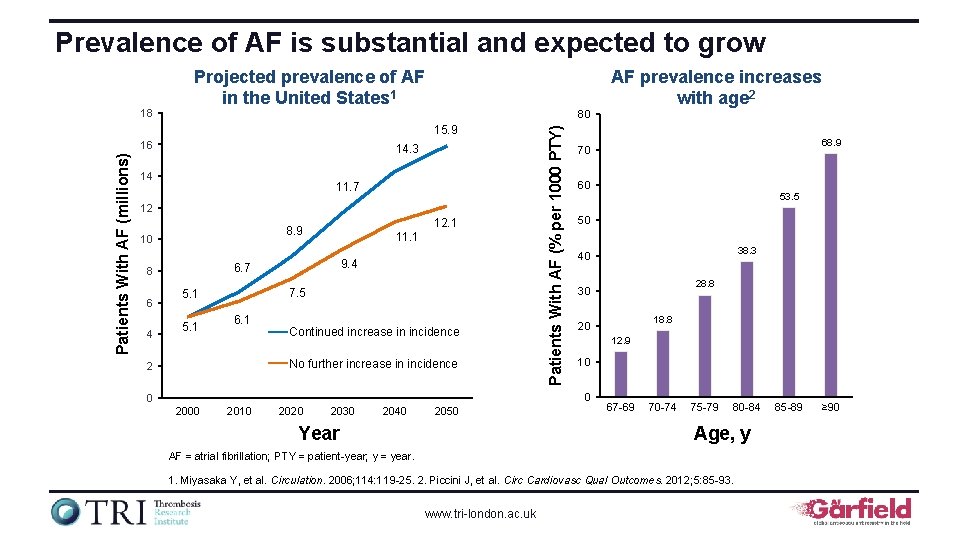
Prevalence of AF is substantial and expected to grow AF prevalence increases with age 2 80 15. 9 Patients With AF (millions) 16 14. 3 14 11. 7 12 10 4 11. 1 9. 4 6. 7 8 6 12. 1 8. 9 7. 5 5. 1 6. 1 Continued increase in incidence No further increase in incidence 2 Patients With AF (% per 1000 PTY) 18 Projected prevalence of AF in the United States 1 60 2000 2010 2020 2030 2040 2050 Year 53. 5 50 38. 3 40 28. 8 30 18. 8 20 12. 9 10 0 0 68. 9 70 67 -69 70 -74 75 -79 80 -84 Age, y AF = atrial fibrillation; PTY = patient-year; y = year. 1. Miyasaka Y, et al. Circulation. 2006; 114: 119 -25. 2. Piccini J, et al. Circ Cardiovasc Qual Outcomes. 2012; 5: 85 -93. www. tri-london. ac. uk 85 -89 ≥ 90

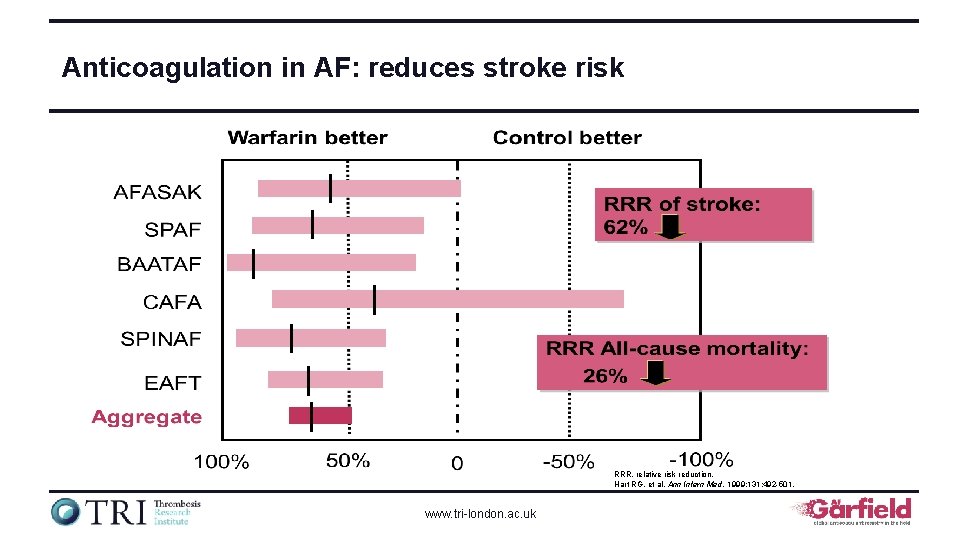
Anticoagulation in AF: reduces stroke risk RRR, relative risk reduction. Hart RG, et al. Ann Intern Med. 1999; 131: 492 -501. www. tri-london. ac. uk

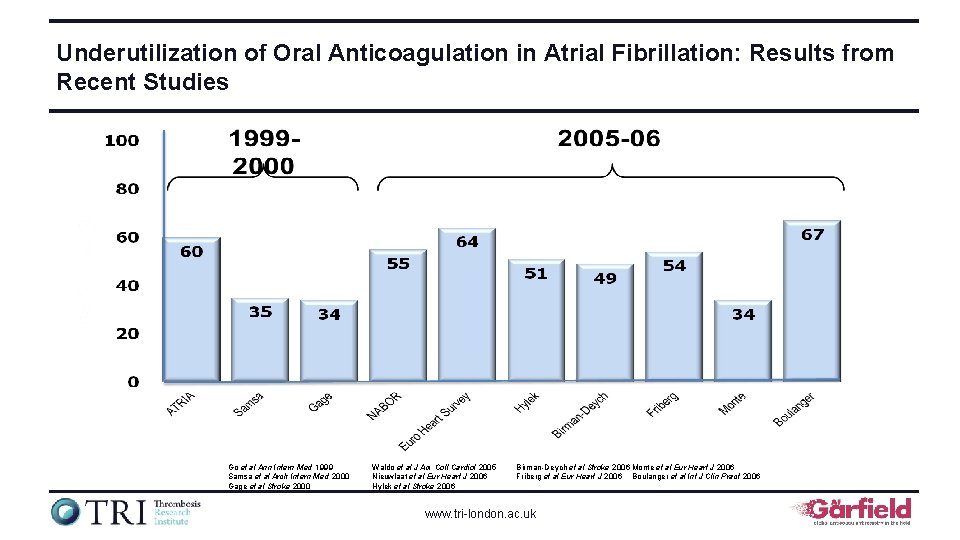
Underutilization of Oral Anticoagulation in Atrial Fibrillation: Results from Recent Studies Go et al Ann Intern Med 1999 Samsa et al Arch Intern Med 2000 Gage et al Stroke 2000 Waldo et al J Am Coll Cardiol 2005 Nieuwlaat et al Eur Heart J 2006 Hylek et al Stroke 2006 Birman-Deych et al Stroke 2006 Monte et al Eur Heart J 2006 Friberg et al Eur Heart J 2006 Boulanger et al Int J Clin Pract 2006 www. tri-london. ac. uk

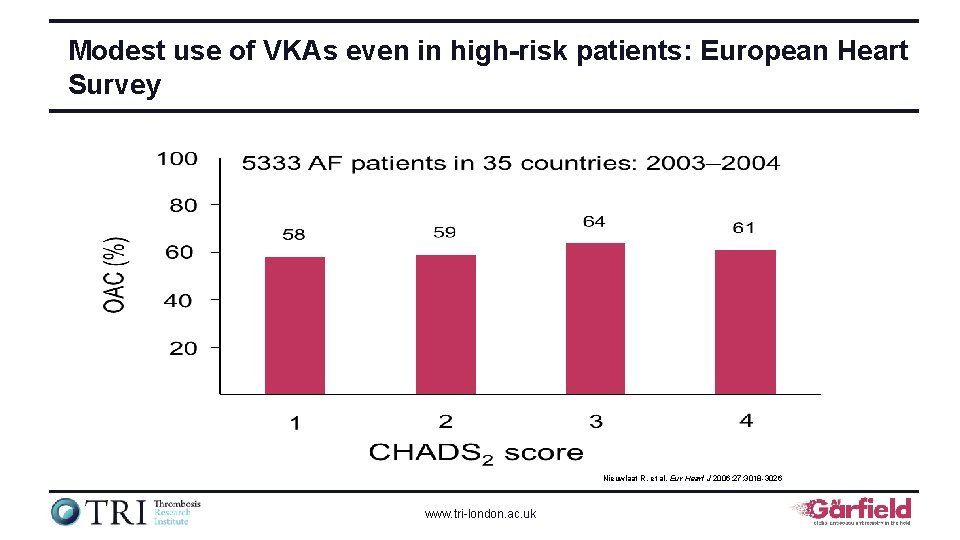
Modest use of VKAs even in high-risk patients: European Heart Survey Nieuwlaat R, et al. Eur Heart J 2006; 27: 3018 -3026 www. tri-london. ac. uk

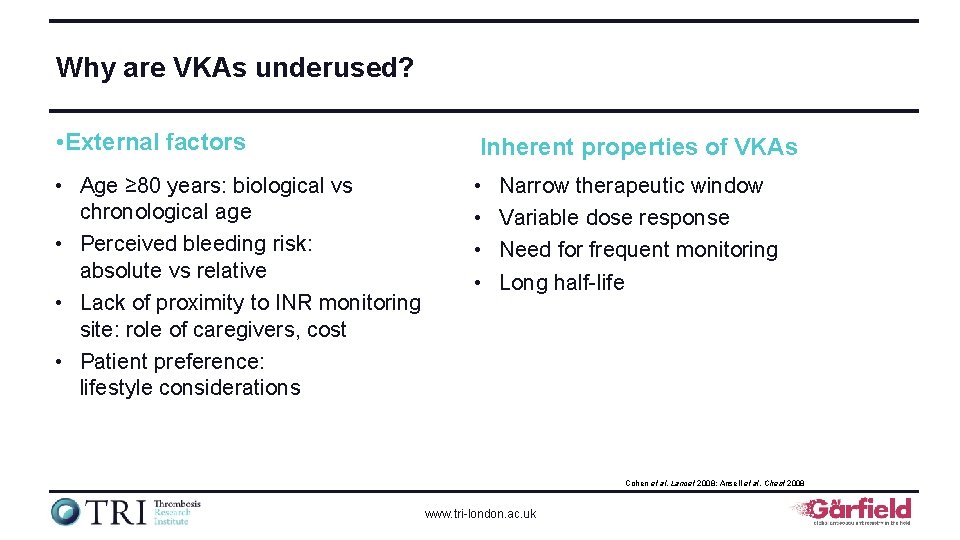
Why are VKAs underused? • External factors Inherent properties of VKAs • Age ≥ 80 years: biological vs chronological age • Perceived bleeding risk: absolute vs relative • Lack of proximity to INR monitoring site: role of caregivers, cost • Patient preference: lifestyle considerations • • Narrow therapeutic window Variable dose response Need for frequent monitoring Long half-life Cohen et al. Lancet 2008; Ansell et al. Chest 2008 www. tri-london. ac. uk

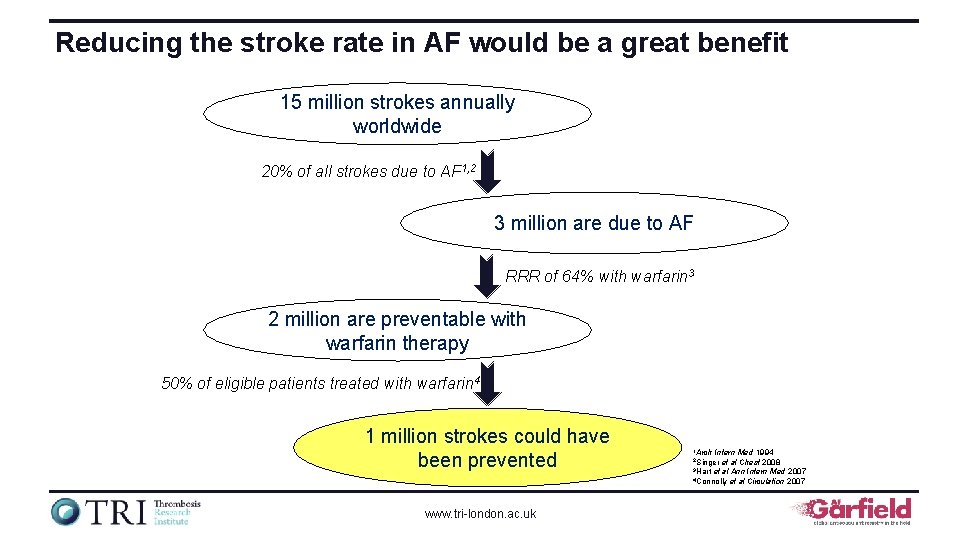
Reducing the stroke rate in AF would be a great benefit 15 million strokes annually worldwide 20% of all strokes due to AF 1, 2 3 million are due to AF RRR of 64% with warfarin 3 2 million are preventable with warfarin therapy 50% of eligible patients treated with warfarin 4 1 million strokes could have been prevented www. tri-london. ac. uk 1 Arch Intern Med 1994 et al Chest 2008 3 Hart et al Ann Intern Med 2007 4 Connolly et al Circulation 2007 2 Singer

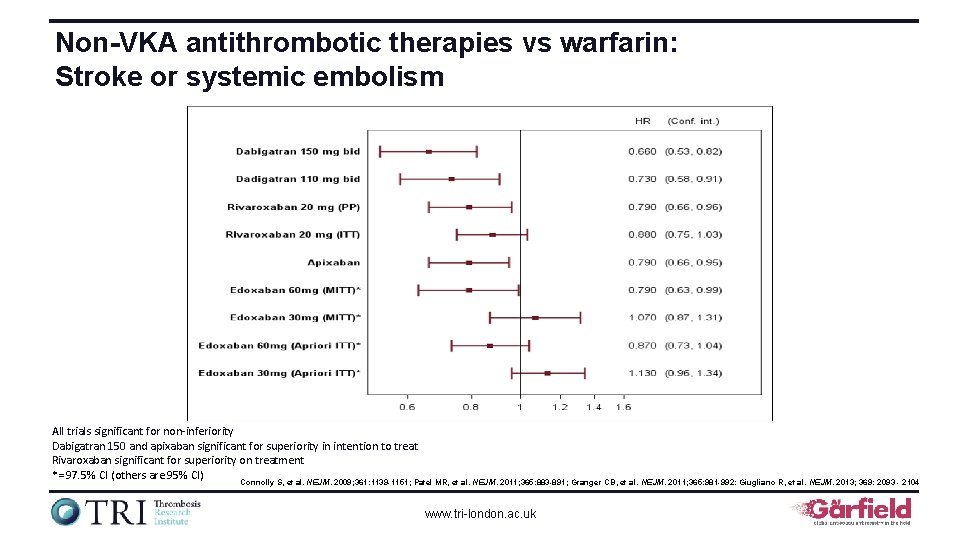
Non-VKA antithrombotic therapies vs warfarin: Stroke or systemic embolism All trials significant for non-inferiority Dabigatran 150 and apixaban significant for superiority in intention to treat Rivaroxaban significant for superiority on treatment *= 97. 5% CI (others are 95% CI) Connolly S, et al. NEJM. 2009; 361: 1139 -1151; Patel MR, et al. NEJM. 2011; 365: 883 -891; Granger CB, et al. NEJM. 2011; 365: 981 -992: Giugliano R, et al. NEJM. 2013; 369: 2093 - 2104 www. tri-london. ac. uk

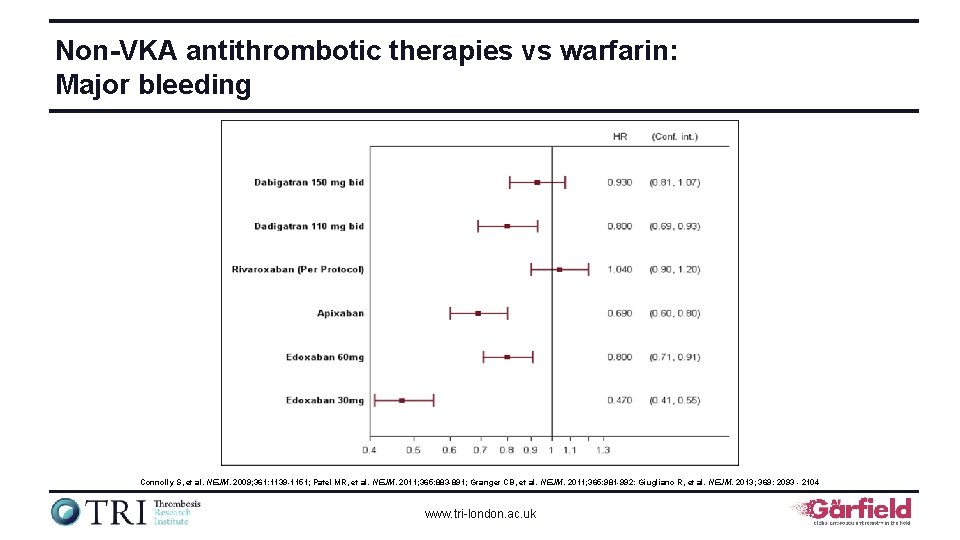
Non-VKA antithrombotic therapies vs warfarin: Major bleeding Connolly S, et al. NEJM. 2009; 361: 1139 -1151; Patel MR, et al. NEJM. 2011; 365: 883 -891; Granger CB, et al. NEJM. 2011; 365: 981 -992: Giugliano R, et al. NEJM. 2013; 369: 2093 - 2104 www. tri-london. ac. uk

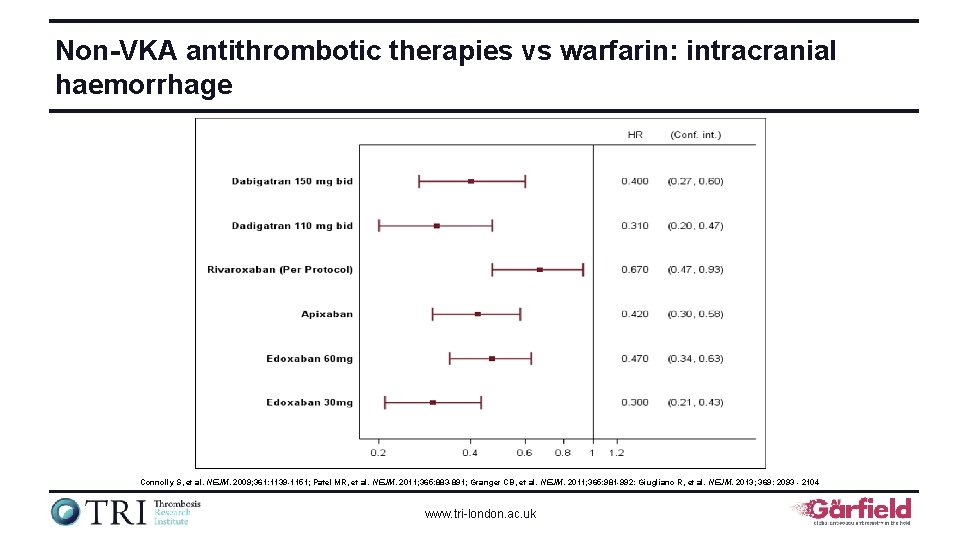
Non-VKA antithrombotic therapies vs warfarin: intracranial haemorrhage Connolly S, et al. NEJM. 2009; 361: 1139 -1151; Patel MR, et al. NEJM. 2011; 365: 883 -891; Granger CB, et al. NEJM. 2011; 365: 981 -992: Giugliano R, et al. NEJM. 2013; 369: 2093 - 2104 www. tri-london. ac. uk

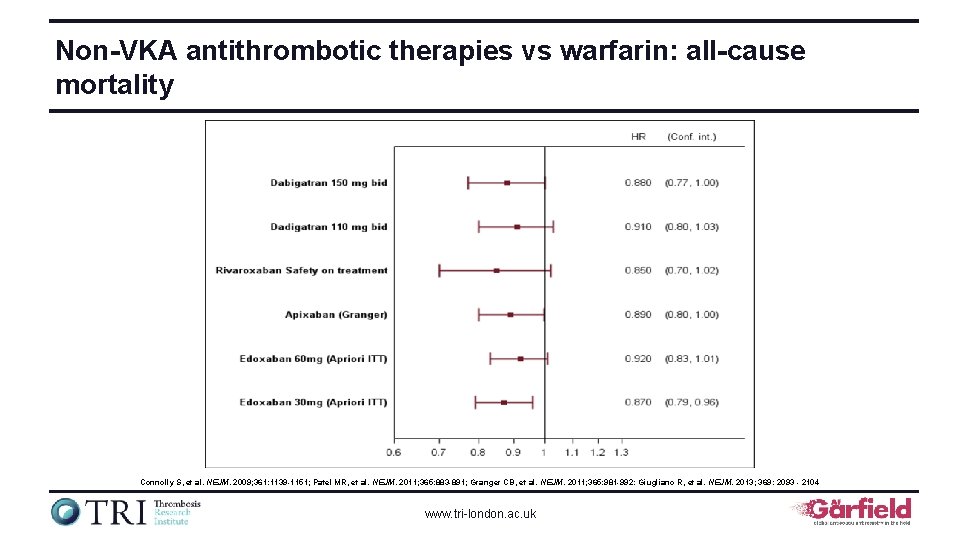
Non-VKA antithrombotic therapies vs warfarin: all-cause mortality Connolly S, et al. NEJM. 2009; 361: 1139 -1151; Patel MR, et al. NEJM. 2011; 365: 883 -891; Granger CB, et al. NEJM. 2011; 365: 981 -992: Giugliano R, et al. NEJM. 2013; 369: 2093 - 2104 www. tri-london. ac. uk

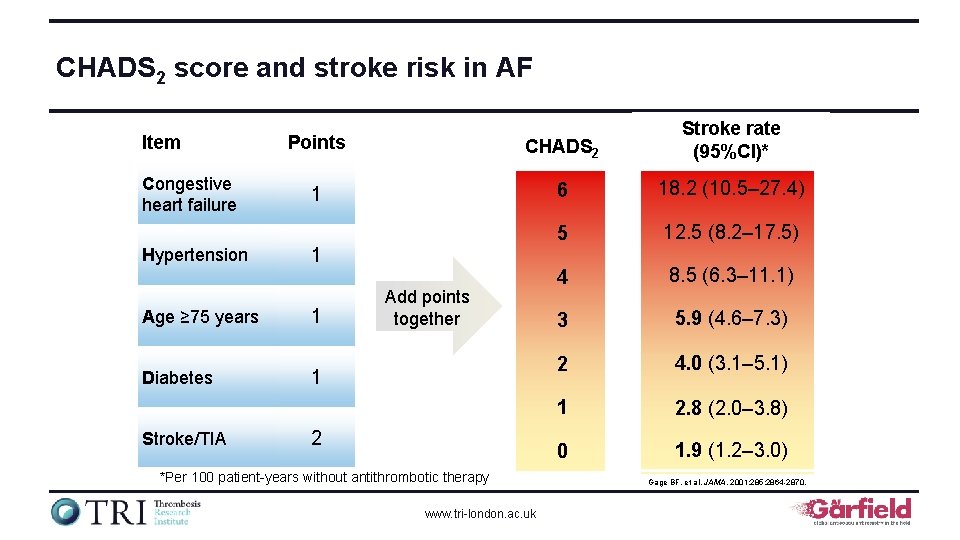
CHADS 2 score and stroke risk in AF Item Congestive heart failure Hypertension Age ≥ 75 years Diabetes Stroke/TIA Points CHADS 2 Stroke rate (95%CI)* 1 6 18. 2 (10. 5– 27. 4) 5 12. 5 (8. 2– 17. 5) 4 8. 5 (6. 3– 11. 1) 3 5. 9 (4. 6– 7. 3) 2 4. 0 (3. 1– 5. 1) 1 2. 8 (2. 0– 3. 8) 0 1. 9 (1. 2– 3. 0) 1 1 Add points together 1 2 *Per 100 patient-years without antithrombotic therapy www. tri-london. ac. uk Gage BF, et al. JAMA. 2001; 285: 2864 -2870.

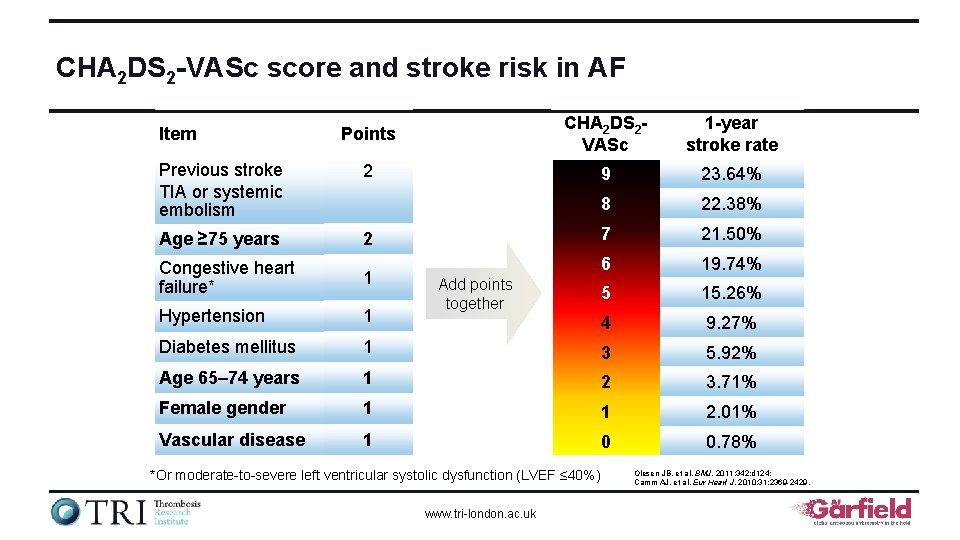
CHA 2 DS 2 -VASc score and stroke risk in AF Points CHA 2 DS 2 VASc 1 -year stroke rate Previous stroke TIA or systemic embolism 2 9 23. 64% 8 22. 38% Age ≥ 75 years 2 7 21. 50% Congestive heart failure* 1 6 19. 74% 5 15. 26% Hypertension 1 4 9. 27% Diabetes mellitus 1 3 5. 92% Age 65– 74 years 1 2 3. 71% Female gender 1 1 2. 01% Vascular disease 1 0 0. 78% Item Add points together *Or moderate-to-severe left ventricular systolic dysfunction (LVEF ≤ 40%) www. tri-london. ac. uk Olesen JB, et al. BMJ. 2011; 342: d 124; Camm AJ, et al. Eur Heart J. 2010; 31: 2369 -2429.

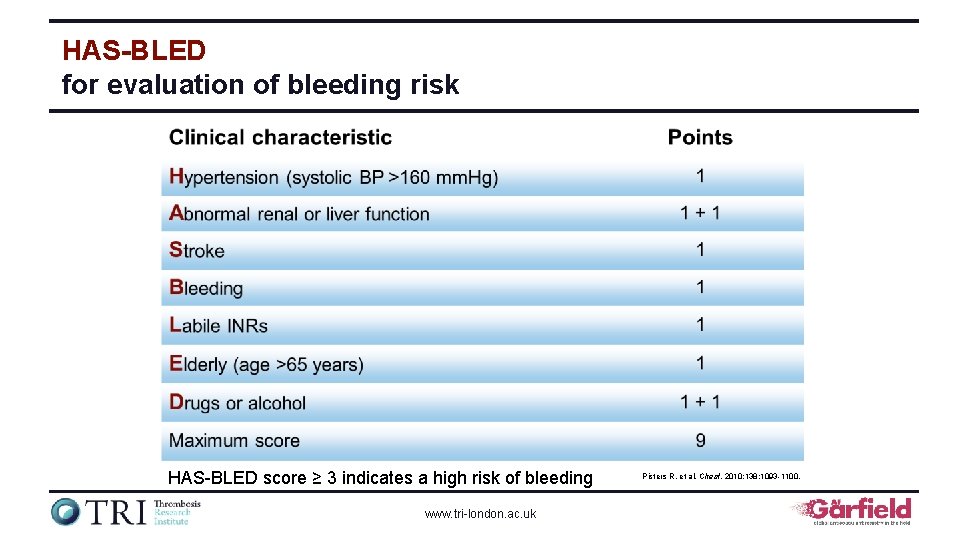
HAS-BLED for evaluation of bleeding risk HAS-BLED score ≥ 3 indicates a high risk of bleeding www. tri-london. ac. uk Pisters R, et al. Chest. 2010; 138: 1093 -1100.

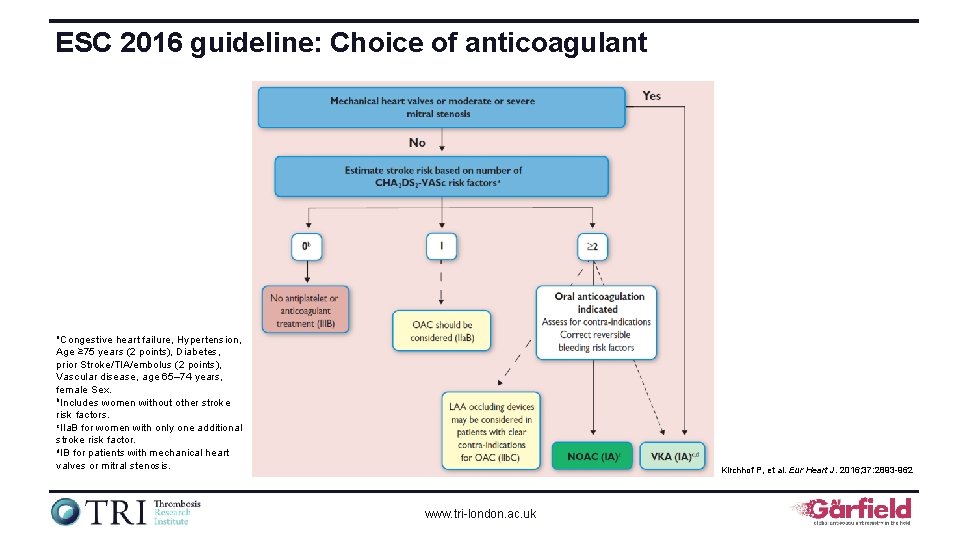
ESC 2016 guideline: Choice of anticoagulant a. Congestive heart failure, Hypertension, Age ≥ 75 years (2 points), Diabetes, prior Stroke/TIA/embolus (2 points), Vascular disease, age 65– 74 years, female Sex. b. Includes women without other stroke risk factors. c. IIa. B for women with only one additional stroke risk factor. d. IB for patients with mechanical heart valves or mitral stenosis. Kirchhof P, et al. Eur Heart J. 2016; 37: 2893 -962 www. tri-london. ac. uk

Outcomes research: Advancing the science of registries The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

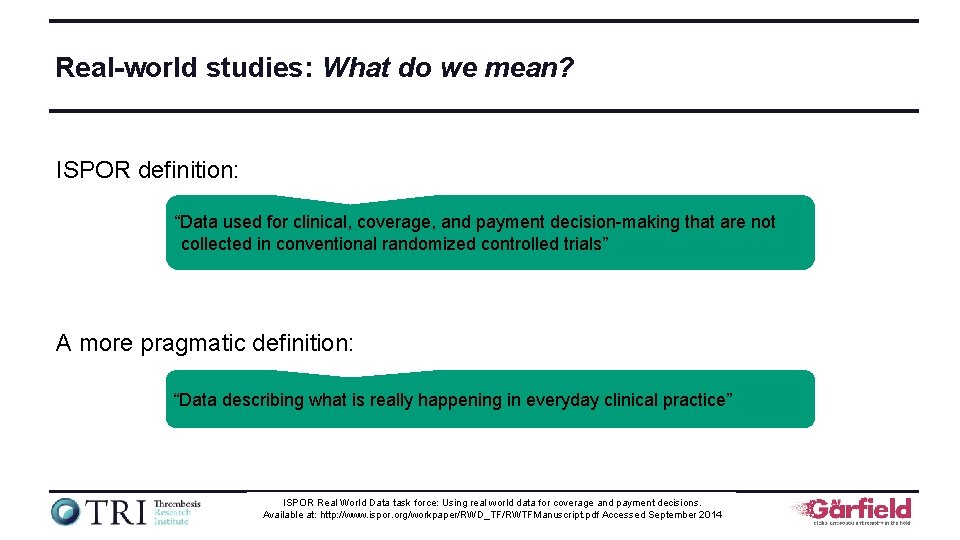
Real-world studies: What do we mean? ISPOR definition: “Data used for clinical, coverage, and payment decision-making that are not collected in conventional randomized controlled trials” A more pragmatic definition: “Data describing what is really happening in everyday clinical practice” ISPOR Real World Data task force: Using real world data for coverage and payment decisions. www. tri-london. ac. uk Available at: http: //www. ispor. org/workpaper/RWD_TF/RWTFManuscript. pdf Accessed September 2014

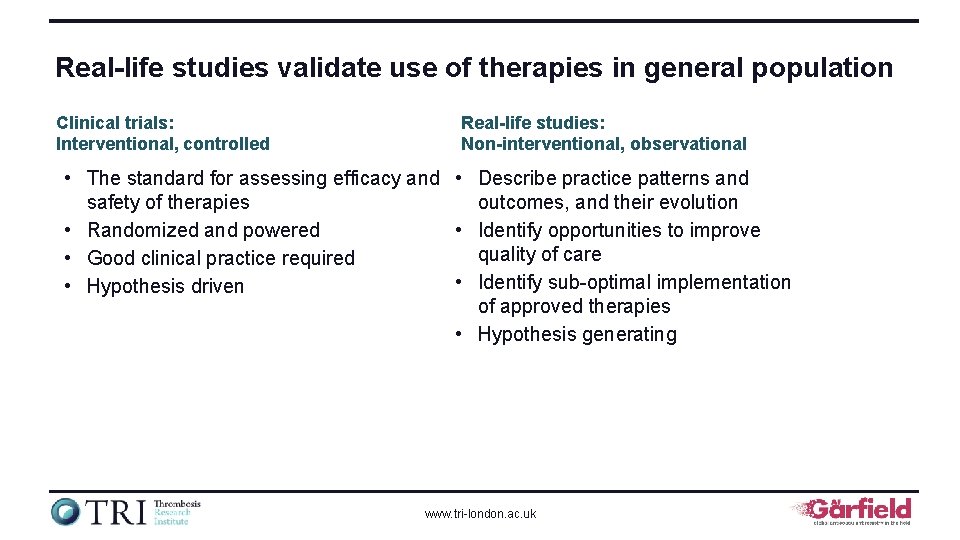
Real-life studies validate use of therapies in general population Clinical trials: Interventional, controlled Real-life studies: Non-interventional, observational • The standard for assessing efficacy and • Describe practice patterns and safety of therapies outcomes, and their evolution • Randomized and powered • Identify opportunities to improve quality of care • Good clinical practice required • Identify sub-optimal implementation • Hypothesis driven of approved therapies • Hypothesis generating www. tri-london. ac. uk

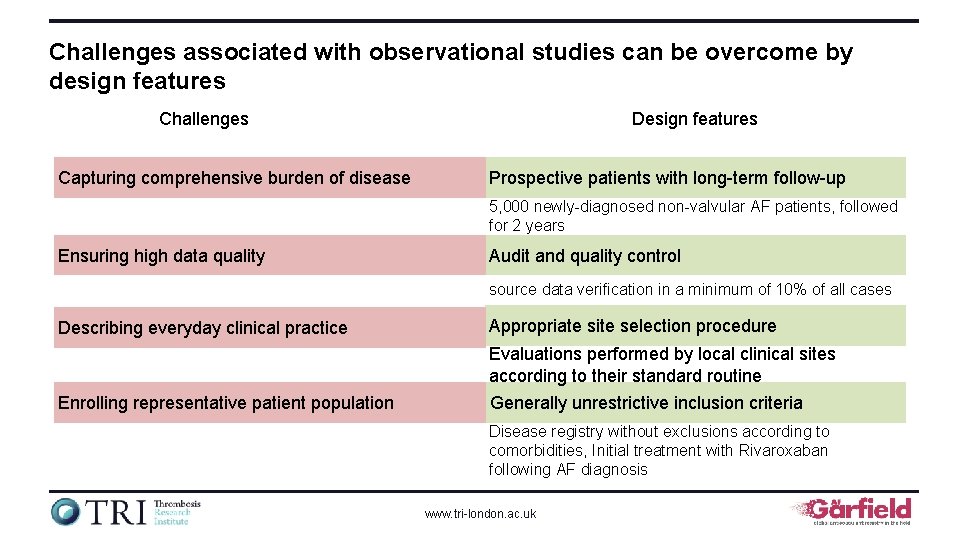
Challenges associated with observational studies can be overcome by design features Challenges Capturing comprehensive burden of disease Design features Prospective patients with long-term follow-up 5, 000 newly-diagnosed non-valvular AF patients, followed for 2 years Ensuring high data quality Audit and quality control source data verification in a minimum of 10% of all cases Describing everyday clinical practice Appropriate site selection procedure Evaluations performed by local clinical sites according to their standard routine Enrolling representative patient population Generally unrestrictive inclusion criteria Disease registry without exclusions according to comorbidities, Initial treatment with Rivaroxaban following AF diagnosis www. tri-london. ac. uk

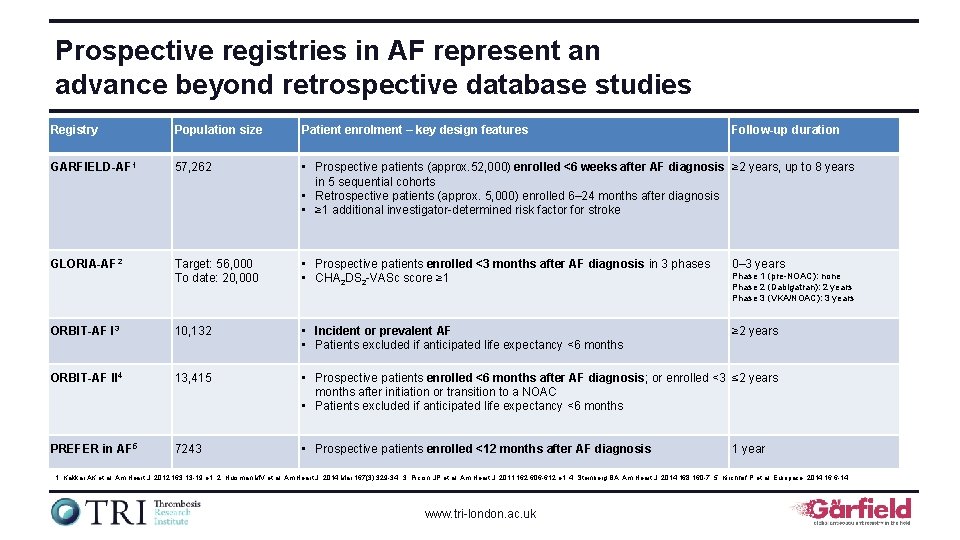
Prospective registries in AF represent an advance beyond retrospective database studies Registry Population size Patient enrolment – key design features Follow-up duration GARFIELD-AF 1 57, 262 • Prospective patients (approx. 52, 000) enrolled <6 weeks after AF diagnosis ≥ 2 years, up to 8 years in 5 sequential cohorts • Retrospective patients (approx. 5, 000) enrolled 6– 24 months after diagnosis • ≥ 1 additional investigator-determined risk factor for stroke GLORIA-AF 2 Target: 56, 000 To date: 20, 000 • Prospective patients enrolled <3 months after AF diagnosis in 3 phases • CHA 2 DS 2 -VASc score ≥ 1 0– 3 years ORBIT-AF I 3 10, 132 • Incident or prevalent AF • Patients excluded if anticipated life expectancy <6 months ≥ 2 years ORBIT-AF II 4 13, 415 • Prospective patients enrolled <6 months after AF diagnosis; or enrolled <3 ≤ 2 years months after initiation or transition to a NOAC • Patients excluded if anticipated life expectancy <6 months PREFER in AF 5 7243 • Prospective patients enrolled <12 months after AF diagnosis Phase 1 (pre-NOAC): none Phase 2 (Dabigatran): 2 years Phase 3 (VKA/NOAC): 3 years 1 year 1. Kakkar AK et al. Am Heart J. 2012; 163: 13 -19 e 1; 2. Huisman MV et al. Am Heart J. 2014 Mar; 167(3): 329 -34; 3. Piccini JP et al. Am Heart J. 2011; 162: 606 -612. e 1; 4. Steinberg BA. Am Heart J. 2014; 168: 160 -7. 5. Kirchhof P et al. Europace. 2014; 16: 6 -14. www. tri-london. ac. uk

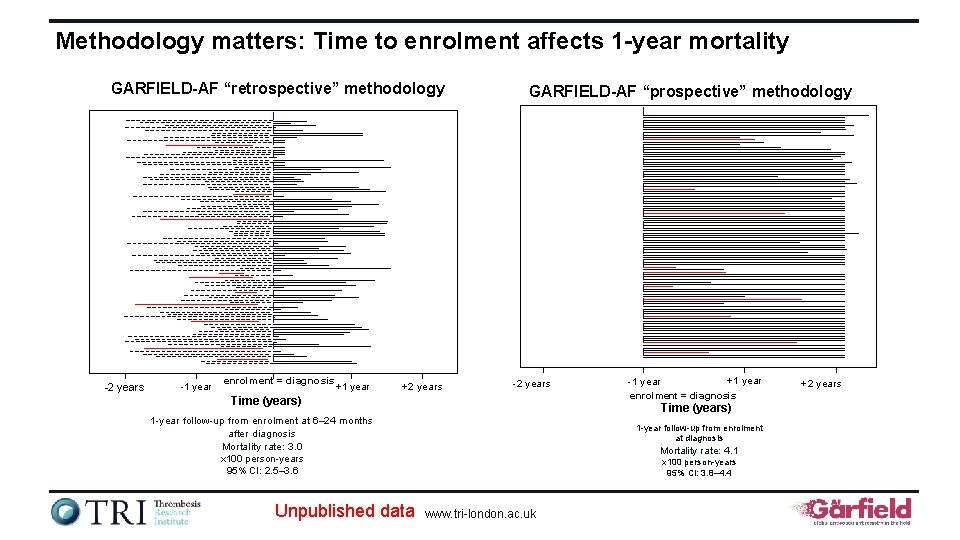
Methodology matters: Time to enrolment affects 1 -year mortality GARFIELD-AF “retrospective” methodology -2 years -1 year enrolment = diagnosis +1 year +2 years GARFIELD-AF “prospective” methodology -2 years Time (years) 1 -year follow-up from enrolment at 6– 24 months after diagnosis Mortality rate: 3. 0 x 100 person-years 95% CI: 2. 5– 3. 6 Unpublished data +1 year -1 year enrolment = diagnosis 1 -year follow-up from enrolment at diagnosis Mortality rate: 4. 1 x 100 person-years 95% CI: 3. 8– 4. 4 www. tri-london. ac. uk +2 years

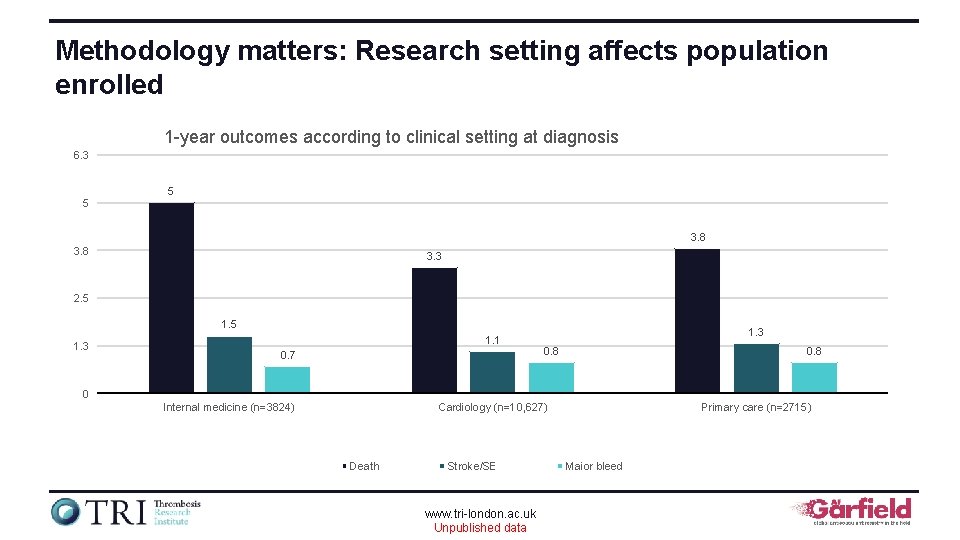
Methodology matters: Research setting affects population enrolled 1 -year outcomes according to clinical setting at diagnosis 6. 3 5 5 3. 8 3. 3 2. 5 1. 3 1. 1 0. 7 1. 3 0. 8 0 Internal medicine (n=3824) Cardiology (n=10, 627) Death Stroke/SE www. tri-london. ac. uk Unpublished data Primary care (n=2715) Major bleed

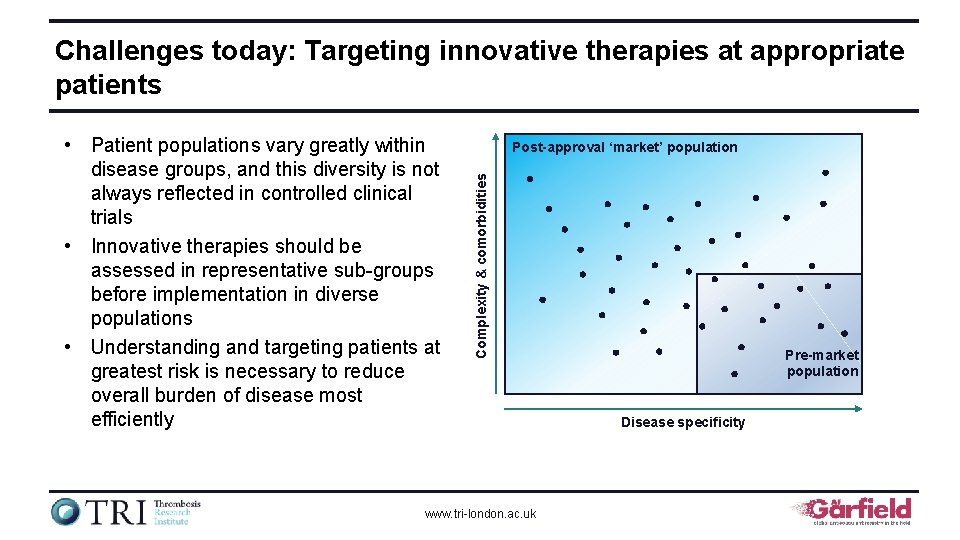
Challenges today: Targeting innovative therapies at appropriate patients Post-approval ‘market’ population Complexity & comorbidities • Patient populations vary greatly within disease groups, and this diversity is not always reflected in controlled clinical trials • Innovative therapies should be assessed in representative sub-groups before implementation in diverse populations • Understanding and targeting patients at greatest risk is necessary to reduce overall burden of disease most efficiently www. tri-london. ac. uk Pre-market population Disease specificity

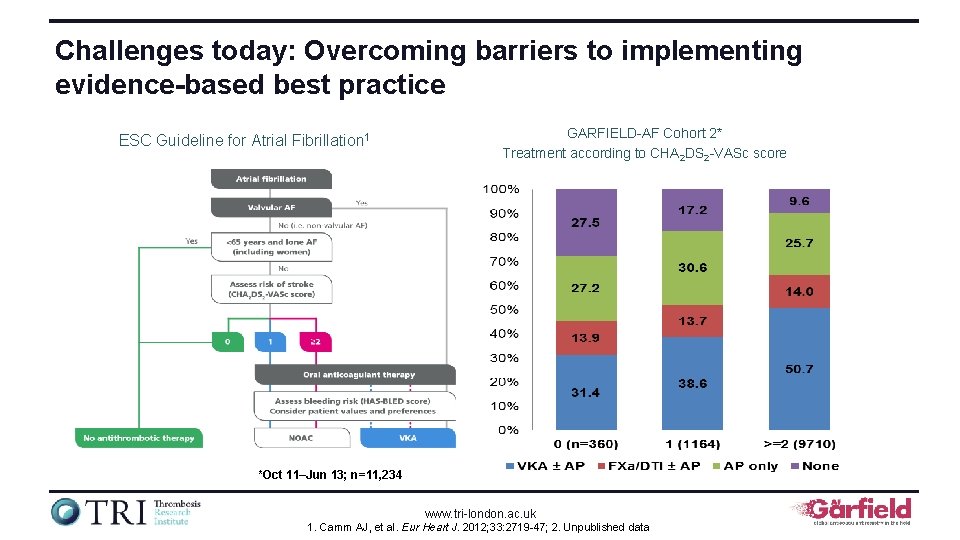
Challenges today: Overcoming barriers to implementing evidence-based best practice ESC Guideline for Atrial Fibrillation 1 GARFIELD-AF Cohort 2* Treatment according to CHA 2 DS 2 -VASc score *Oct 11–Jun 13; n=11, 234 www. tri-london. ac. uk 1. Camm AJ, et al. Eur Heart J. 2012; 33: 2719 -47; 2. Unpublished data

Challenges today: Understanding the patient perspective and its rise up the agenda • Systematically recording and evaluating patient experiences is key when assessing quality of healthcare – Patients views of their own health measured using PROMs are important treatment effects and complement conventional clinical endpoints • Internet and other media have increased number of information sources for patients – Patients are aware of competing anticoagulant therapies and look beyond clinical trials for information • The patient ‘vote’ is increasingly important – Their views and views of their representatives (patient groups) need to be listened to PROM = Patient-reported outcome measure www. tri-london. ac. uk

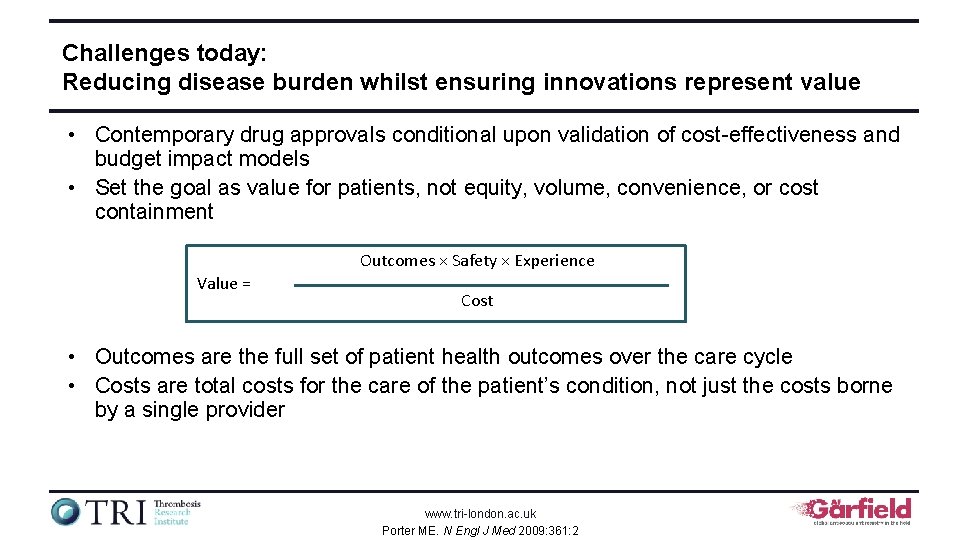
Challenges today: Reducing disease burden whilst ensuring innovations represent value • Contemporary drug approvals conditional upon validation of cost-effectiveness and budget impact models • Set the goal as value for patients, not equity, volume, convenience, or cost containment Outcomes × Safety × Experience Value = Cost • Outcomes are the full set of patient health outcomes over the care cycle • Costs are total costs for the care of the patient’s condition, not just the costs borne by a single provider www. tri-london. ac. uk Porter ME. N Engl J Med 2009: 361: 2

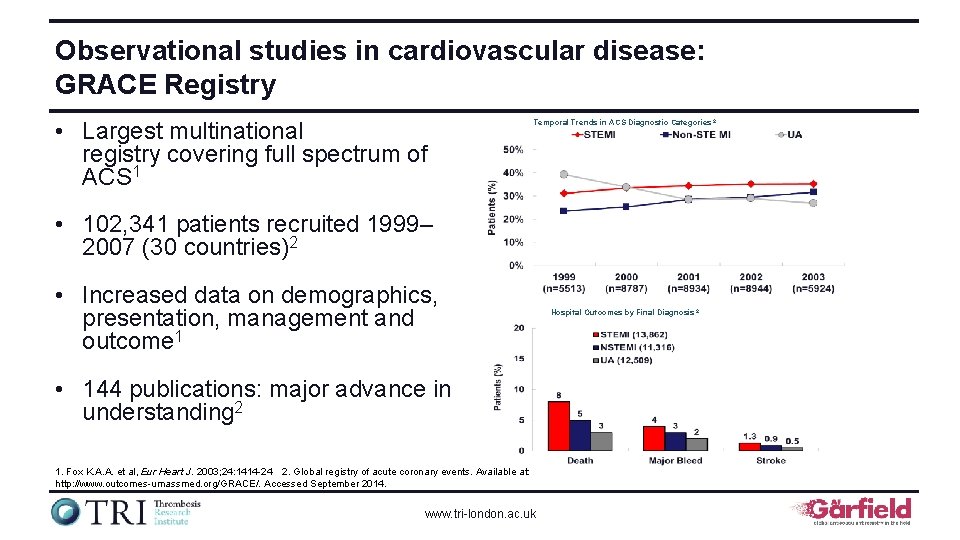
Observational studies in cardiovascular disease: GRACE Registry • Largest multinational registry covering full spectrum of ACS 1 Temporal Trends in ACS Diagnostic Categories 2 • 102, 341 patients recruited 1999– 2007 (30 countries)2 • Increased data on demographics, presentation, management and outcome 1 • 144 publications: major advance in understanding 2 1. Fox K. A. A. et al, Eur Heart J. 2003; 24: 1414 -24 2. Global registry of acute coronary events. Available at: http: //www. outcomes-umassmed. org/GRACE/. Accessed September 2014. www. tri-london. ac. uk Hospital Outcomes by Final Diagnosis 2

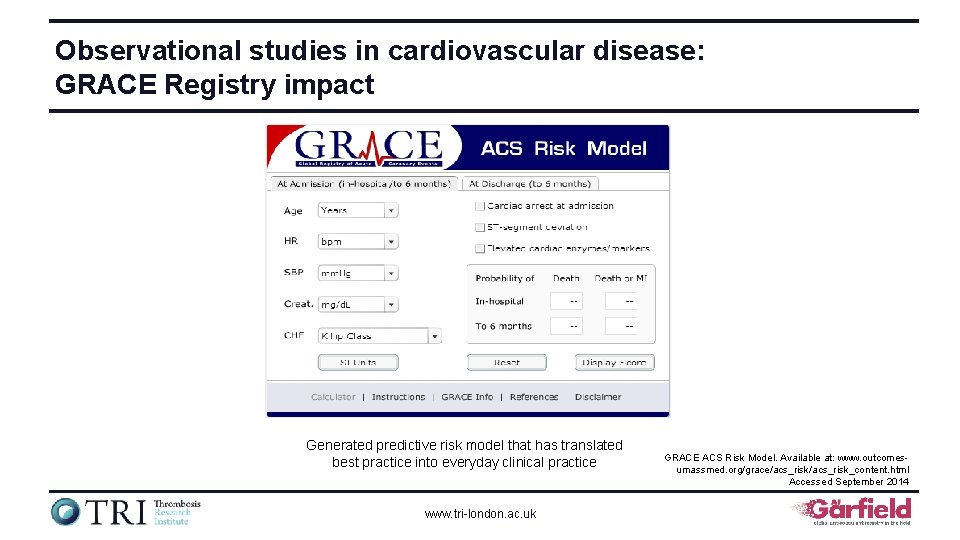
Observational studies in cardiovascular disease: GRACE Registry impact Generated predictive risk model that has translated best practice into everyday clinical practice www. tri-london. ac. uk GRACE ACS Risk Model. Available at: www. outcomesumassmed. org/grace/acs_risk_content. html Accessed September 2014

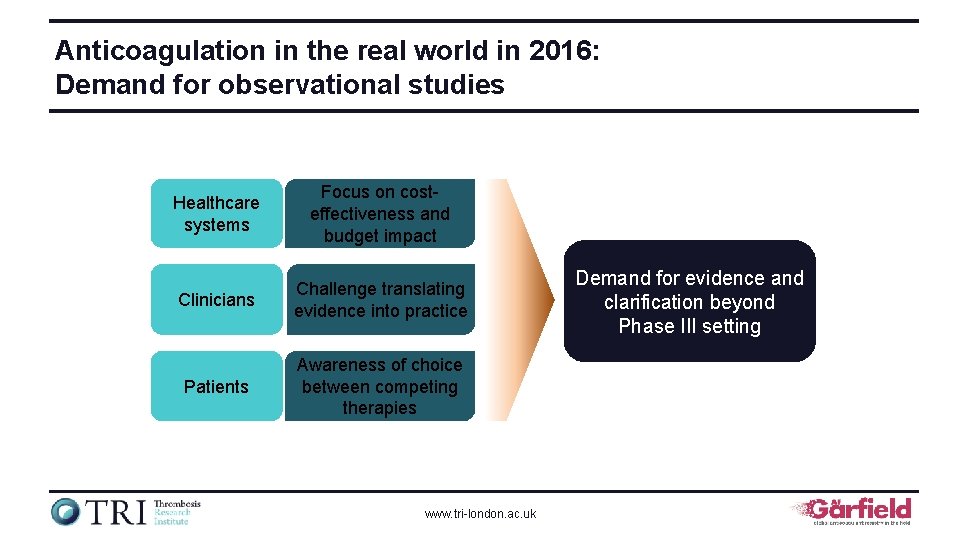
Anticoagulation in the real world in 2016: Demand for observational studies Healthcare systems Focus on costeffectiveness and budget impact Clinicians Challenge translating evidence into practice Patients Awareness of choice between competing therapies www. tri-london. ac. uk Demand for evidence and clarification beyond Phase III setting

Why a global AF registry? • Uncertainty persists about how AF is managed in the real world and how these treatment patterns affect patient and population outcomes • This is a problem – in relation to treatment guidelines – in specific patient populations – across various care settings www. tri-london. ac. uk

Why a global AF registry? (Cont. 1) • Large burden of preventable stroke in patients with AF • Historical underutilization and poor control of VKAs for stroke prevention in AF • Recent introduction of new oral anticoagulants for stroke prevention in AF www. tri-london. ac. uk

Why a global AF registry? (Cont. 2) • How are we treating patients with AF in the new oral anticoagulant era? • What is the rate of stroke and systemic embolism? • What is the incidence of bleeding complications? • What is the rate of therapy persistence? www. tri-london. ac. uk

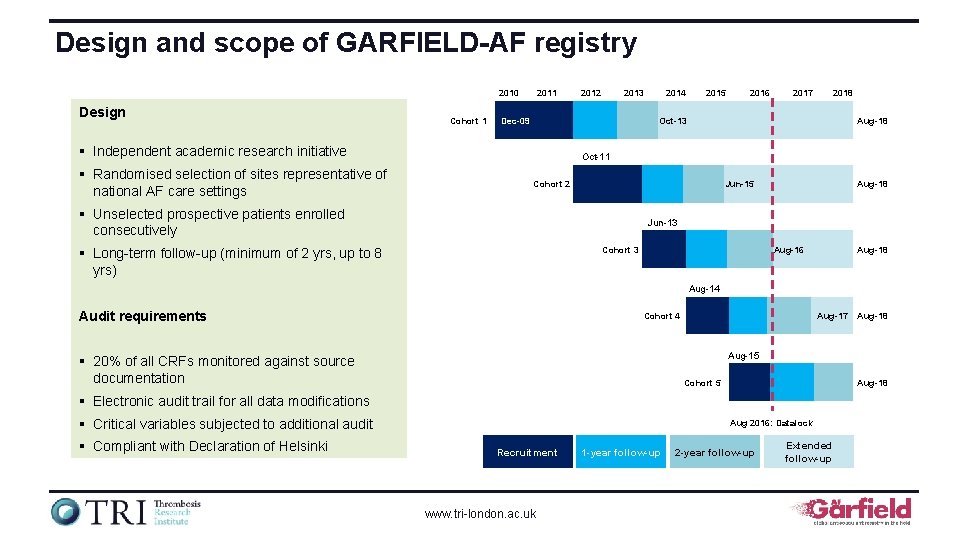
Design and scope of GARFIELD-AF registry 2010 Design Cohort 1 2013 Dec-09 2014 2015 2016 2017 2018 Oct-13 § Independent academic research initiative § Randomised selection of sites representative of national AF care settings 2012 Aug-18 Oct-11 Cohort 2 Jun-15 § Unselected prospective patients enrolled consecutively Aug-18 Jun-13 Cohort 3 § Long-term follow-up (minimum of 2 yrs, up to 8 yrs) Aug-16 Aug-18 Aug-14 Audit requirements Cohort 4 Aug-17 Aug-15 § 20% of all CRFs monitored against source documentation Cohort 5 Aug-18 § Electronic audit trail for all data modifications § Critical variables subjected to additional audit § Compliant with Declaration of Helsinki Aug-18 Aug 2016: Datalock Recruitment www. tri-london. ac. uk 1 -year follow-up 2 -year follow-up Extended follow-up

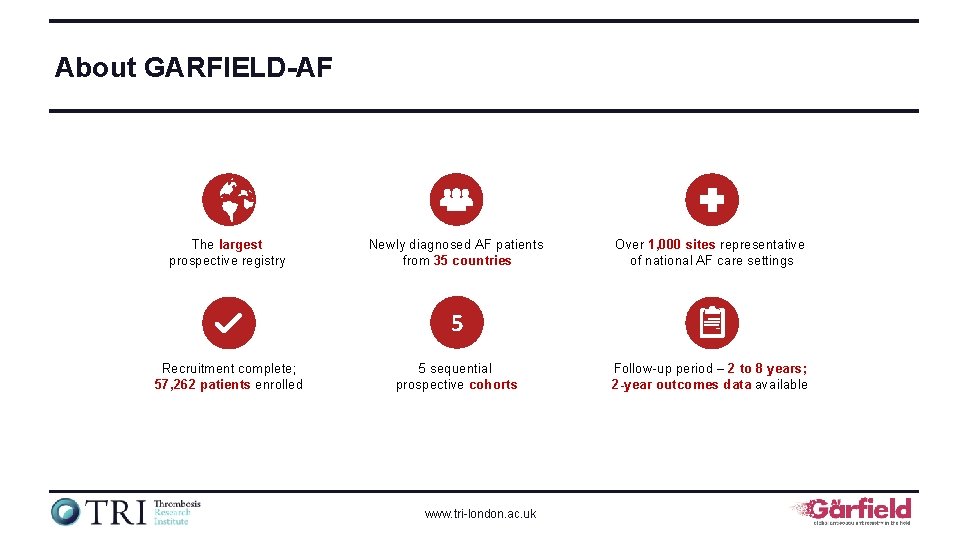
About GARFIELD-AF The largest prospective registry Newly diagnosed AF patients from 35 countries Over 1, 000 sites representative of national AF care settings 5 Recruitment complete; 57, 262 patients enrolled 5 sequential prospective cohorts www. tri-london. ac. uk Follow-up period – 2 to 8 years; 2 -year outcomes data available

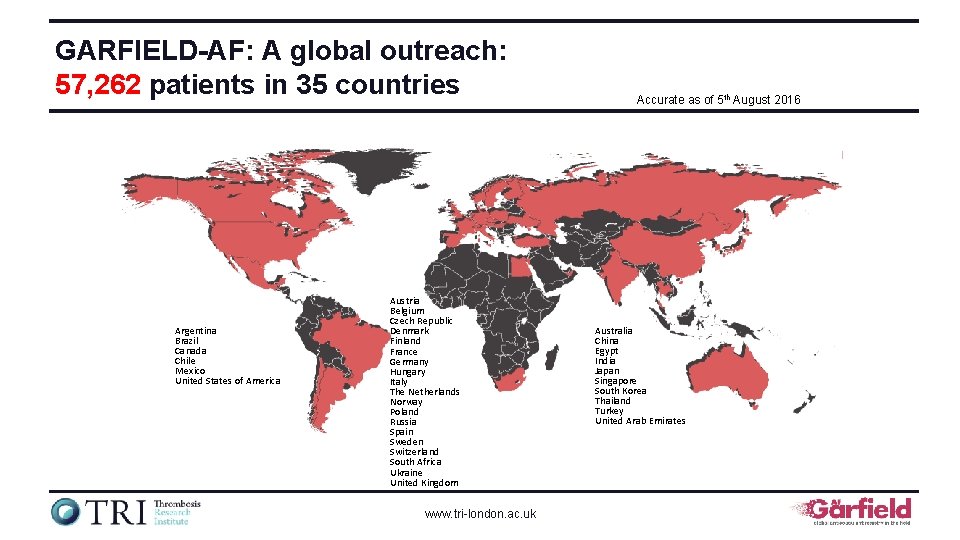
GARFIELD-AF: A global outreach: 57, 262 patients in 35 countries Argentina Brazil Canada Chile Mexico United States of America Austria Belgium Czech Republic Denmark Finland France Germany Hungary Italy The Netherlands Norway Poland Russia Spain Sweden Switzerland South Africa Ukraine United Kingdom www. tri-london. ac. uk Accurate as of 5 th August 2016 Australia China Egypt India Japan Singapore South Korea Thailand Turkey United Arab Emirates

GARFIELD-AF data highlights The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

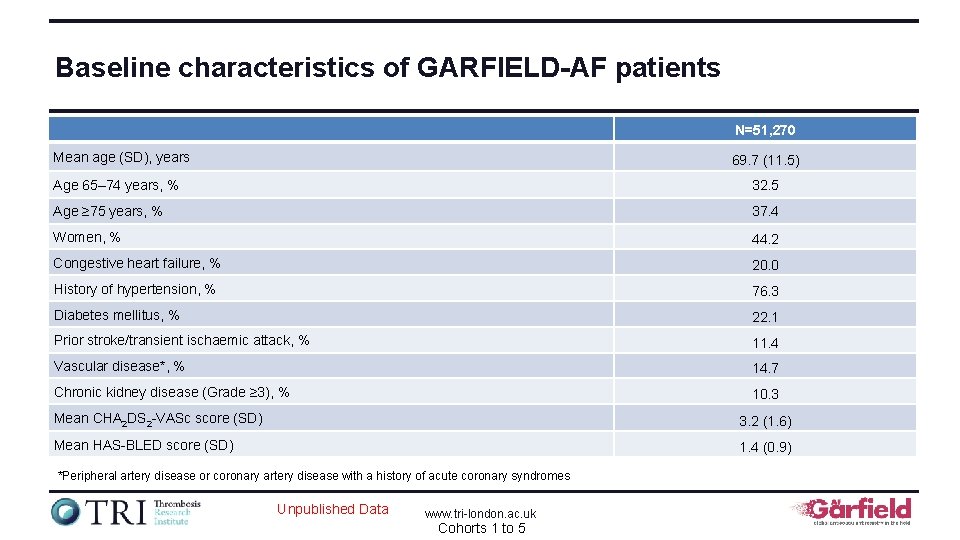
Baseline characteristics of GARFIELD-AF patients N=51, 270 Mean age (SD), years 69. 7 (11. 5) Age 65– 74 years, % 32. 5 Age ≥ 75 years, % 37. 4 Women, % 44. 2 Congestive heart failure, % 20. 0 History of hypertension, % 76. 3 Diabetes mellitus, % 22. 1 Prior stroke/transient ischaemic attack, % 11. 4 Vascular disease*, % 14. 7 Chronic kidney disease (Grade ≥ 3), % 10. 3 Mean CHA 2 DS 2 -VASc score (SD) 3. 2 (1. 6) Mean HAS-BLED score (SD) 1. 4 (0. 9) *Peripheral artery disease or coronary artery disease with a history of acute coronary syndromes Unpublished Data www. tri-london. ac. uk Cohorts 1 to 5

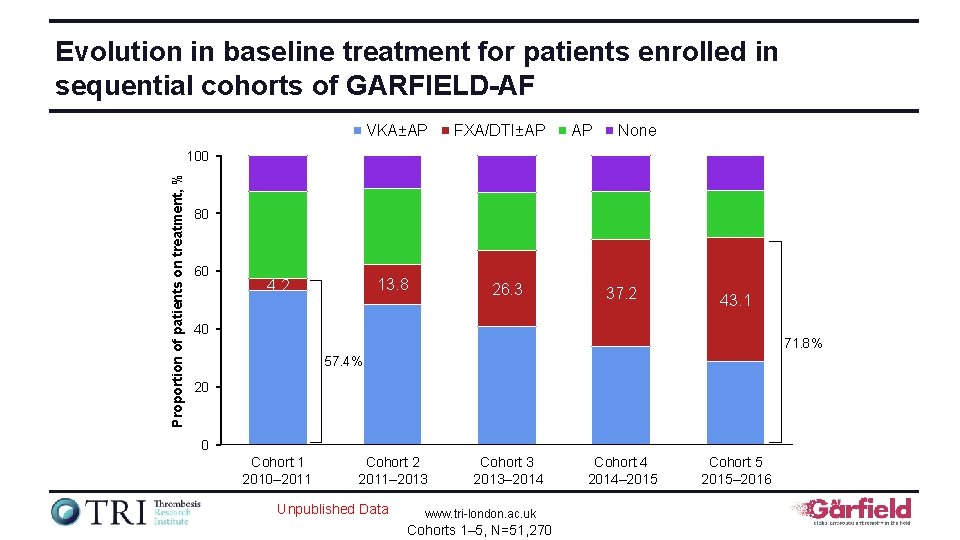
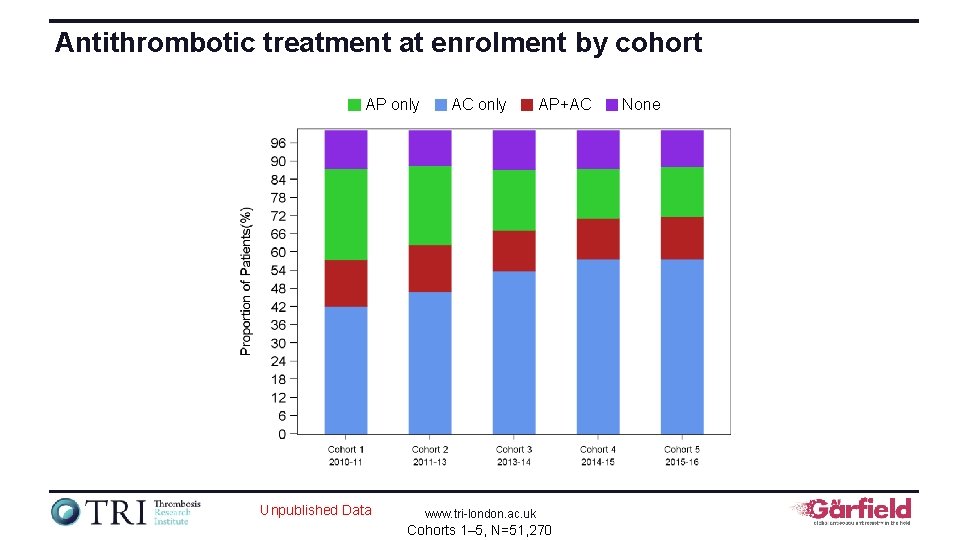
Evolution in baseline treatment for patients enrolled in sequential cohorts of GARFIELD-AF VKA±AP FXA/DTI±AP AP None Proportion of patients on treatment, % 100 80 60 13. 8 4. 2 26. 3 37. 2 43. 1 40 71. 8% 57. 4% 20 0 Cohort 1 2010– 2011 Cohort 2 2011– 2013 Unpublished Data Cohort 3 2013– 2014 www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 Cohort 4 2014– 2015 Cohort 5 2015– 2016

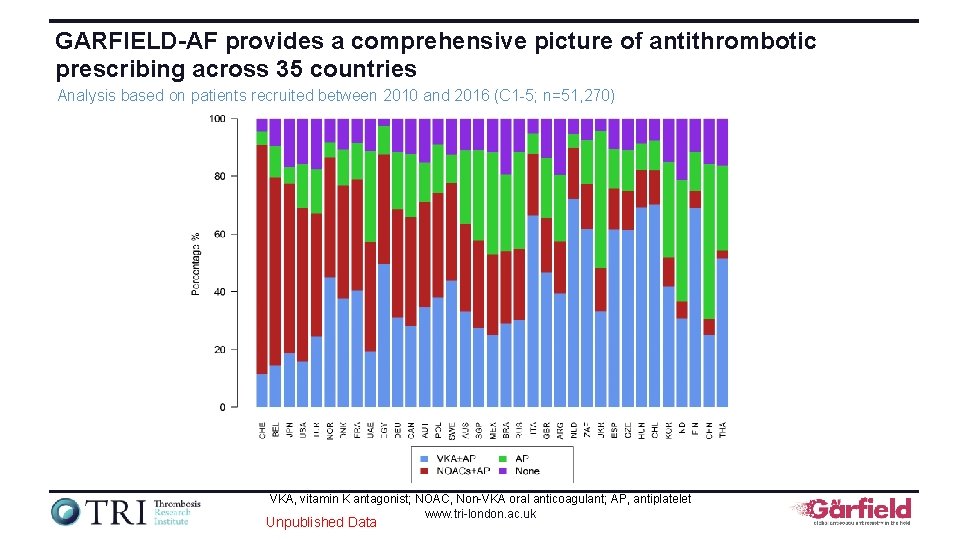
GARFIELD-AF provides a comprehensive picture of antithrombotic prescribing across 35 countries Analysis based on patients recruited between 2010 and 2016 (C 1 -5; n=51, 270) VKA, vitamin K antagonist; NOAC, Non-VKA oral anticoagulant; AP, antiplatelet www. tri-london. ac. uk Unpublished Data

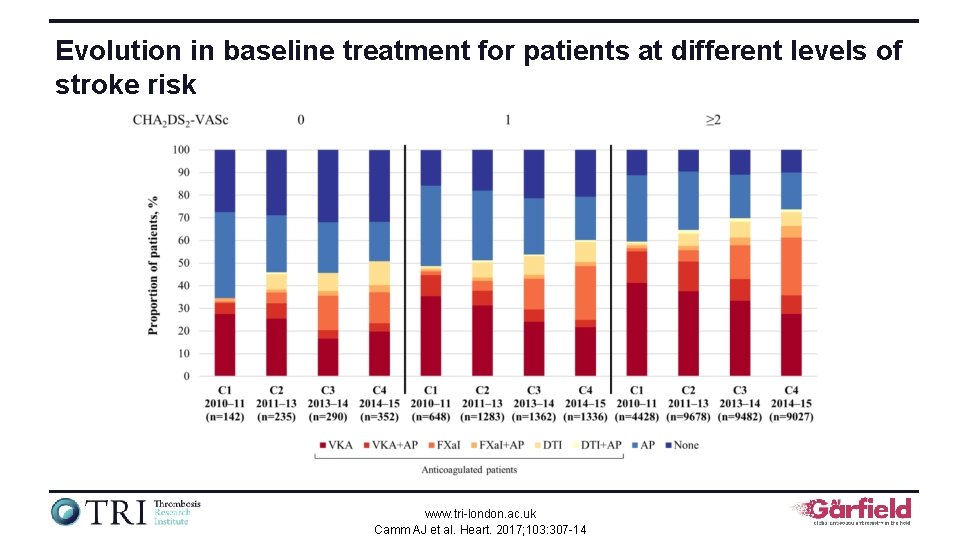
Evolution in baseline treatment for patients at different levels of stroke risk www. tri-london. ac. uk Camm AJ et al. Heart. 2017; 103: 307 -14

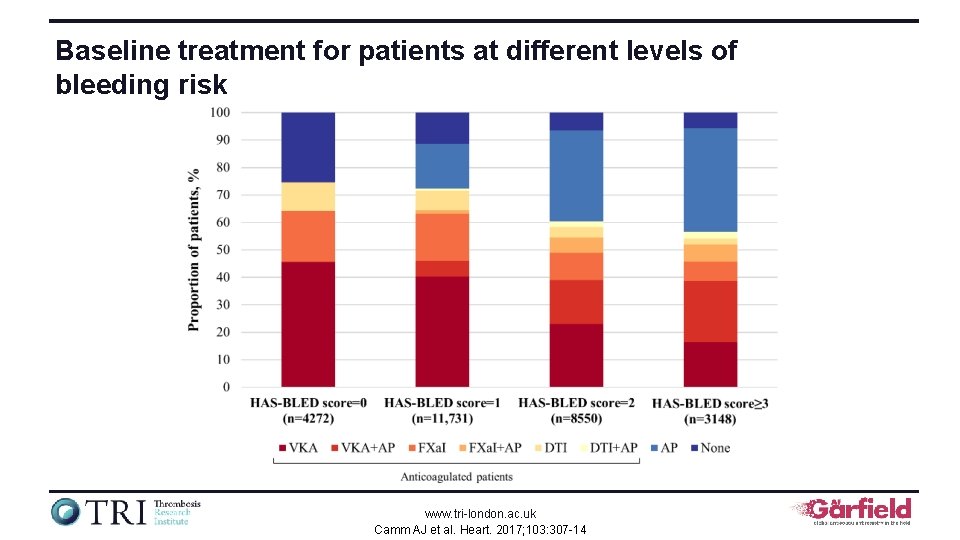
Baseline treatment for patients at different levels of bleeding risk www. tri-london. ac. uk Camm AJ et al. Heart. 2017; 103: 307 -14

2 -year outcomes data for cohorts 1 and 2 The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

Research questions In patients with newly diagnosed AF who have been enrolled in GARFIELD-AF for 2 years: • What were the rates of stroke/systemic embolism (SE) major bleeding and all -cause mortality? • What types of stroke/SE and major bleeding events were recorded, and how severe were they? • What were the causes of death? • Which variables were associated with increased risk of stroke/SE, major bleeding and all-cause mortality? www. tri-london. ac. uk

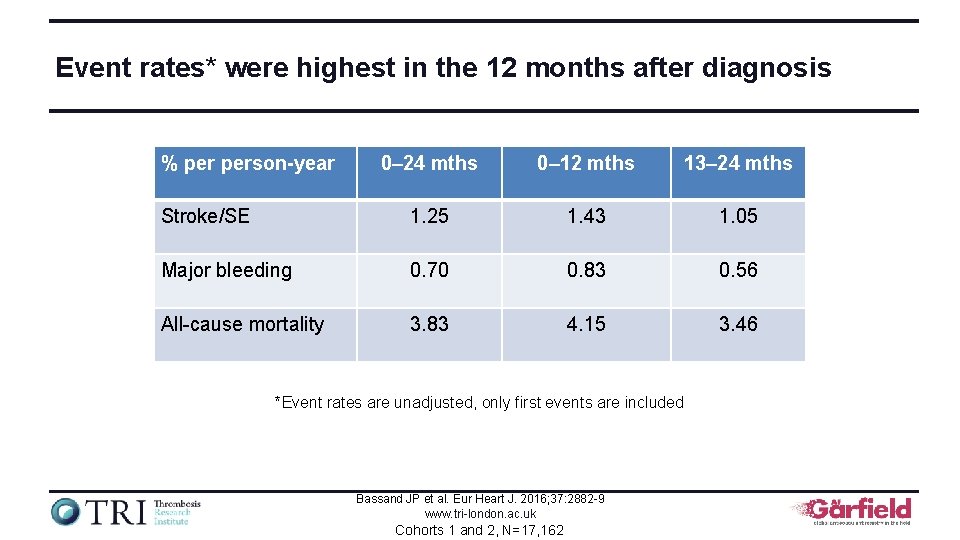
Event rates* were highest in the 12 months after diagnosis % person-year 0– 24 mths 0– 12 mths 13– 24 mths Stroke/SE 1. 25 1. 43 1. 05 Major bleeding 0. 70 0. 83 0. 56 All-cause mortality 3. 83 4. 15 3. 46 *Event rates are unadjusted, only first events are included Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9 www. tri-london. ac. uk Cohorts 1 and 2, N=17, 162

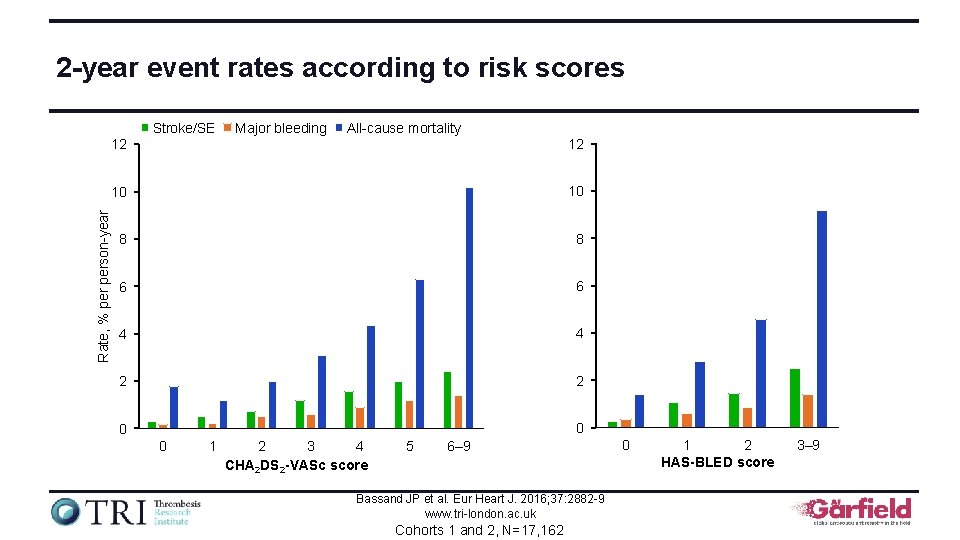
2 -year event rates according to risk scores Rate, % person-year Stroke/SE Major bleeding All-cause mortality 12 12 10 10 8 8 6 6 4 4 2 2 0 0 0 1 2 3 4 CHA 2 DS 2 -VASc score 5 6– 9 Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9 www. tri-london. ac. uk Cohorts 1 and 2, N=17, 162 0 1 2 HAS-BLED score 3– 9

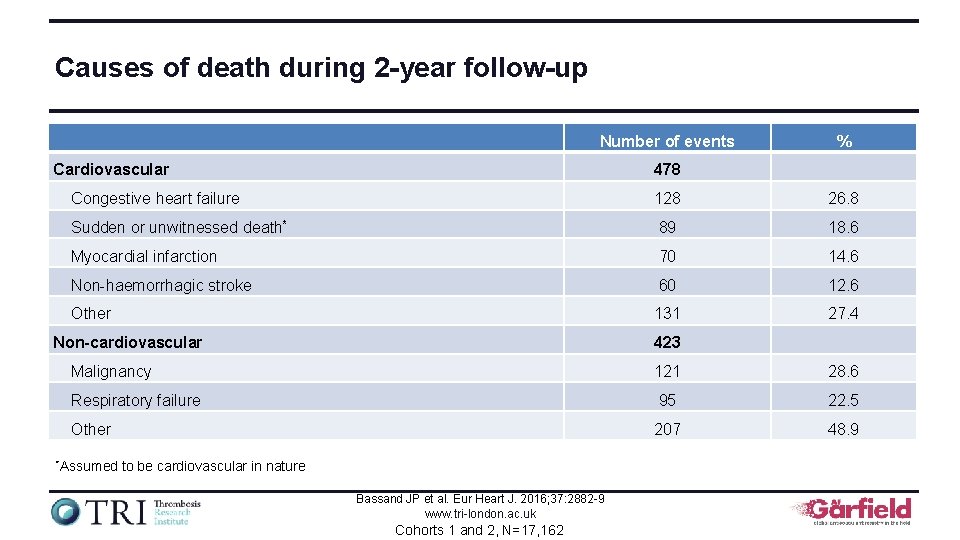
Causes of death during 2 -year follow-up Number of events Cardiovascular % 478 Congestive heart failure 128 26. 8 Sudden or unwitnessed death* 89 18. 6 Myocardial infarction 70 14. 6 Non-haemorrhagic stroke 60 12. 6 Other 131 27. 4 Non-cardiovascular 423 Malignancy 121 28. 6 Respiratory failure 95 22. 5 Other 207 48. 9 *Assumed to be cardiovascular in nature Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9 www. tri-london. ac. uk Cohorts 1 and 2, N=17, 162

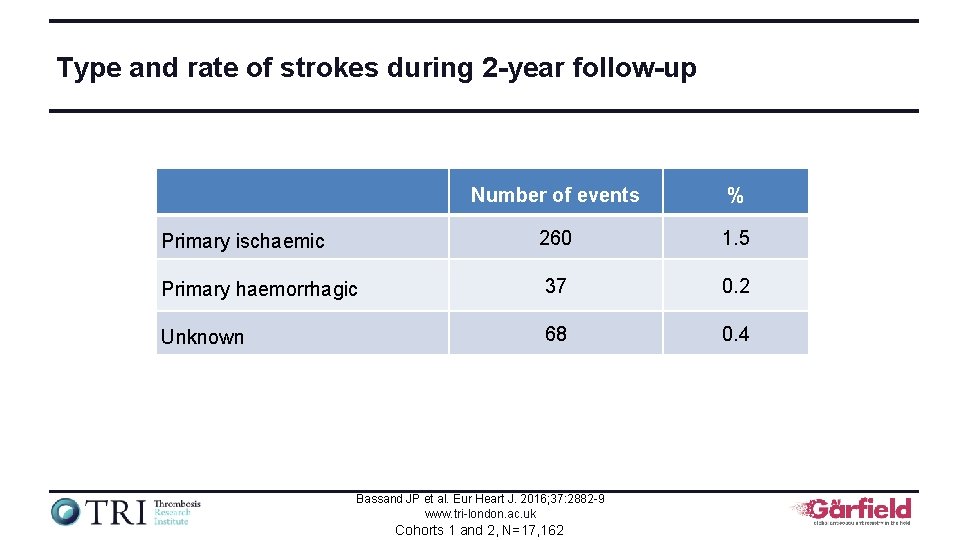
Type and rate of strokes during 2 -year follow-up Number of events % Primary ischaemic 260 1. 5 Primary haemorrhagic 37 0. 2 Unknown 68 0. 4 Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9 www. tri-london. ac. uk Cohorts 1 and 2, N=17, 162

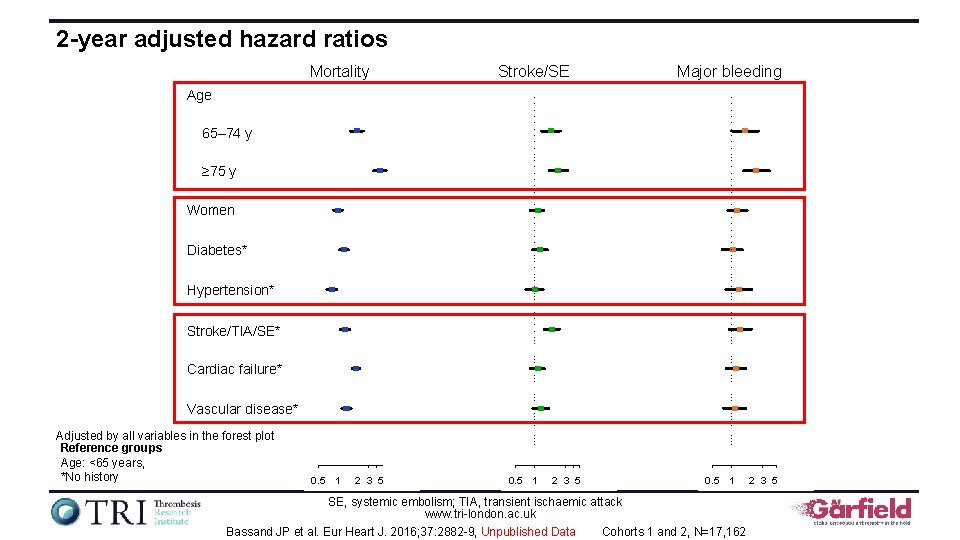
2 -year adjusted hazard ratios Mortality Stroke/SE Major bleeding Age 65– 74 y ≥ 75 y Women Diabetes* Hypertension* Stroke/TIA/SE* Cardiac failure* Vascular disease* Adjusted by all variables in the forest plot Reference groups Age: <65 years, *No history 0. 5 1 2 3 5 0. 5 1 SE, systemic embolism; TIA, transient ischaemic attack www. tri-london. ac. uk Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9, Unpublished Data Cohorts 1 and 2, N=17, 162 2 3 5

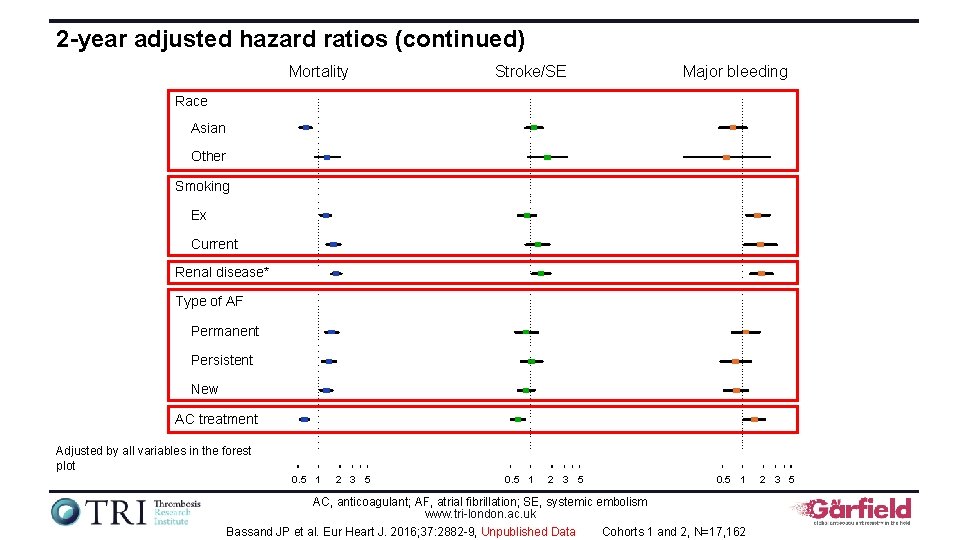
2 -year adjusted hazard ratios (continued) Mortality Stroke/SE Major bleeding Race Asian Other Smoking Ex Current Renal disease* Type of AF Permanent Persistent New AC treatment Adjusted by all variables in the forest plot 0. 5 1 2 3 5 0. 5 1 AC, anticoagulant; AF, atrial fibrillation; SE, systemic embolism www. tri-london. ac. uk Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9, Unpublished Data Cohorts 1 and 2, N=17, 162 2 3 5

Treating low-risk patients with nonvalvular atrial fibrillation The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

Objectives • To describe baseline characteristics of patients at different levels of stroke risk conforming to guideline recommendations • To evaluate treatment patterns in patients at different levels of stroke risk • Analyse the 1 -year rates of clinical outcomes (stroke / systemic embolism [SE], major bleeding and all-cause mortality) by risk and anticoagulant therapy in patients enrolled between Mar 2010 to Sep 2015 www. tri-london. ac. uk Cohorts 1– 4, N=39, 898

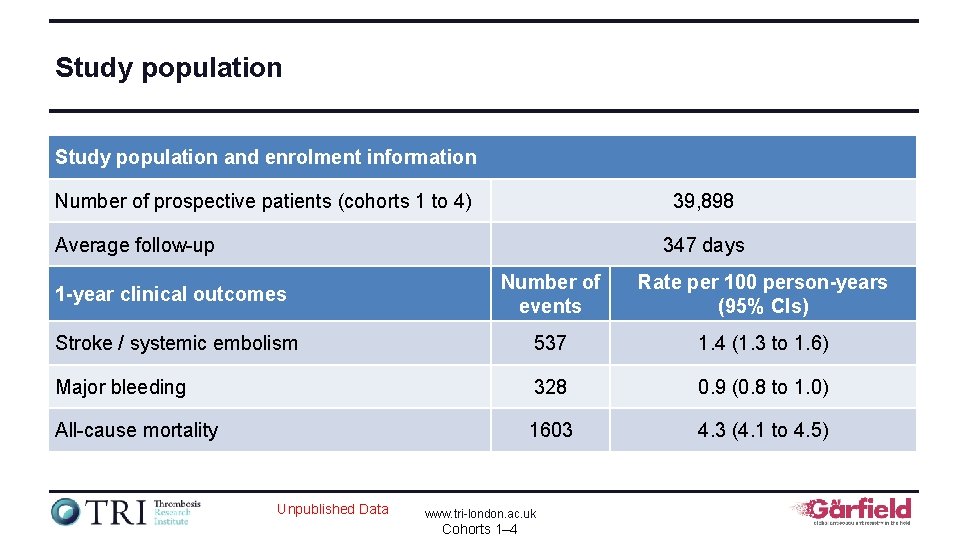
Study population and enrolment information Number of prospective patients (cohorts 1 to 4) 39, 898 Average follow-up 347 days 1 -year clinical outcomes Number of events Rate per 100 person-years (95% CIs) Stroke / systemic embolism 537 1. 4 (1. 3 to 1. 6) Major bleeding 328 0. 9 (0. 8 to 1. 0) All-cause mortality 1603 4. 3 (4. 1 to 4. 5) Unpublished Data www. tri-london. ac. uk Cohorts 1– 4

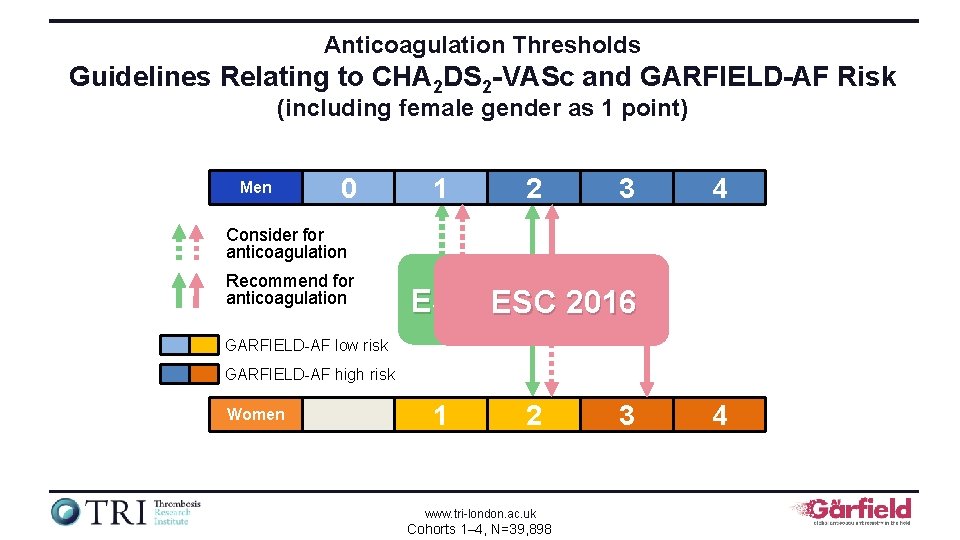
Anticoagulation Thresholds Guidelines Relating to CHA 2 DS 2 -VASc and GARFIELD-AF Risk (including female gender as 1 point) Men 0 1 2 3 4 Consider for anticoagulation Recommend for anticoagulation ESC 2012 ESC 2016 GARFIELD-AF low risk GARFIELD-AF high risk Women 1 2 www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 3 4

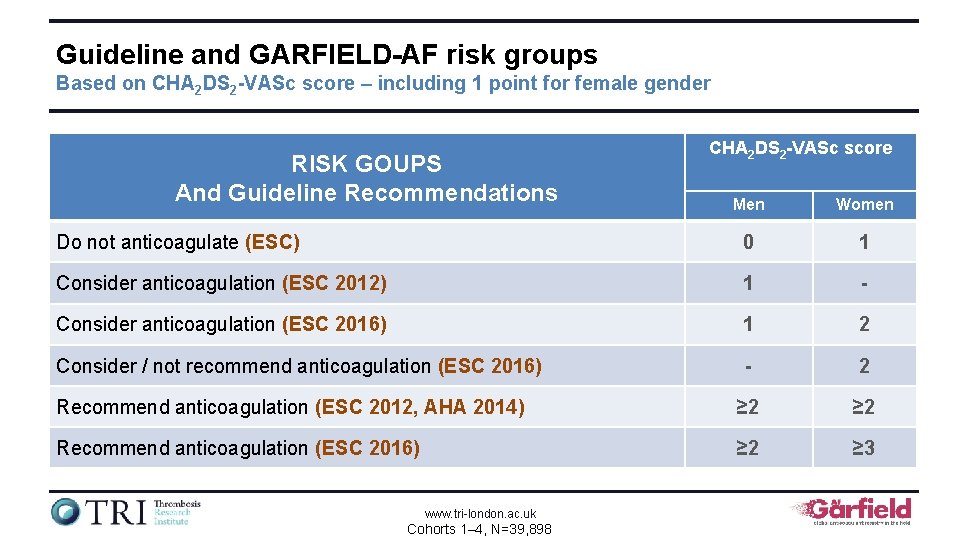
Guideline and GARFIELD-AF risk groups Based on CHA 2 DS 2 -VASc score – including 1 point for female gender RISK GOUPS And Guideline Recommendations CHA 2 DS 2 -VASc score Men Women Do not anticoagulate (ESC) 0 1 Consider anticoagulation (ESC 2012) 1 - Consider anticoagulation (ESC 2016) 1 2 Consider / not recommend anticoagulation (ESC 2016) - 2 Recommend anticoagulation (ESC 2012, AHA 2014) ≥ 2 Recommend anticoagulation (ESC 2016) ≥ 2 ≥ 3 www. tri-london. ac. uk Cohorts 1– 4, N=39, 898

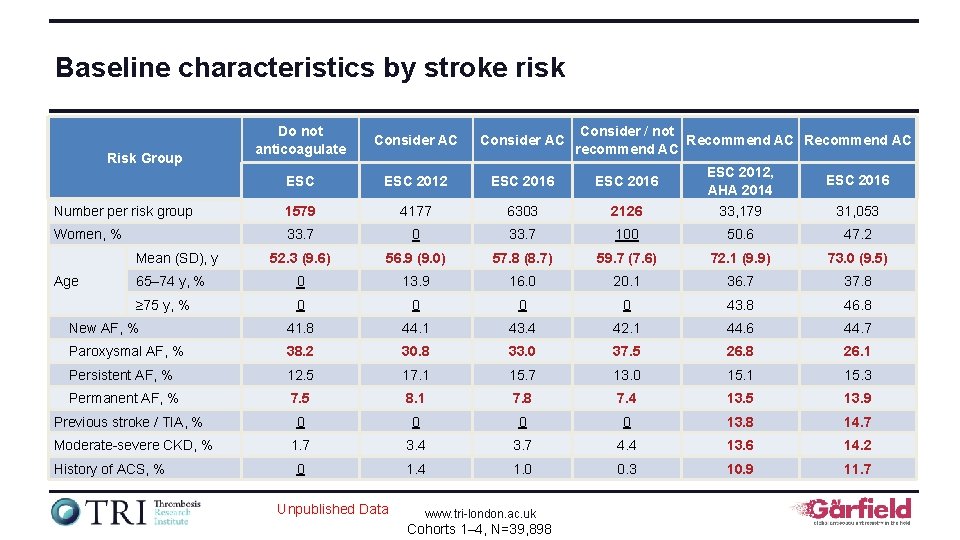
Baseline characteristics by stroke risk Do not anticoagulate Consider AC ESC 2012 ESC 2016 Number per risk group 1579 4177 6303 2126 ESC 2012, AHA 2014 33, 179 Women, % 33. 7 0 33. 7 100 50. 6 47. 2 52. 3 (9. 6) 56. 9 (9. 0) 57. 8 (8. 7) 59. 7 (7. 6) 72. 1 (9. 9) 73. 0 (9. 5) 65– 74 y, % 0 13. 9 16. 0 20. 1 36. 7 37. 8 ≥ 75 y, % 0 0 43. 8 46. 8 New AF, % 41. 8 44. 1 43. 4 42. 1 44. 6 44. 7 Paroxysmal AF, % 38. 2 30. 8 33. 0 37. 5 26. 8 26. 1 Persistent AF, % 12. 5 17. 1 15. 7 13. 0 15. 1 15. 3 Permanent AF, % 7. 5 8. 1 7. 8 7. 4 13. 5 13. 9 0 0 13. 8 14. 7 1. 7 3. 4 3. 7 4. 4 13. 6 14. 2 0 1. 4 1. 0 0. 3 10. 9 11. 7 Risk Group Mean (SD), y Age Previous stroke / TIA, % Moderate-severe CKD, % History of ACS, % Unpublished Data www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 Consider / not Recommend AC recommend AC ESC 2016 31, 053

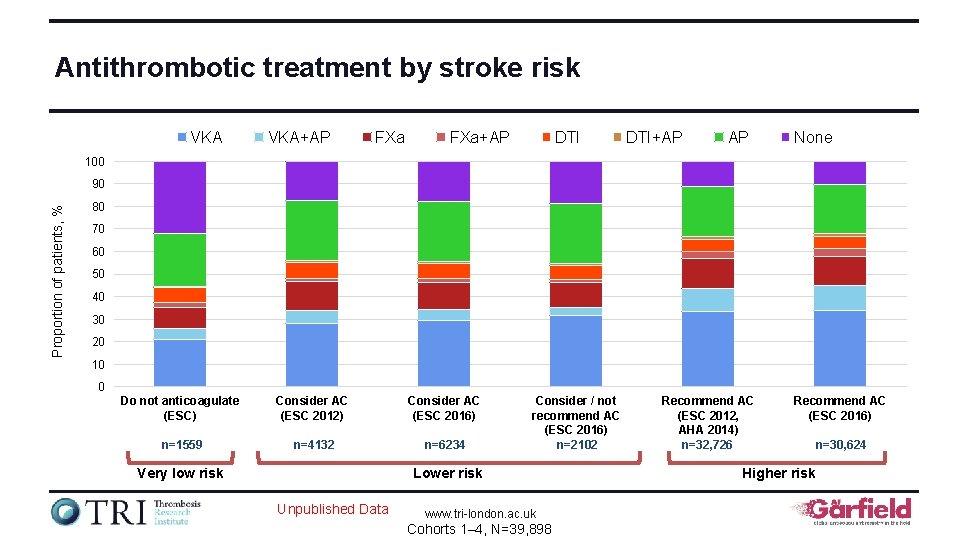
Antithrombotic treatment by stroke risk VKA+AP FXa+AP DTI+AP AP None 100 Proportion of patients, % 90 80 70 60 50 40 30 20 10 0 Do not anticoagulate (ESC) Consider AC (ESC 2012) Consider AC (ESC 2016) n=1559 n=4132 n=6234 Very low risk Consider / not recommend AC (ESC 2016) n=2102 Lower risk Unpublished Data www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 Recommend AC (ESC 2012, AHA 2014) n=32, 726 Recommend AC (ESC 2016) Higher risk n=30, 624

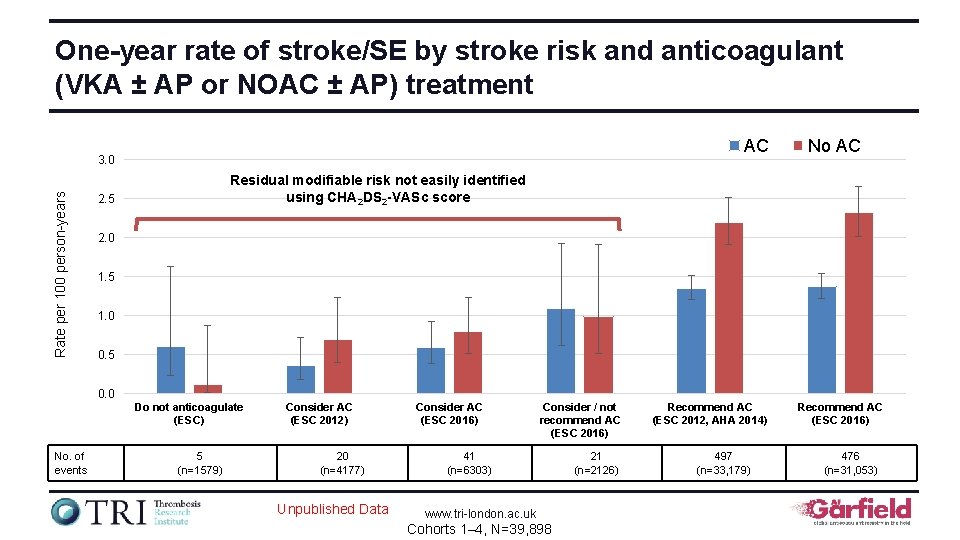
One-year rate of stroke/SE by stroke risk and anticoagulant (VKA ± AP or NOAC ± AP) treatment AC Rate per 100 person-years 3. 0 No AC Residual modifiable risk not easily identified using CHA 2 DS 2 -VASc score 2. 5 2. 0 1. 5 1. 0 0. 5 0. 0 Do not anticoagulate (ESC) No. of events 5 (n=1579) Consider AC (ESC 2012) 20 (n=4177) Unpublished Data Consider AC (ESC 2016) Consider / not recommend AC (ESC 2016) 41 (n=6303) www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 21 (n=2126) Recommend AC (ESC 2012, AHA 2014) 497 (n=33, 179) Recommend AC (ESC 2016) 476 (n=31, 053)

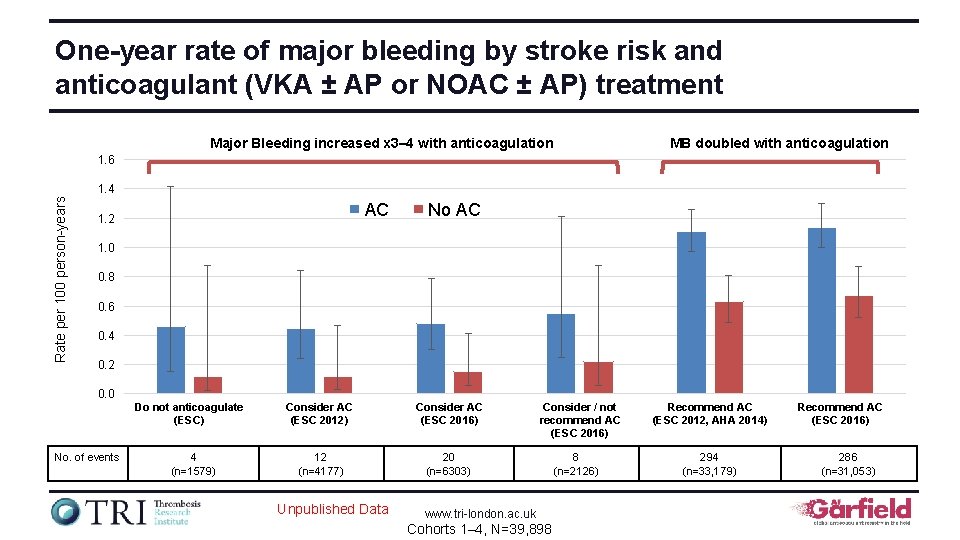
One-year rate of major bleeding by stroke risk and anticoagulant (VKA ± AP or NOAC ± AP) treatment Major Bleeding increased x 3– 4 with anticoagulation MB doubled with anticoagulation 1. 6 Rate per 100 person-years 1. 4 AC 1. 2 No AC 1. 0 0. 8 0. 6 0. 4 0. 2 0. 0 Do not anticoagulate (ESC) No. of events 4 (n=1579) Consider AC (ESC 2012) Consider AC (ESC 2016) 12 (n=4177) 20 (n=6303) Unpublished Data Consider / not recommend AC (ESC 2016) www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 8 (n=2126) Recommend AC (ESC 2012, AHA 2014) 294 (n=33, 179) Recommend AC (ESC 2016) 286 (n=31, 053)

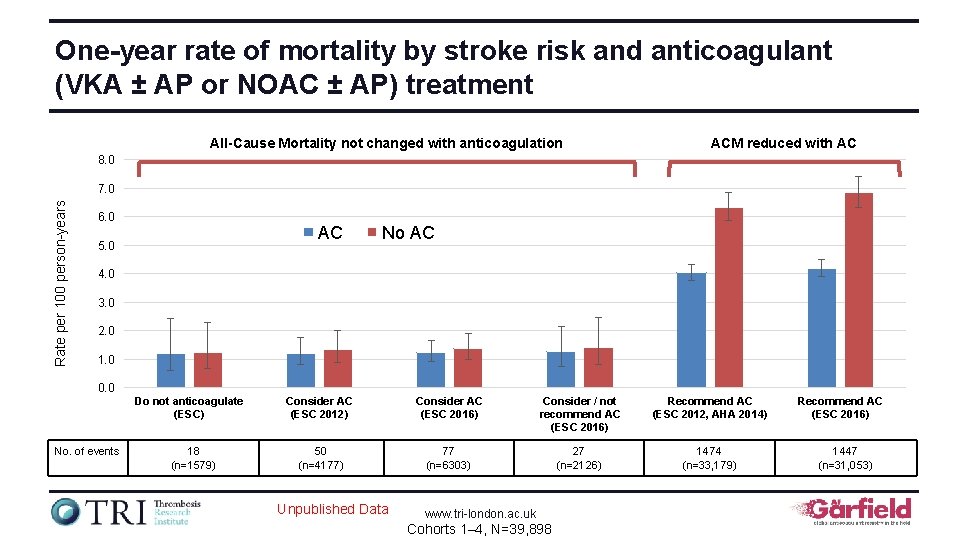
One-year rate of mortality by stroke risk and anticoagulant (VKA ± AP or NOAC ± AP) treatment All-Cause Mortality not changed with anticoagulation ACM reduced with AC 8. 0 Rate per 100 person-years 7. 0 6. 0 AC 5. 0 No AC 4. 0 3. 0 2. 0 1. 0 0. 0 Do not anticoagulate (ESC) No. of events 18 (n=1579) Consider AC (ESC 2012) Consider AC (ESC 2016) Consider / not recommend AC (ESC 2016) Recommend AC (ESC 2012, AHA 2014) 50 (n=4177) 77 (n=6303) 27 (n=2126) 1474 (n=33, 179) Unpublished Data www. tri-london. ac. uk Cohorts 1– 4, N=39, 898 Recommend AC (ESC 2016) 1447 (n=31, 053)

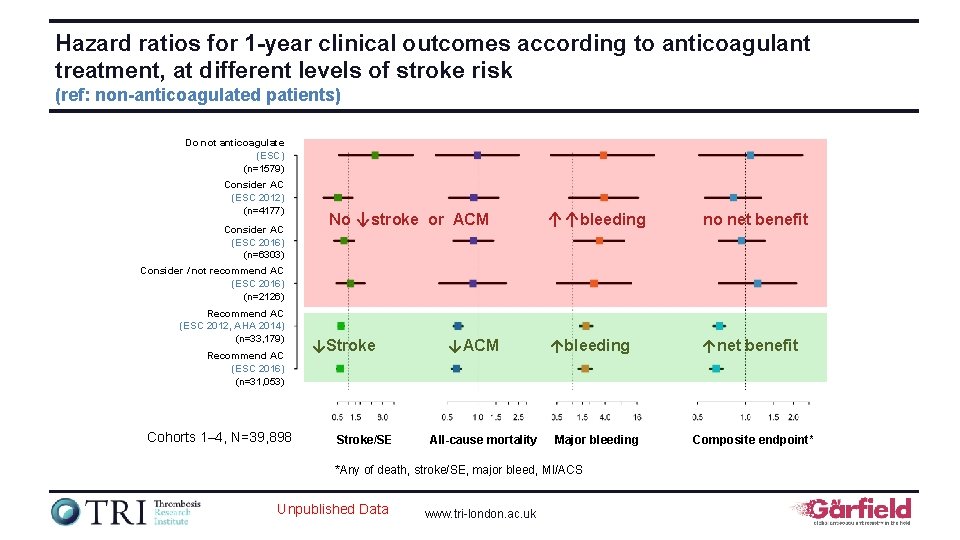
Hazard ratios for 1 -year clinical outcomes according to anticoagulant treatment, at different levels of stroke risk (ref: non-anticoagulated patients) Do not anticoagulate (ESC) (n=1579) Consider AC (ESC 2012) (n=4177) Consider AC (ESC 2016) (n=6303) No ↓stroke or ACM ↑↑bleeding no net benefit ↑bleeding ↑net benefit Consider / not recommend AC (ESC 2016) (n=2126) Recommend AC (ESC 2012, AHA 2014) (n=33, 179) Recommend AC (ESC 2016) (n=31, 053) Cohorts 1– 4, N=39, 898 ↓Stroke/SE ↓ACM All-cause mortality Major bleeding *Any of death, stroke/SE, major bleed, MI/ACS Unpublished Data www. tri-london. ac. uk Composite endpoint*

Risk scores for patients with nonvalvular AF The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

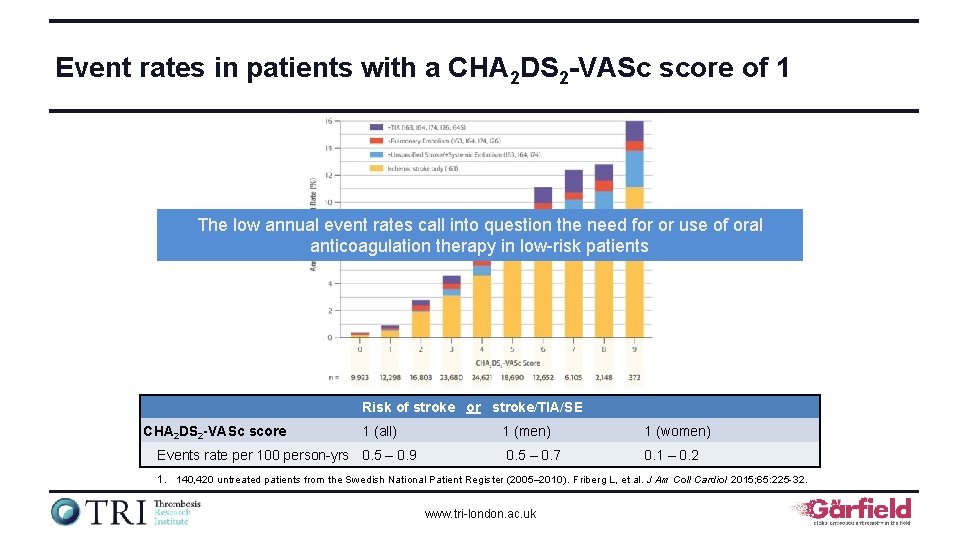
Event rates in patients with a CHA 2 DS 2 -VASc score of 1 The low annual event rates call into question the need for or use of oral anticoagulation therapy in low-risk patients Risk of stroke or stroke/TIA/SE CHA 2 DS 2 -VASc score 1 (all) Events rate per 100 person-yrs 0. 5 – 0. 9 1 (men) 1 (women) 0. 5 – 0. 7 0. 1 – 0. 2 1. 140, 420 untreated patients from the Swedish National Patient Register (2005– 2010). Friberg L, et al. J Am Coll Cardiol 2015; 65: 225 -32. www. tri-london. ac. uk

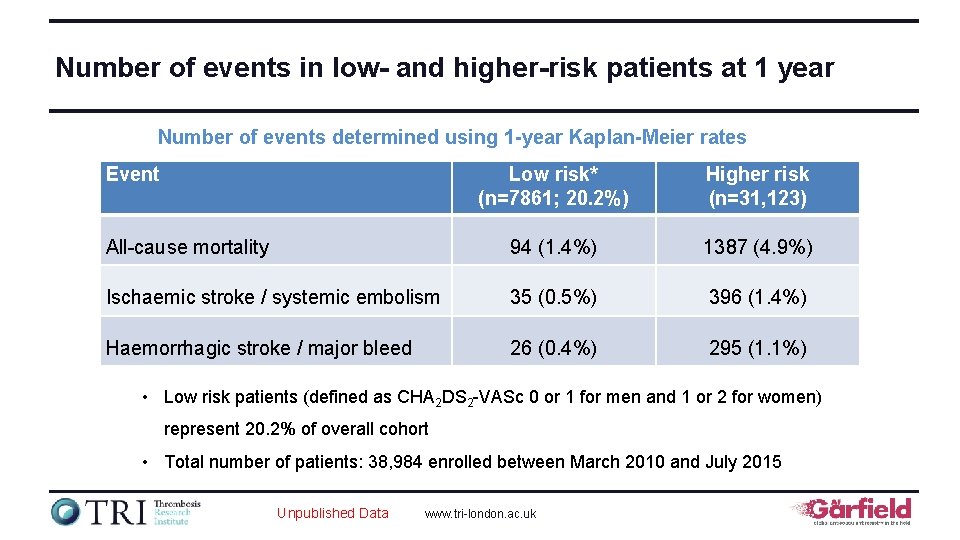
Number of events in low- and higher-risk patients at 1 year Number of events determined using 1 -year Kaplan-Meier rates Event Low risk* (n=7861; 20. 2%) Higher risk (n=31, 123) All-cause mortality 94 (1. 4%) 1387 (4. 9%) Ischaemic stroke / systemic embolism 35 (0. 5%) 396 (1. 4%) Haemorrhagic stroke / major bleed 26 (0. 4%) 295 (1. 1%) • Low risk patients (defined as CHA 2 DS 2 -VASc 0 or 1 for men and 1 or 2 for women) represent 20. 2% of overall cohort • Total number of patients: 38, 984 enrolled between March 2010 and July 2015 Unpublished Data www. tri-london. ac. uk

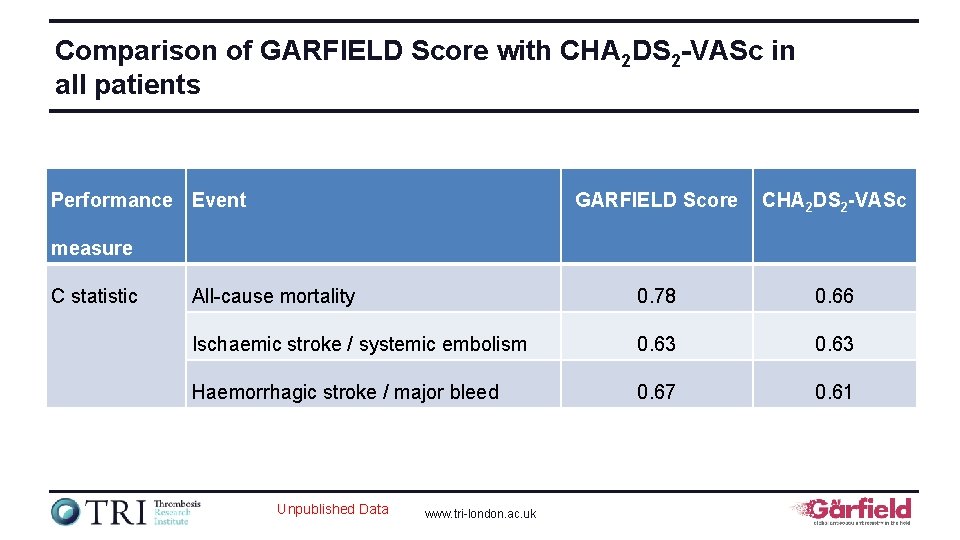
Comparison of GARFIELD Score with CHA 2 DS 2 -VASc in all patients Performance Event GARFIELD Score CHA 2 DS 2 -VASc All-cause mortality 0. 78 0. 66 Ischaemic stroke / systemic embolism 0. 63 Haemorrhagic stroke / major bleed 0. 67 0. 61 measure C statistic Unpublished Data www. tri-london. ac. uk

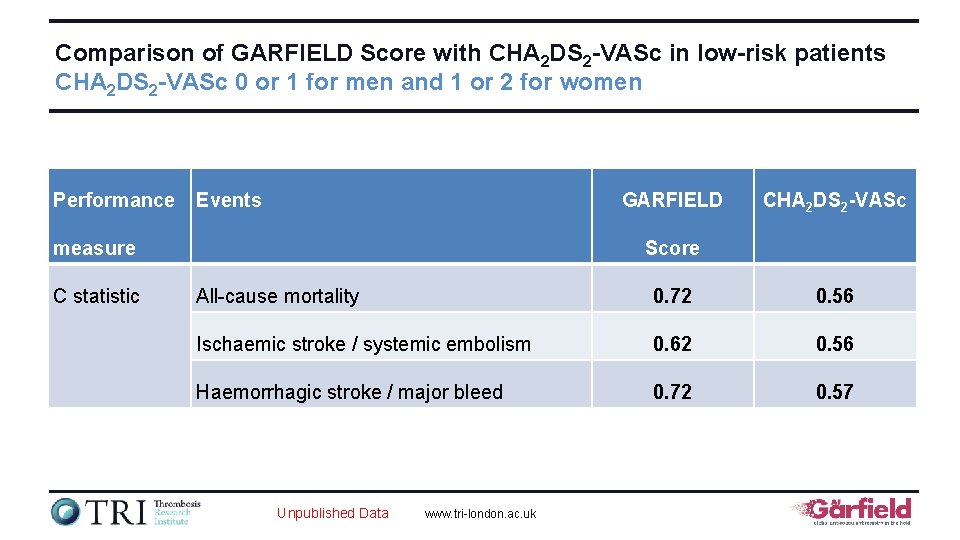
Comparison of GARFIELD Score with CHA 2 DS 2 -VASc in low-risk patients CHA 2 DS 2 -VASc 0 or 1 for men and 1 or 2 for women Performance Events GARFIELD measure C statistic CHA 2 DS 2 -VASc Score All-cause mortality 0. 72 0. 56 Ischaemic stroke / systemic embolism 0. 62 0. 56 Haemorrhagic stroke / major bleed 0. 72 0. 57 Unpublished Data www. tri-london. ac. uk

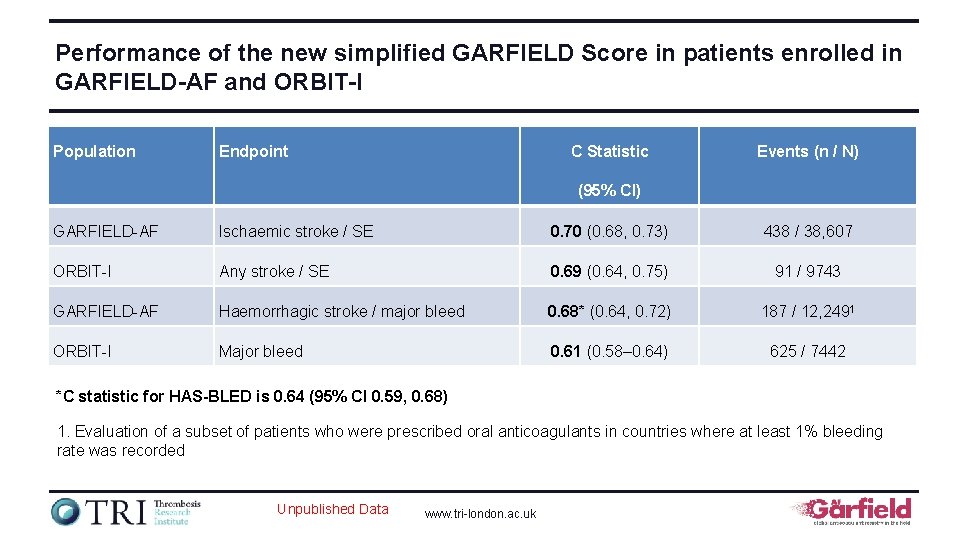
Performance of the new simplified GARFIELD Score in patients enrolled in GARFIELD-AF and ORBIT-I Population Endpoint C Statistic Events (n / N) (95% CI) GARFIELD-AF Ischaemic stroke / SE 0. 70 (0. 68, 0. 73) 438 / 38, 607 ORBIT-I Any stroke / SE 0. 69 (0. 64, 0. 75) 91 / 9743 GARFIELD-AF Haemorrhagic stroke / major bleed 0. 68* (0. 64, 0. 72) 187 / 12, 2491 ORBIT-I Major bleed 0. 61 (0. 58– 0. 64) 625 / 7442 *C statistic for HAS-BLED is 0. 64 (95% CI 0. 59, 0. 68) 1. Evaluation of a subset of patients who were prescribed oral anticoagulants in countries where at least 1% bleeding rate was recorded Unpublished Data www. tri-london. ac. uk

Early mortality in newly diagnosed nonvalvular AF The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

Background • GARFIELD-AF recruits recent onset AF (less than 6 weeks) • The average patient is recruited 2 weeks after the diagnosis of AF • This recruitment time is sooner than GLORIA-AF (12 weeks), ORBIT-AF (6 months), and PREFER in AF (12 months) www. tri-london. ac. uk

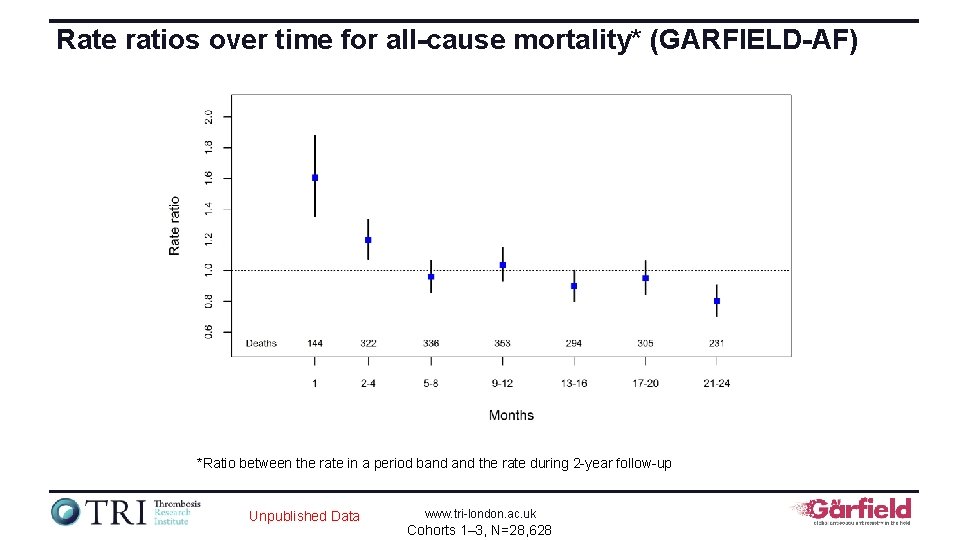
Rate ratios over time for all-cause mortality* (GARFIELD-AF) *Ratio between the rate in a period band the rate during 2 -year follow-up Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

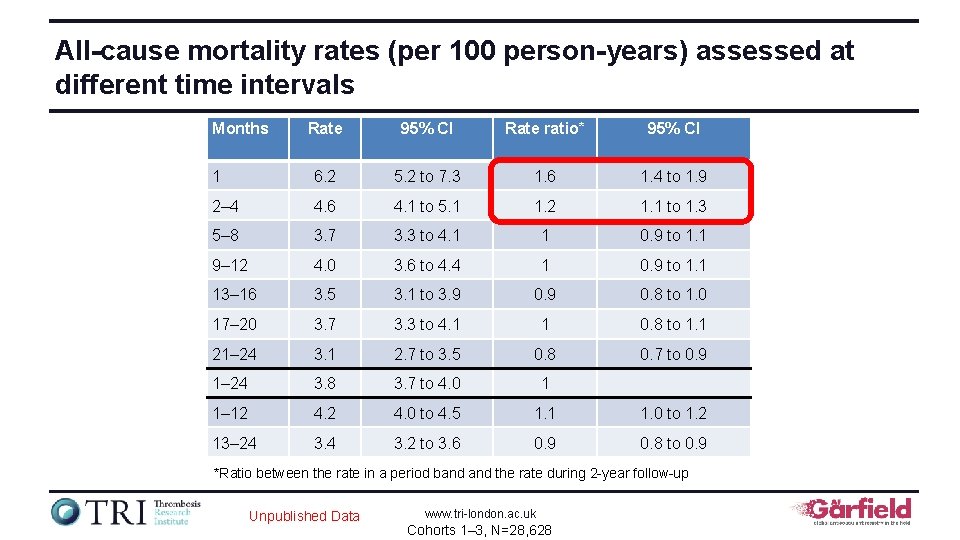
All-cause mortality rates (per 100 person-years) assessed at different time intervals Months Rate 95% CI Rate ratio* 95% CI 1 6. 2 5. 2 to 7. 3 1. 6 1. 4 to 1. 9 2– 4 4. 6 4. 1 to 5. 1 1. 2 1. 1 to 1. 3 5– 8 3. 7 3. 3 to 4. 1 1 0. 9 to 1. 1 9– 12 4. 0 3. 6 to 4. 4 1 0. 9 to 1. 1 13– 16 3. 5 3. 1 to 3. 9 0. 8 to 1. 0 17– 20 3. 7 3. 3 to 4. 1 1 0. 8 to 1. 1 21– 24 3. 1 2. 7 to 3. 5 0. 8 0. 7 to 0. 9 1– 24 3. 8 3. 7 to 4. 0 1 1– 12 4. 0 to 4. 5 1. 1 1. 0 to 1. 2 13– 24 3. 2 to 3. 6 0. 9 0. 8 to 0. 9 *Ratio between the rate in a period band the rate during 2 -year follow-up Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

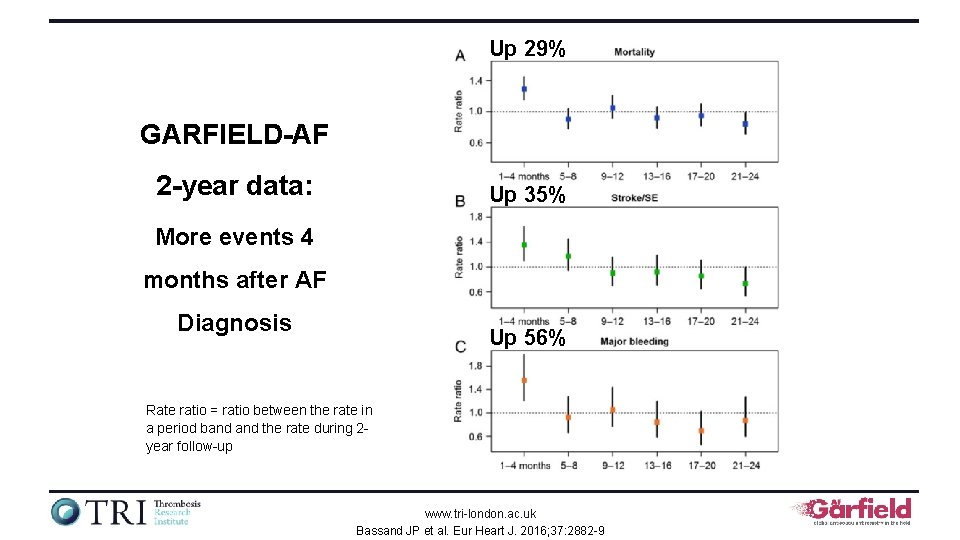
Up 29% GARFIELD-AF 2 -year data: Up 35% More events 4 months after AF Diagnosis Up 56% Rate ratio = ratio between the rate in a period band the rate during 2 year follow-up www. tri-london. ac. uk Bassand JP et al. Eur Heart J. 2016; 37: 2882 -9

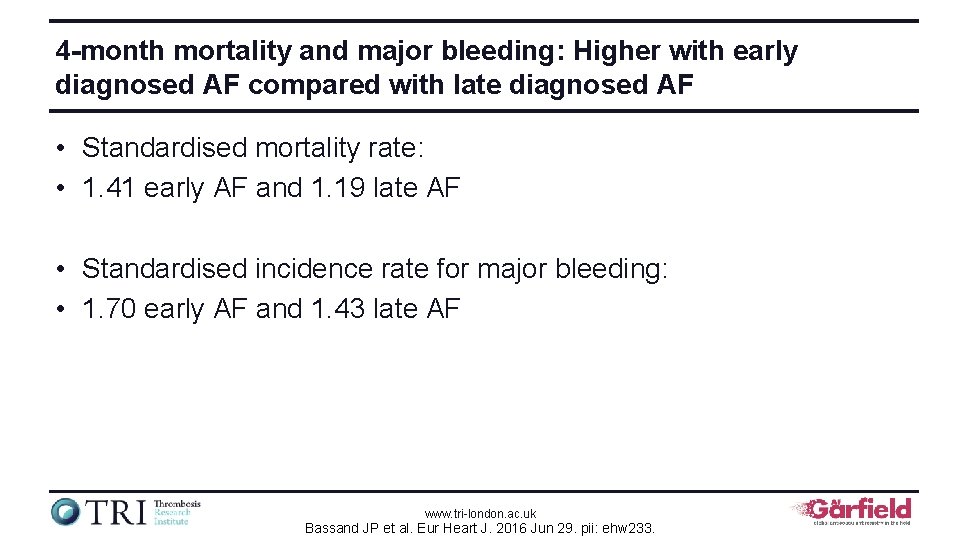
4 -month mortality and major bleeding: Higher with early diagnosed AF compared with late diagnosed AF • Standardised mortality rate: • 1. 41 early AF and 1. 19 late AF • Standardised incidence rate for major bleeding: • 1. 70 early AF and 1. 43 late AF www. tri-london. ac. uk Bassand JP et al. Eur Heart J. 2016 Jun 29. pii: ehw 233.

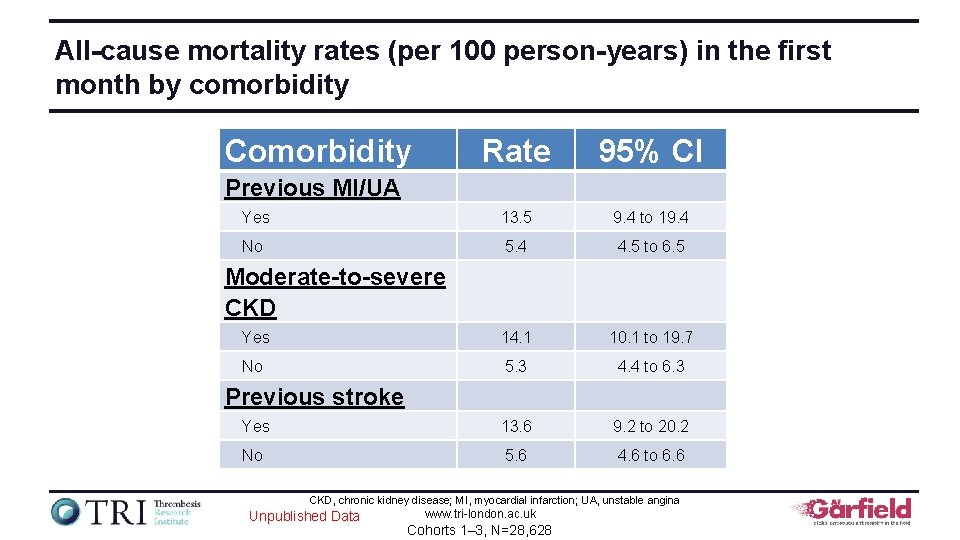
All-cause mortality rates (per 100 person-years) in the first month by comorbidity Comorbidity Rate 95% CI Yes 13. 5 9. 4 to 19. 4 No 5. 4 4. 5 to 6. 5 Yes 14. 1 10. 1 to 19. 7 No 5. 3 4. 4 to 6. 3 Yes 13. 6 9. 2 to 20. 2 No 5. 6 4. 6 to 6. 6 Previous MI/UA Moderate-to-severe CKD Previous stroke CKD, chronic kidney disease; MI, myocardial infarction; UA, unstable angina Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

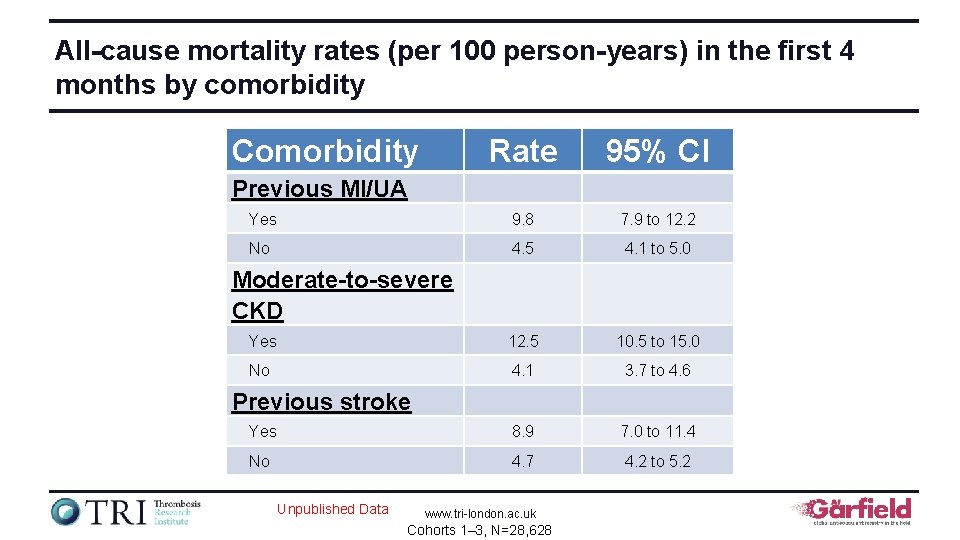
All-cause mortality rates (per 100 person-years) in the first 4 months by comorbidity Comorbidity Rate 95% CI Yes 9. 8 7. 9 to 12. 2 No 4. 5 4. 1 to 5. 0 Yes 12. 5 10. 5 to 15. 0 No 4. 1 3. 7 to 4. 6 Yes 8. 9 7. 0 to 11. 4 No 4. 7 4. 2 to 5. 2 Previous MI/UA Moderate-to-severe CKD Previous stroke Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

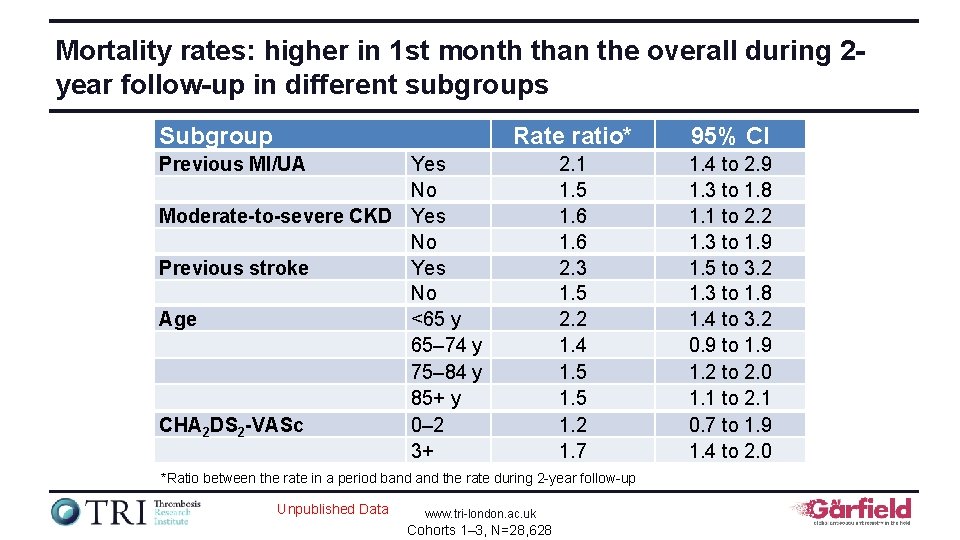
Mortality rates: higher in 1 st month than the overall during 2 year follow-up in different subgroups Subgroup Rate ratio* 95% CI 2. 1 1. 5 1. 6 2. 3 1. 5 2. 2 1. 4 1. 5 1. 2 1. 7 1. 4 to 2. 9 1. 3 to 1. 8 1. 1 to 2. 2 1. 3 to 1. 9 1. 5 to 3. 2 1. 3 to 1. 8 1. 4 to 3. 2 0. 9 to 1. 9 1. 2 to 2. 0 1. 1 to 2. 1 0. 7 to 1. 9 1. 4 to 2. 0 Previous MI/UA Yes No Moderate-to-severe CKD Yes No Previous stroke Yes No Age <65 y 65– 74 y 75– 84 y 85+ y CHA 2 DS 2 -VASc 0– 2 3+ *Ratio between the rate in a period band the rate during 2 -year follow-up Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

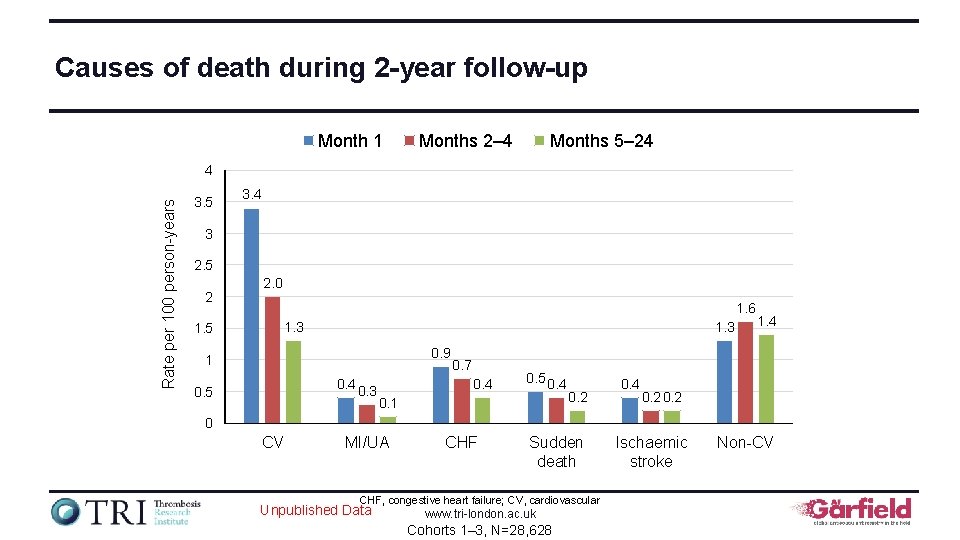
Causes of death during 2 -year follow-up Month 1 Months 2– 4 Months 5– 24 Rate per 100 person-years 4 3. 5 3. 4 3 2. 5 2 2. 0 1. 6 1. 3 1. 5 1. 3 0. 9 1 0. 4 0. 3 0. 5 0. 7 0. 4 0. 5 0. 4 0. 1 0. 2 0. 4 1. 4 0. 2 0 CV MI/UA CHF Sudden death CHF, congestive heart failure; CV, cardiovascular Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628 Ischaemic stroke Non-CV

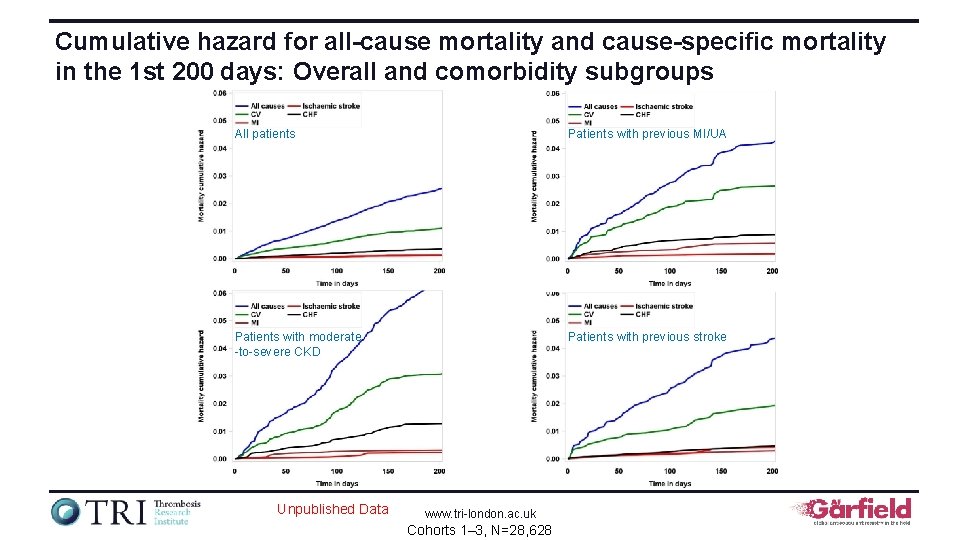
Cumulative hazard for all-cause mortality and cause-specific mortality in the 1 st 200 days: Overall and comorbidity subgroups All patients Patients with previous MI/UA Patients with moderate -to-severe CKD Patients with previous stroke Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

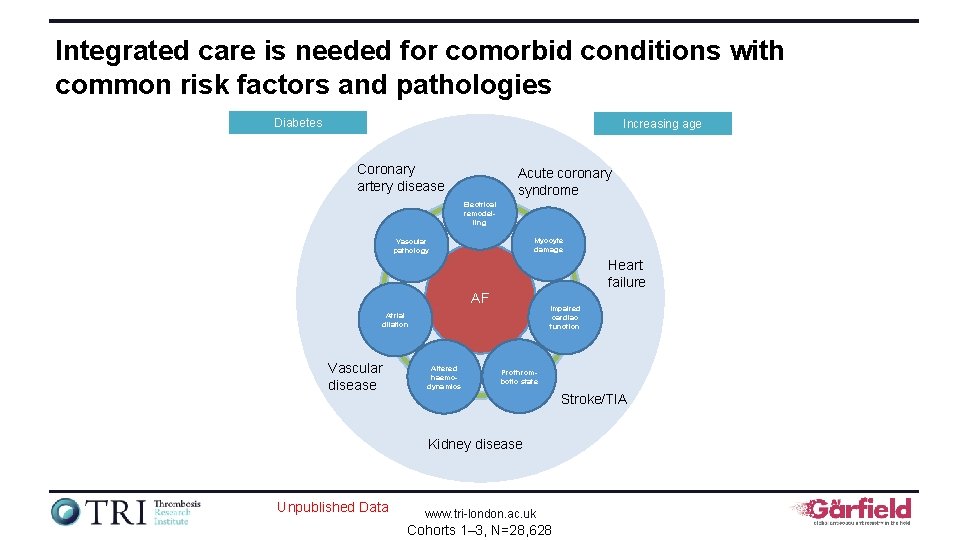
Integrated care is needed for comorbid conditions with common risk factors and pathologies Diabetes Increasing age Coronary artery disease Acute coronary syndrome Electrical remodelling Myocyte damage Vascular pathology Heart failure AF Impaired cardiac function Atrial dilation Vascular disease Altered haemodynamics Prothrombotic state Stroke/TIA Kidney disease Unpublished Data www. tri-london. ac. uk Cohorts 1– 3, N=28, 628

Quality of VKA control and outcomes The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

Objectives • To show the TTR (INR 2. 0 to 3. 0) for different types of VKAs used in GARFIELD-AF • To analyse the quality of VKA control using TTR and its impact on 1 -year outcomes (stroke / systemic embolism, major bleeding, allcause mortality) • To analyse the 4 -month rates of events according to TTR www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

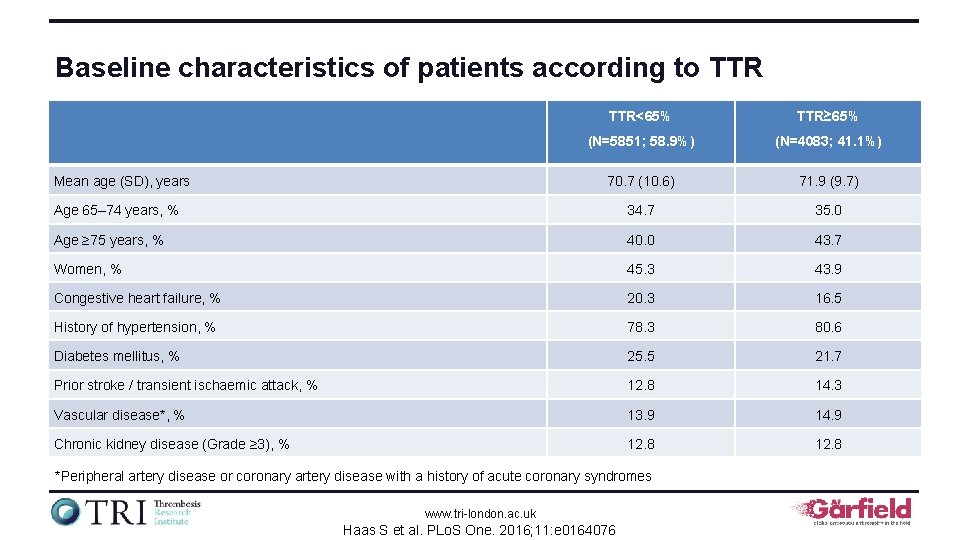
Baseline characteristics of patients according to TTR<65% TTR≥ 65% (N=5851; 58. 9%) (N=4083; 41. 1%) 70. 7 (10. 6) 71. 9 (9. 7) Age 65– 74 years, % 34. 7 35. 0 Age ≥ 75 years, % 40. 0 43. 7 Women, % 45. 3 43. 9 Congestive heart failure, % 20. 3 16. 5 History of hypertension, % 78. 3 80. 6 Diabetes mellitus, % 25. 5 21. 7 Prior stroke / transient ischaemic attack, % 12. 8 14. 3 Vascular disease*, % 13. 9 14. 9 Chronic kidney disease (Grade ≥ 3), % 12. 8 Mean age (SD), years *Peripheral artery disease or coronary artery disease with a history of acute coronary syndromes www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

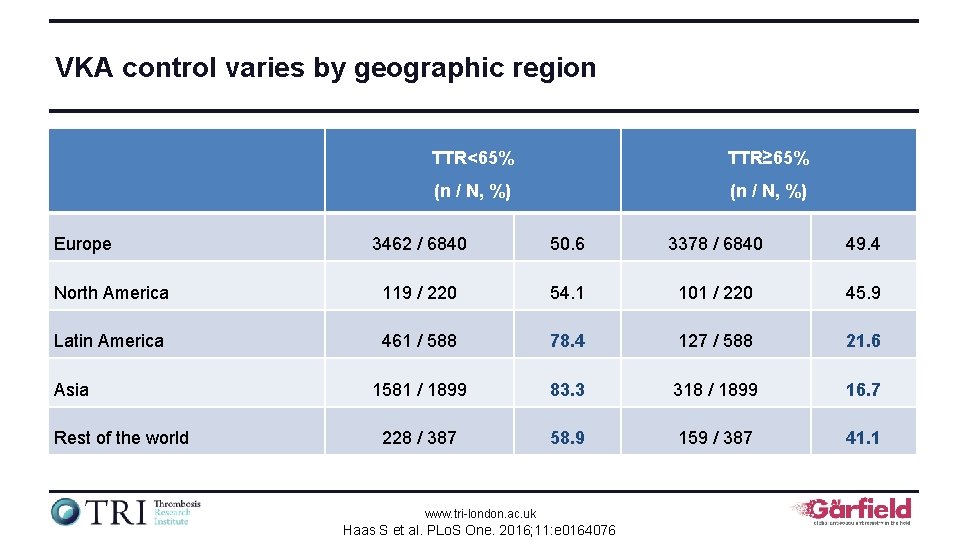
VKA control varies by geographic region Europe TTR<65% TTR≥ 65% (n / N, %) 3462 / 6840 50. 6 3378 / 6840 49. 4 North America 119 / 220 54. 1 101 / 220 45. 9 Latin America 461 / 588 78. 4 127 / 588 21. 6 1581 / 1899 83. 3 318 / 1899 16. 7 228 / 387 58. 9 159 / 387 41. 1 Asia Rest of the world www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

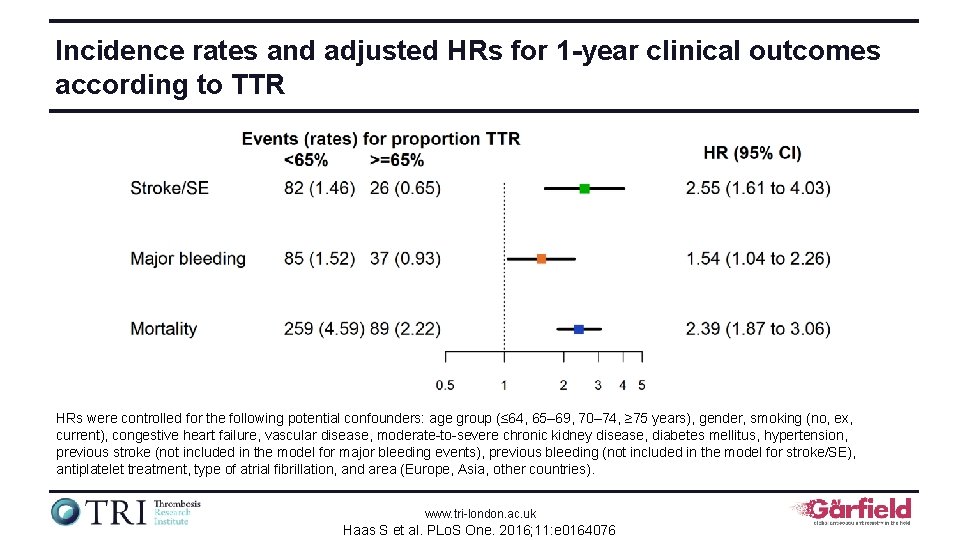
Incidence rates and adjusted HRs for 1 -year clinical outcomes according to TTR HRs were controlled for the following potential confounders: age group (≤ 64, 65– 69, 70– 74, ≥ 75 years), gender, smoking (no, ex, current), congestive heart failure, vascular disease, moderate-to-severe chronic kidney disease, diabetes mellitus, hypertension, previous stroke (not included in the model for major bleeding events), previous bleeding (not included in the model for stroke/SE), antiplatelet treatment, type of atrial fibrillation, and area (Europe, Asia, other countries). www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

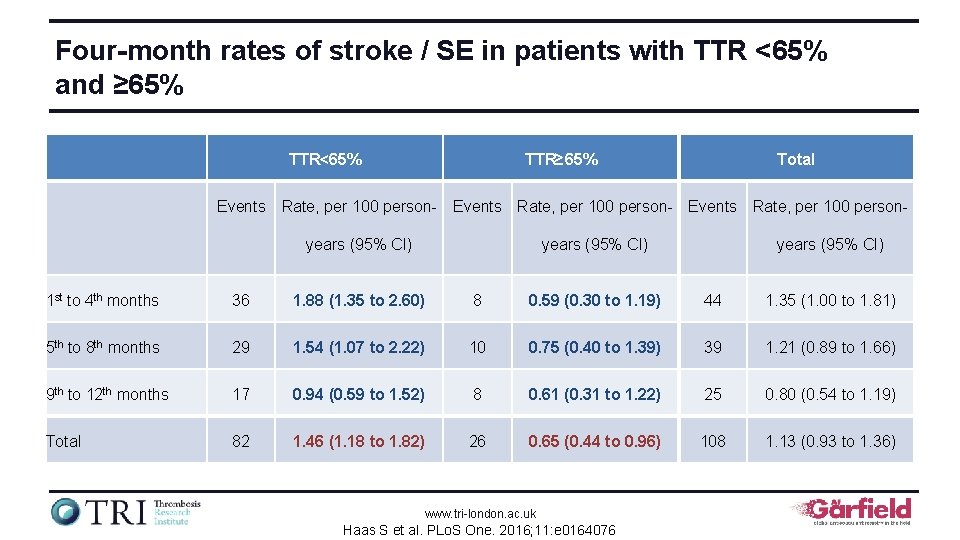
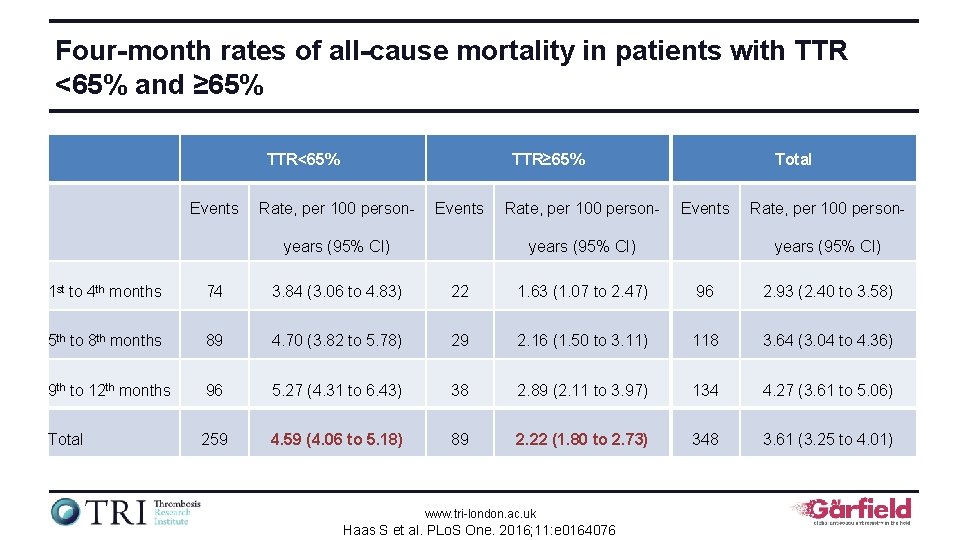
Four-month rates of stroke / SE in patients with TTR <65% and ≥ 65% TTR<65% TTR≥ 65% Total Events Rate, per 100 person- Events Rate, per 100 personyears (95% CI) 1 st to 4 th months 36 1. 88 (1. 35 to 2. 60) 8 0. 59 (0. 30 to 1. 19) 44 1. 35 (1. 00 to 1. 81) 5 th to 8 th months 29 1. 54 (1. 07 to 2. 22) 10 0. 75 (0. 40 to 1. 39) 39 1. 21 (0. 89 to 1. 66) 9 th to 12 th months 17 0. 94 (0. 59 to 1. 52) 8 0. 61 (0. 31 to 1. 22) 25 0. 80 (0. 54 to 1. 19) Total 82 1. 46 (1. 18 to 1. 82) 26 0. 65 (0. 44 to 0. 96) 108 1. 13 (0. 93 to 1. 36) www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

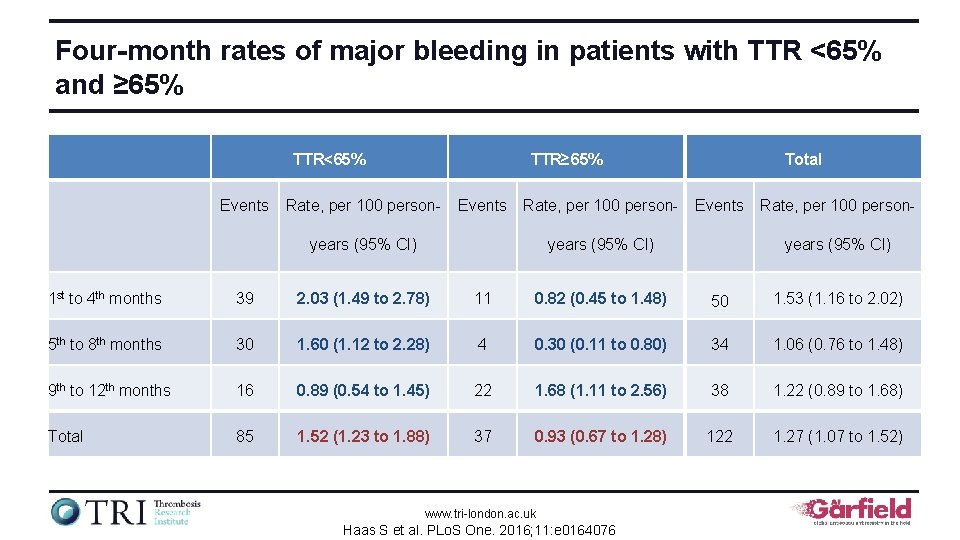
Four-month rates of major bleeding in patients with TTR <65% and ≥ 65% TTR<65% Events TTR≥ 65% Rate, per 100 person- Events Rate, per 100 person- years (95% CI) Total Events years (95% CI) Rate, per 100 personyears (95% CI) 1 st to 4 th months 39 2. 03 (1. 49 to 2. 78) 11 0. 82 (0. 45 to 1. 48) 50 1. 53 (1. 16 to 2. 02) 5 th to 8 th months 30 1. 60 (1. 12 to 2. 28) 4 0. 30 (0. 11 to 0. 80) 34 1. 06 (0. 76 to 1. 48) 9 th to 12 th months 16 0. 89 (0. 54 to 1. 45) 22 1. 68 (1. 11 to 2. 56) 38 1. 22 (0. 89 to 1. 68) Total 85 1. 52 (1. 23 to 1. 88) 37 0. 93 (0. 67 to 1. 28) 122 1. 27 (1. 07 to 1. 52) www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

Four-month rates of all-cause mortality in patients with TTR <65% and ≥ 65% TTR<65% Events TTR≥ 65% Rate, per 100 person- Events years (95% CI) Rate, per 100 person- Total Events years (95% CI) Rate, per 100 personyears (95% CI) 1 st to 4 th months 74 3. 84 (3. 06 to 4. 83) 22 1. 63 (1. 07 to 2. 47) 96 2. 93 (2. 40 to 3. 58) 5 th to 8 th months 89 4. 70 (3. 82 to 5. 78) 29 2. 16 (1. 50 to 3. 11) 118 3. 64 (3. 04 to 4. 36) 9 th to 12 th months 96 5. 27 (4. 31 to 6. 43) 38 2. 89 (2. 11 to 3. 97) 134 4. 27 (3. 61 to 5. 06) Total 259 4. 59 (4. 06 to 5. 18) 89 2. 22 (1. 80 to 2. 73) 348 3. 61 (3. 25 to 4. 01) www. tri-london. ac. uk Haas S et al. PLo. S One. 2016; 11: e 0164076

Characteristics of patients with nonvalvular AF receiving antiplatelet therapy The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

Background • ESC guidelines on atrial fibrillation (AF) do not recommend the routine use of antiplatelet (AP) therapy for stroke prevention, even not in patients at a very low risk of stroke 1 • American guidelines recommend aspirin, oral anticoagulants, or no antithrombotic therapy in patients with a CHA 2 DS 2 -VASc score of 1, but the recommendation is weak (IIb)2 1. Camm AJ et al. Europace. 2012; 14(10): 1385 -413. 2. January CT et al. J Am Coll Cardiol. 2014; 64(21): e 1 -76. www. tri-london. ac. uk

Objectives • To describe the characteristics of patients in GARFIELD-AF who use AP therapy only, anticoagulant (AC) therapy only, or AP + AC at enrolment • To evaluate the factors associated with prescription of AP only versus AC only • To describe the treatment pattern over time in the 5 cohorts, and by CHA 2 DS 2 -VASc and country economic status www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

Methods • This analysis includes patients in cohorts 1 to 5 of GARFIELD-AF, enrolled between March 2010 and July 2016 • The associations between use of AP alone (versus AC alone) and baseline characteristics was assessed by using logistic regression models • Stepwise selection was used to select the baseline variables, with α=0. 05 for exclusion www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

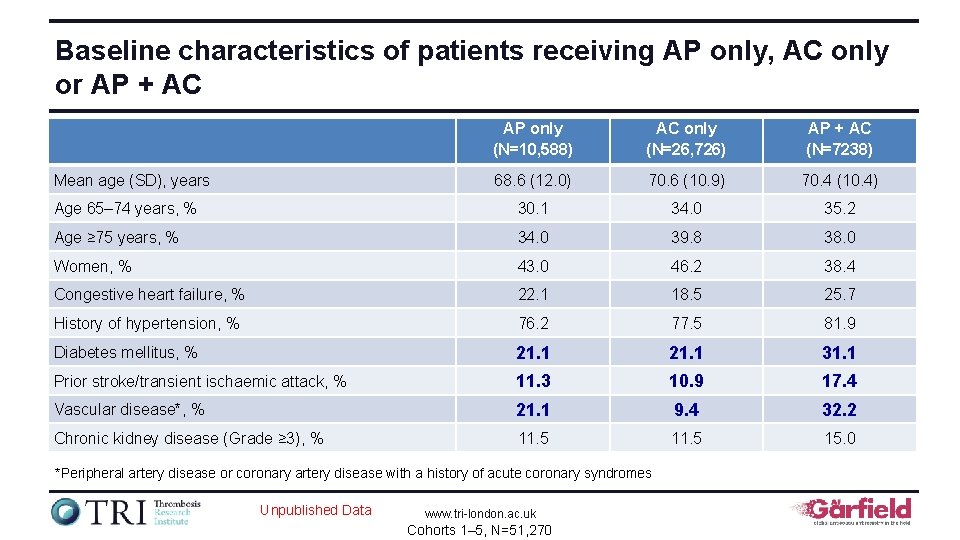
Baseline characteristics of patients receiving AP only, AC only or AP + AC AP only (N=10, 588) AC only (N=26, 726) AP + AC (N=7238) 68. 6 (12. 0) 70. 6 (10. 9) 70. 4 (10. 4) Age 65– 74 years, % 30. 1 34. 0 35. 2 Age ≥ 75 years, % 34. 0 39. 8 38. 0 Women, % 43. 0 46. 2 38. 4 Congestive heart failure, % 22. 1 18. 5 25. 7 History of hypertension, % 76. 2 77. 5 81. 9 Diabetes mellitus, % 21. 1 31. 1 Prior stroke/transient ischaemic attack, % 11. 3 10. 9 17. 4 Vascular disease*, % 21. 1 9. 4 32. 2 Chronic kidney disease (Grade ≥ 3), % 11. 5 15. 0 Mean age (SD), years *Peripheral artery disease or coronary artery disease with a history of acute coronary syndromes Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

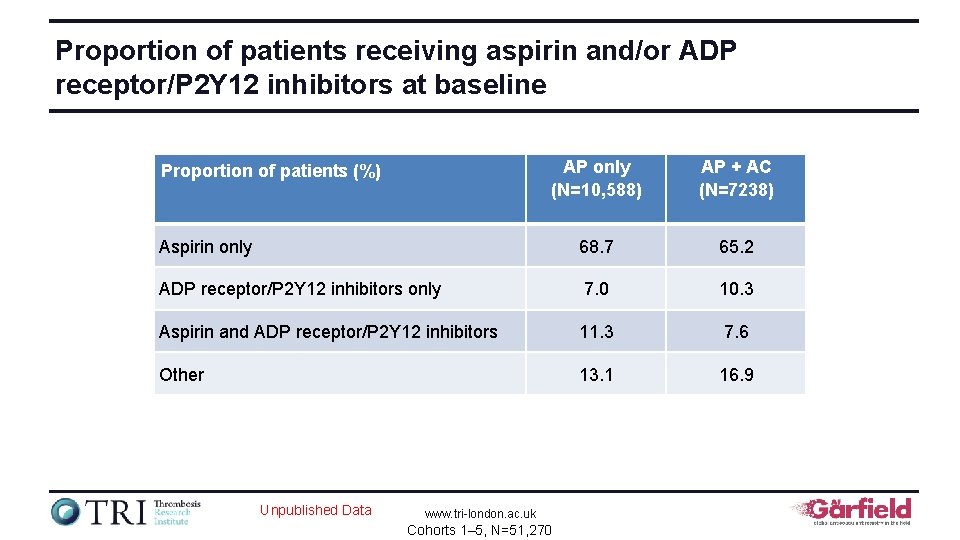
Proportion of patients receiving aspirin and/or ADP receptor/P 2 Y 12 inhibitors at baseline AP only (N=10, 588) AP + AC (N=7238) Aspirin only 68. 7 65. 2 ADP receptor/P 2 Y 12 inhibitors only 7. 0 10. 3 Aspirin and ADP receptor/P 2 Y 12 inhibitors 11. 3 7. 6 Other 13. 1 16. 9 Proportion of patients (%) Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

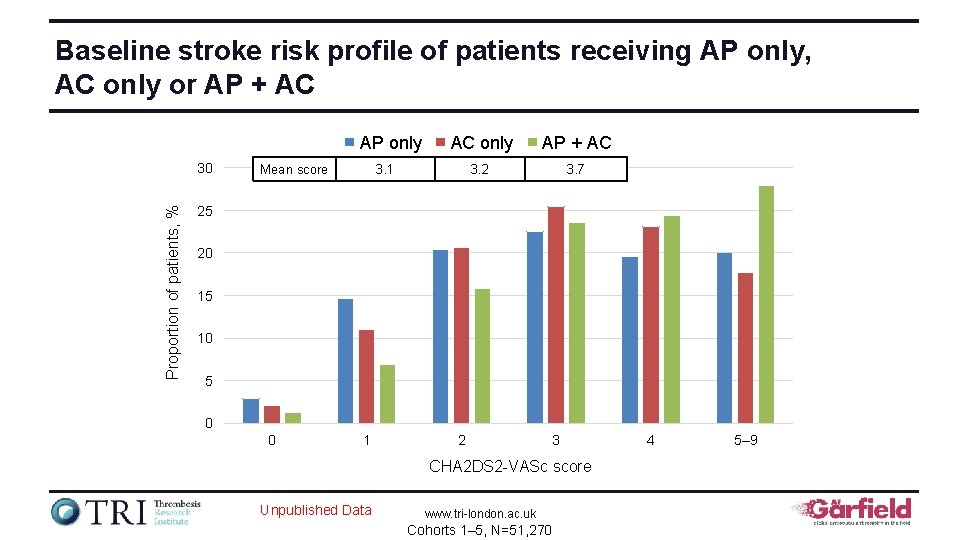
Baseline stroke risk profile of patients receiving AP only, AC only or AP + AC AP only Proportion of patients, % 30 Mean score AC only AP + AC 3. 2 3. 7 3. 1 25 20 15 10 5 0 0 1 2 3 CHA 2 DS 2 -VASc score Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 4 5– 9

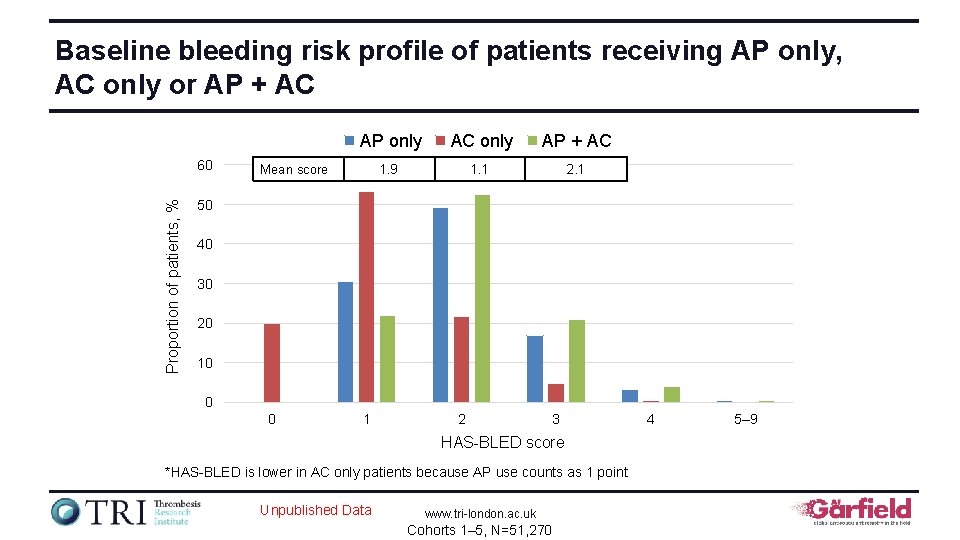
Baseline bleeding risk profile of patients receiving AP only, AC only or AP + AC Proportion of patients, % 60 AP only AC only AP + AC 1. 9 1. 1 2. 1 Mean score 50 40 30 20 10 0 0 1 2 3 HAS-BLED score *HAS-BLED is lower in AC only patients because AP use counts as 1 point Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 4 5– 9

Antithrombotic treatment at enrolment by cohort AP only Unpublished Data AC only AP+AC www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 None

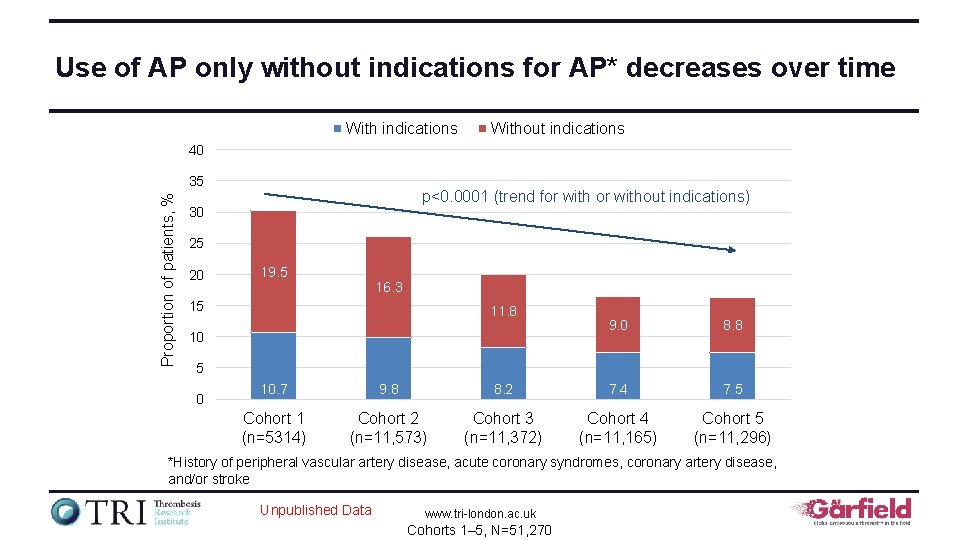
Use of AP only without indications for AP* decreases over time With indications Without indications 40 Proportion of patients, % 35 p<0. 0001 (trend for without indications) 30 25 20 19. 5 16. 3 15 11. 8 10 9. 0 8. 8 5 0 10. 7 9. 8 8. 2 7. 4 7. 5 Cohort 1 (n=5314) Cohort 2 (n=11, 573) Cohort 3 (n=11, 372) Cohort 4 (n=11, 165) Cohort 5 (n=11, 296) *History of peripheral vascular artery disease, acute coronary syndromes, coronary artery disease, and/or stroke Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

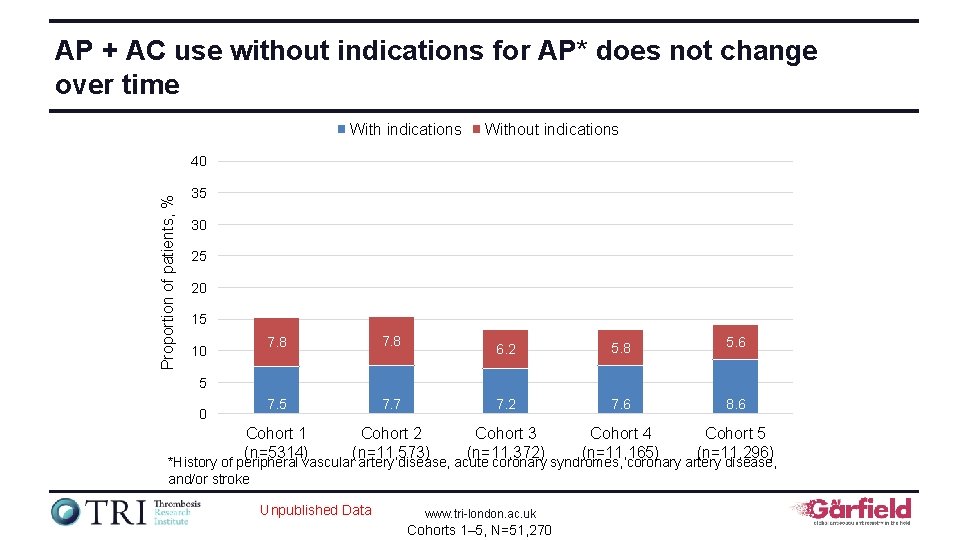
AP + AC use without indications for AP* does not change over time With indications Without indications Proportion of patients, % 40 35 30 25 20 15 10 7. 8 7. 5 Cohort 1 (n=5314) 6. 2 5. 8 5. 6 7. 7 7. 2 7. 6 8. 6 Cohort 2 (n=11, 573) Cohort 3 (n=11, 372) Cohort 4 (n=11, 165) Cohort 5 (n=11, 296) 5 0 *History of peripheral vascular artery disease, acute coronary syndromes, coronary artery disease, and/or stroke Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

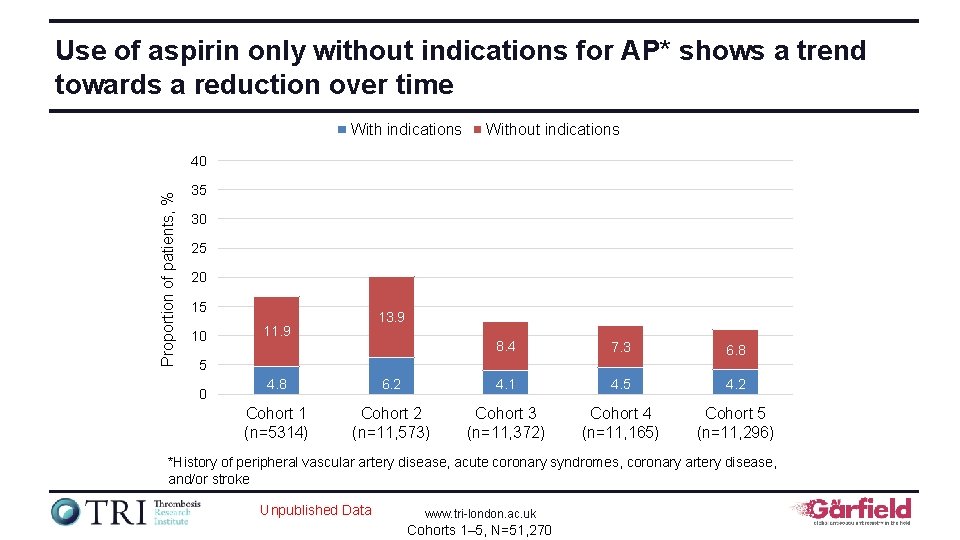
Use of aspirin only without indications for AP* shows a trend towards a reduction over time With indications Without indications Proportion of patients, % 40 35 30 25 20 15 10 13. 9 11. 9 8. 4 7. 3 6. 8 5 0 4. 8 6. 2 4. 1 4. 5 4. 2 Cohort 1 (n=5314) Cohort 2 (n=11, 573) Cohort 3 (n=11, 372) Cohort 4 (n=11, 165) Cohort 5 (n=11, 296) *History of peripheral vascular artery disease, acute coronary syndromes, coronary artery disease, and/or stroke Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

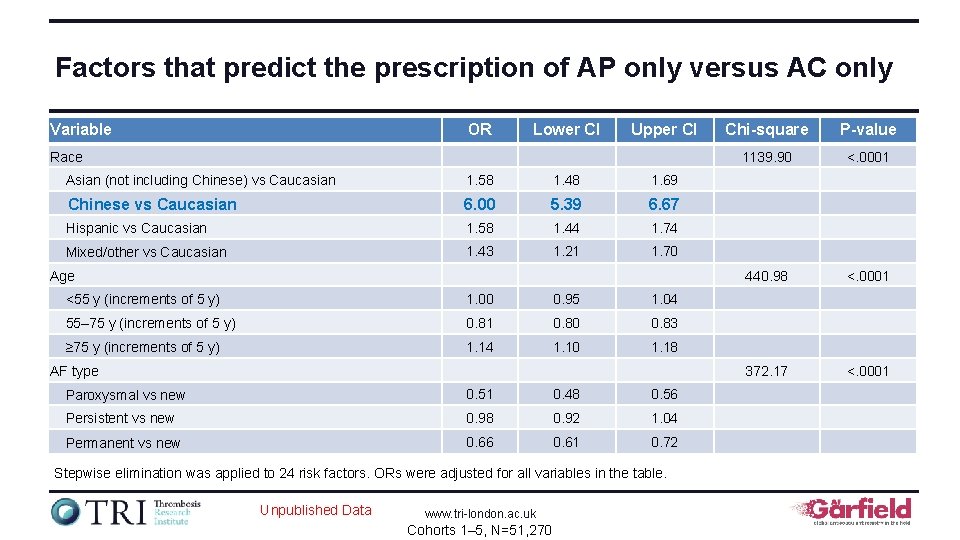
Factors that predict the prescription of AP only versus AC only Variable OR Lower CI Upper CI Race Asian (not including Chinese) vs Caucasian 1. 58 1. 48 1. 69 Chinese vs Caucasian 6. 00 5. 39 6. 67 Hispanic vs Caucasian 1. 58 1. 44 1. 74 Mixed/other vs Caucasian 1. 43 1. 21 1. 70 Age <55 y (increments of 5 y) 1. 00 0. 95 1. 04 55– 75 y (increments of 5 y) 0. 81 0. 80 0. 83 ≥ 75 y (increments of 5 y) 1. 14 1. 10 1. 18 AF type Paroxysmal vs new 0. 51 0. 48 0. 56 Persistent vs new 0. 98 0. 92 1. 04 Permanent vs new 0. 66 0. 61 0. 72 Stepwise elimination was applied to 24 risk factors. ORs were adjusted for all variables in the table. Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 Chi-square P-value 1139. 90 <. 0001 440. 98 <. 0001 372. 17 <. 0001

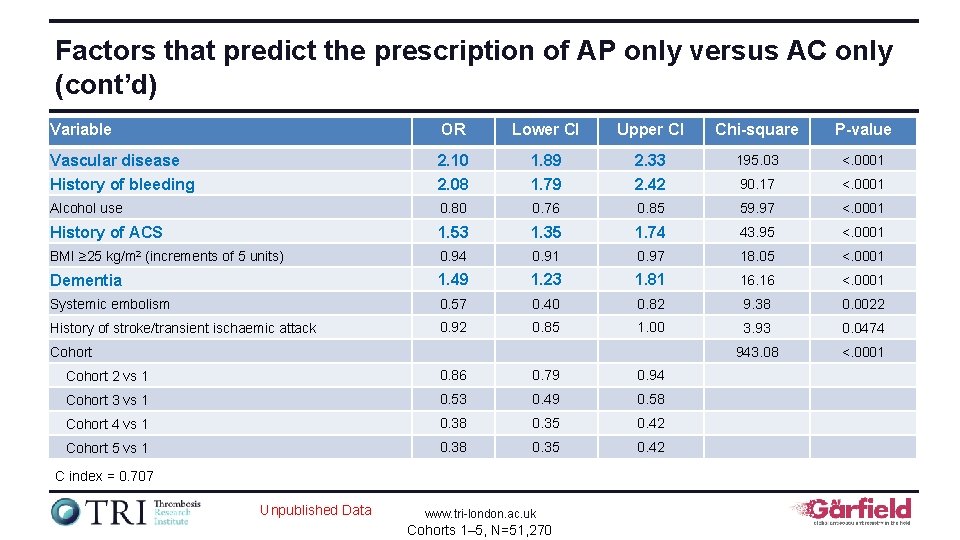
Factors that predict the prescription of AP only versus AC only (cont’d) Variable OR Lower CI Upper CI Chi-square P-value Vascular disease History of bleeding 2. 10 2. 08 1. 89 1. 79 2. 33 2. 42 195. 03 <. 0001 90. 17 <. 0001 Alcohol use 0. 80 0. 76 0. 85 59. 97 <. 0001 History of ACS 1. 53 1. 35 1. 74 43. 95 <. 0001 BMI ≥ 25 kg/m 2 (increments of 5 units) 0. 94 0. 91 0. 97 18. 05 <. 0001 Dementia 1. 49 1. 23 1. 81 16. 16 <. 0001 Systemic embolism 0. 57 0. 40 0. 82 9. 38 0. 0022 History of stroke/transient ischaemic attack 0. 92 0. 85 1. 00 3. 93 0. 0474 943. 08 <. 0001 Cohort 2 vs 1 0. 86 0. 79 0. 94 Cohort 3 vs 1 0. 53 0. 49 0. 58 Cohort 4 vs 1 0. 38 0. 35 0. 42 Cohort 5 vs 1 0. 38 0. 35 0. 42 C index = 0. 707 Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

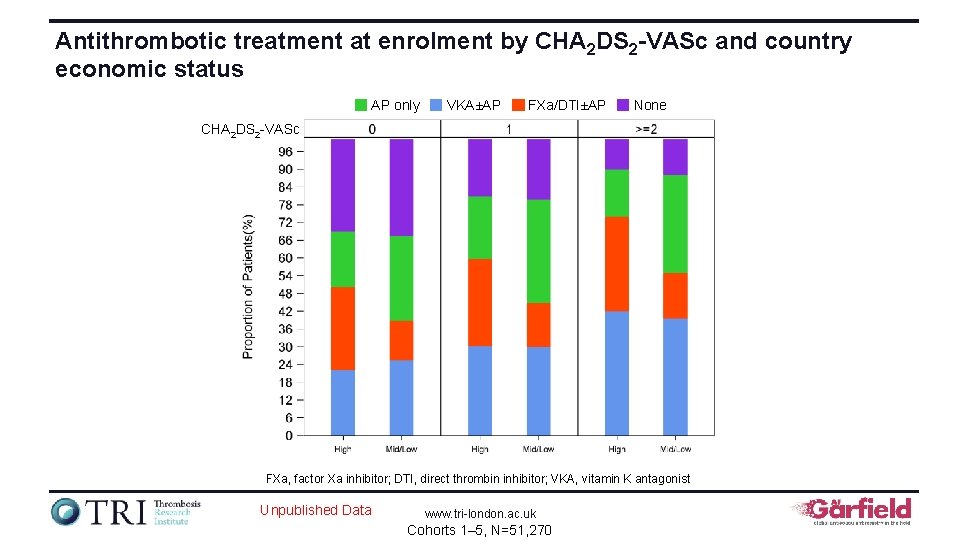
Antithrombotic treatment at enrolment by CHA 2 DS 2 -VASc and country economic status AP only VKA±AP FXa/DTI±AP None CHA 2 DS 2 -VASc FXa, factor Xa inhibitor; DTI, direct thrombin inhibitor; VKA, vitamin K antagonist Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270

Evaluation of prescribing with NOACs vs VKAs in nonvalvular AF The GARFIELD-AF registry is funded by an unrestricted research grant from Bayer AG www. tri-london. ac. uk

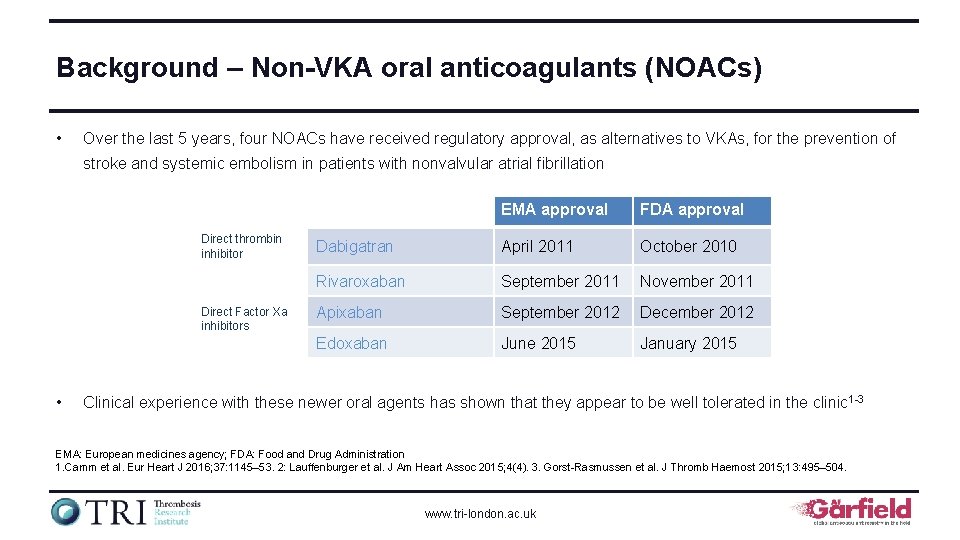
Background – Non-VKA oral anticoagulants (NOACs) • Over the last 5 years, four NOACs have received regulatory approval, as alternatives to VKAs, for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation Direct thrombin inhibitor Direct Factor Xa inhibitors • EMA approval FDA approval Dabigatran April 2011 October 2010 Rivaroxaban September 2011 November 2011 Apixaban September 2012 December 2012 Edoxaban June 2015 January 2015 Clinical experience with these newer oral agents has shown that they appear to be well tolerated in the clinic 1 -3 EMA: European medicines agency; FDA: Food and Drug Administration 1. Camm et al. Eur Heart J 2016; 37: 1145– 53. 2: Lauffenburger et al. J Am Heart Assoc 2015; 4(4). 3. Gorst-Rasmussen et al. J Thromb Haemost 2015; 13: 495– 504. www. tri-london. ac. uk

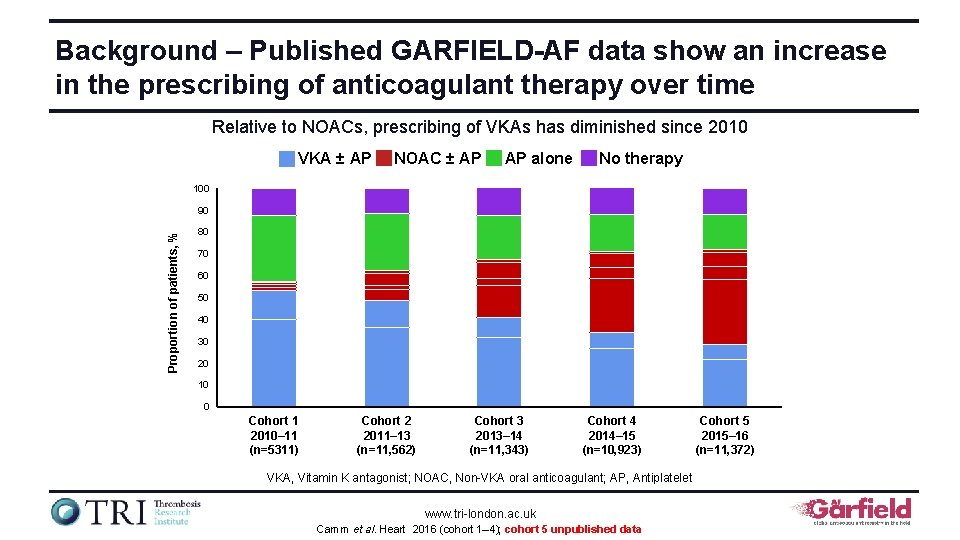
Background – Published GARFIELD-AF data show an increase in the prescribing of anticoagulant therapy over time Relative to NOACs, prescribing of VKAs has diminished since 2010 VKA ± AP NOAC ± AP AP alone No therapy 100 Proportion of patients, % 90 80 70 60 50 40 30 20 10 0 Cohort 1 2010– 11 (n=5311) Cohort 2 2011– 13 (n=11, 562) Cohort 3 2013– 14 (n=11, 343) Cohort 4 2014– 15 (n=10, 923) VKA, Vitamin K antagonist; NOAC, Non-VKA oral anticoagulant; AP, Antiplatelet www. tri-london. ac. uk Camm et al. Heart 2016 (cohort 1– 4); cohort 5 unpublished data Cohort 5 2015– 16 (n=11, 372)

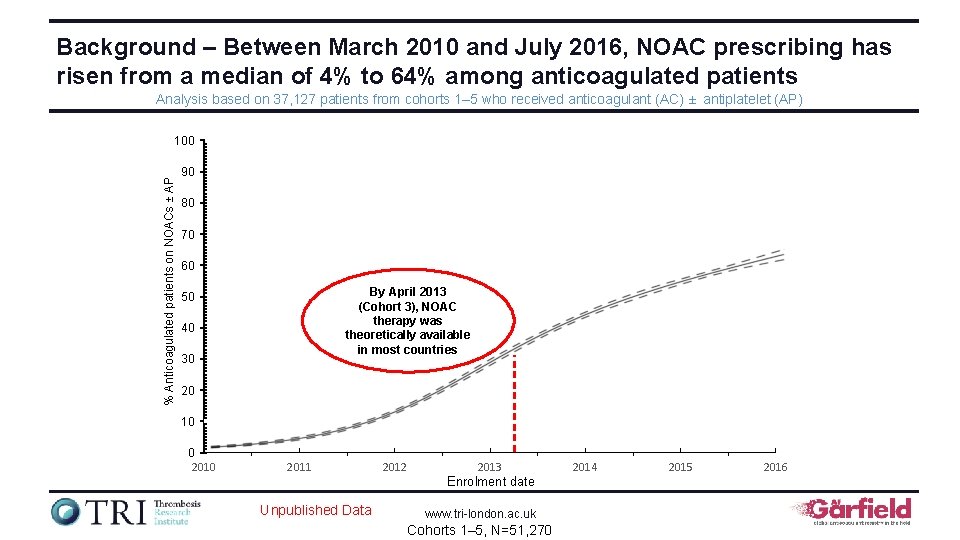
Background – Between March 2010 and July 2016, NOAC prescribing has risen from a median of 4% to 64% among anticoagulated patients Analysis based on 37, 127 patients from cohorts 1– 5 who received anticoagulant (AC) ± antiplatelet (AP) % Anticoagulated patients on NOACs ± AP 100 90 80 70 60 By April 2013 (Cohort 3), NOAC therapy was theoretically available in most countries 50 40 30 20 10 0 2011 2012 2013 Enrolment date Unpublished Data www. tri-london. ac. uk Cohorts 1– 5, N=51, 270 2014 2015 2016

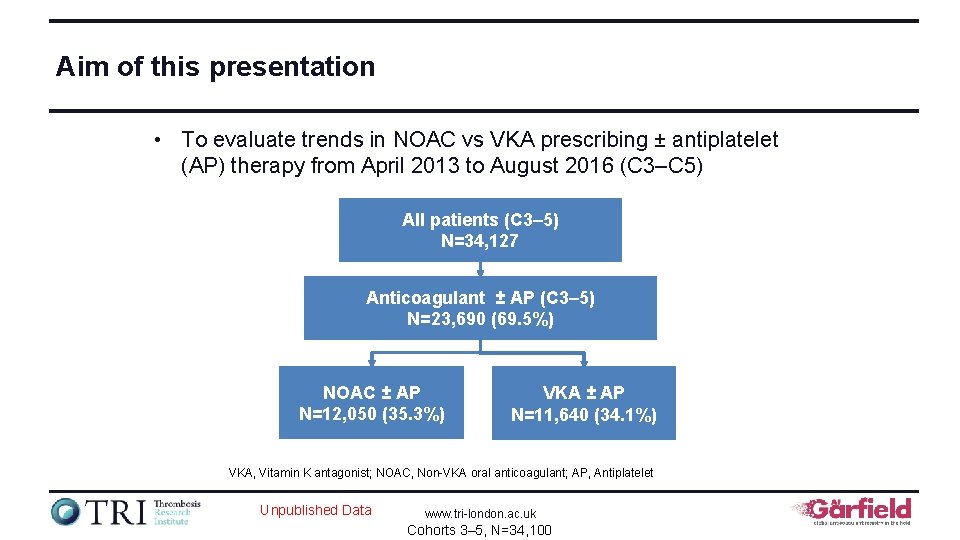
Aim of this presentation • To evaluate trends in NOAC vs VKA prescribing ± antiplatelet (AP) therapy from April 2013 to August 2016 (C 3–C 5) All patients (C 3– 5) N=34, 127 Anticoagulant ± AP (C 3– 5) N=23, 690 (69. 5%) NOAC ± AP N=12, 050 (35. 3%) VKA ± AP N=11, 640 (34. 1%) VKA, Vitamin K antagonist; NOAC, Non-VKA oral anticoagulant; AP, Antiplatelet Unpublished Data www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

Analysis of the relationship between ALL variables and NOAC (vs VKA) use www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

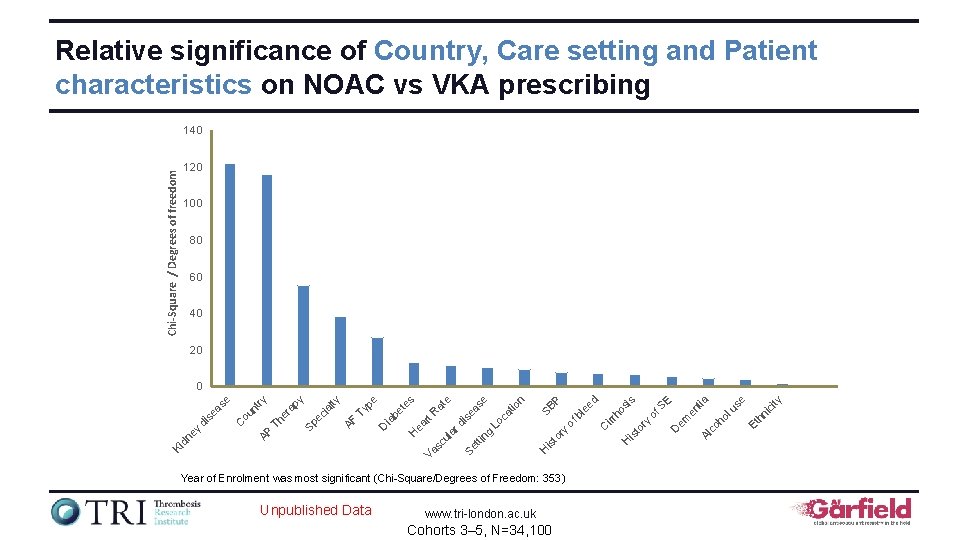
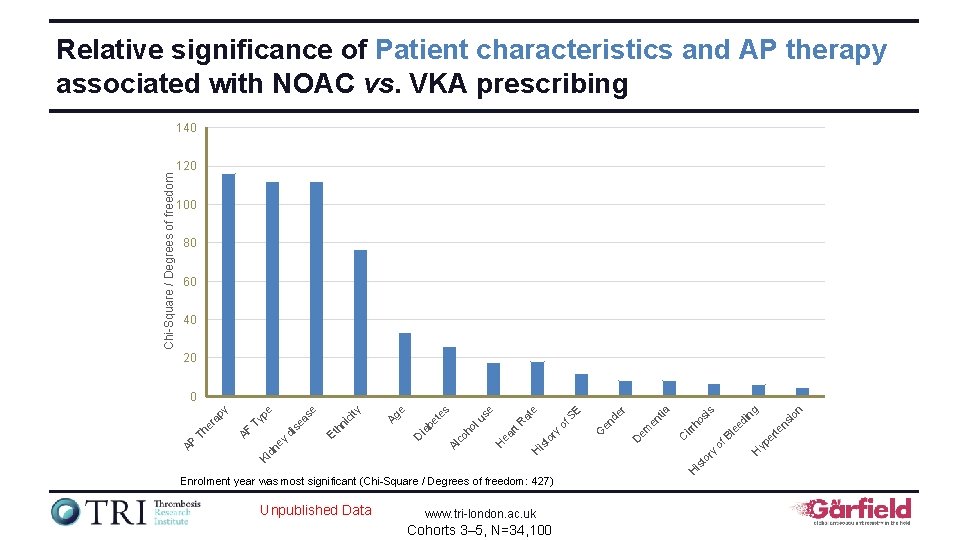
Relative significance of Country, Care setting and Patient characteristics on NOAC vs VKA prescribing Chi-Square / Degrees of freedom 140 120 100 80 60 40 20 Unpublished Data www. tri-london. ac. uk Cohorts 3– 5, N=34, 100 ity ic Et hn se lu co em D ho E en tia Al H is to ry irr of S ho si s d is H Year of Enrolment was most significant (Chi-Square/Degrees of Freedom: 353) C to ry of bl SB ee P n tio as ng is e tti Se Va Lo ca e e rd ul a sc H ea rt R at es be t D ia pe Ty AF ec ia lty py Sp er a Th AP ou n C Ki dn ey di se as e try 0

Analysis of the relationship between NOAC vs VKA use and the following variables: Cohort Care setting – Location: Emergency room, hospital office / anticoagulation clinic – Speciality: Cardiology, internal medicine, neurology, primary care / geriatric Patient characteristics www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

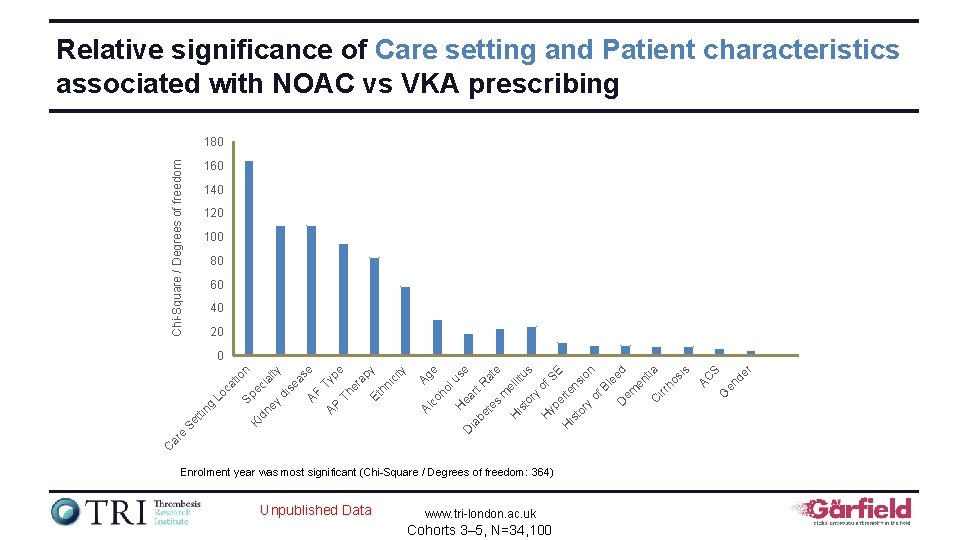
Relative significance of Care setting and Patient characteristics associated with NOAC vs VKA prescribing Chi-Square / Degrees of freedom 180 160 140 120 100 80 60 40 20 Enrolment year was most significant (Chi-Square / Degrees of freedom: 364) Unpublished Data www. tri-london. ac. uk Cohorts 3– 5, N=34, 100 r en de S G AC u ea se ia be rt R at te e s m e H llit is us to ry of H yp SE er H te is ns to io ry n of Bl ee D em d en tia C irr ho si s D H ho l Ag e Al co C ar e Se tti ng Lo ca tio n Sp Ki e ci dn al ey ty di se as AF e AP Typ Th e er ap y Et hn ic ity 0

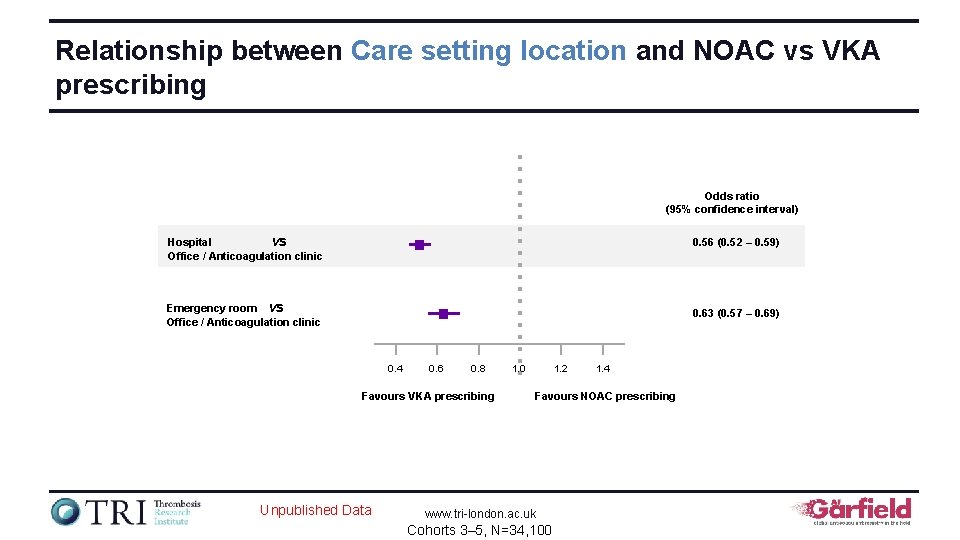
Relationship between Care setting location and NOAC vs VKA prescribing Odds ratio (95% confidence interval) Hospital vs Office / Anticoagulation clinic 0. 56 (0. 52 – 0. 59) Emergency room vs Office / Anticoagulation clinic 0. 63 (0. 57 – 0. 69) 0. 4 0. 6 0. 8 Favours VKA prescribing Unpublished Data 1. 2 1. 0 1. 4 Favours NOAC prescribing www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

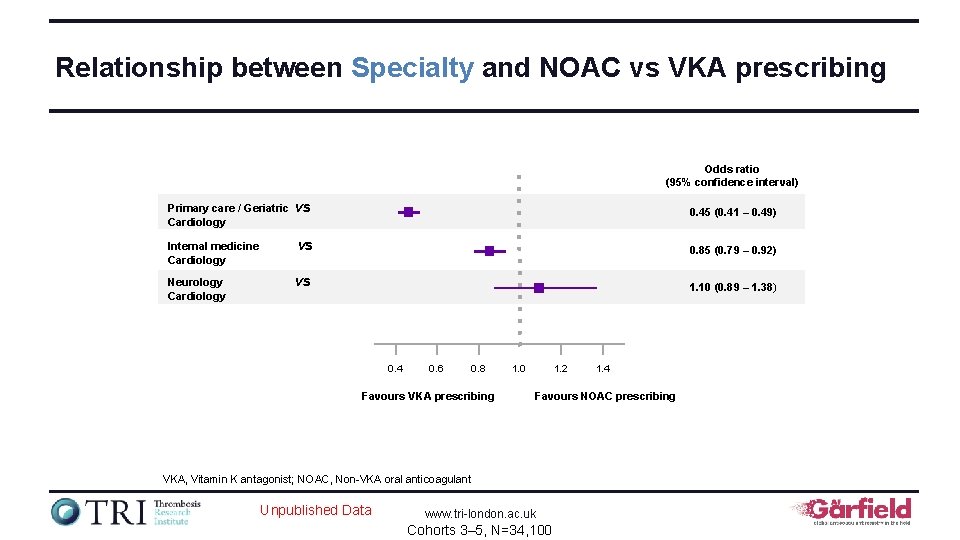
Relationship between Specialty and NOAC vs VKA prescribing Odds ratio (95% confidence interval) Primary care / Geriatric Cardiology vs 0. 45 (0. 41 – 0. 49) Internal medicine Cardiology vs 0. 85 (0. 79 – 0. 92) Neurology Cardiology vs 1. 10 (0. 89 – 1. 38) 0. 4 0. 6 0. 8 Favours VKA prescribing 1. 2 1. 0 Favours NOAC prescribing VKA, Vitamin K antagonist; NOAC, Non-VKA oral anticoagulant Unpublished Data 1. 4 www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

Analysis of the relationship between NOAC vs VKA use and Patient Cohort characteristics • • • Age Gender Ethnicity Life style Clinical factors www. tri-london. ac. uk Cohorts 3– 5, N=34, 100

Relative significance of Patient characteristics and AP therapy associated with NOAC vs. VKA prescribing 140 100 80 60 40 20 www. tri-london. ac. uk Cohorts 3– 5, N=34, 100 n io yp er H of te ee d ns in g s ho si irr C em D is Unpublished Data H Enrolment year was most significant (Chi-Square / Degrees of freedom: 427) Bl r en tia to ry H is to en de of S ry rt R ea H G E e at lu se ho co Al D ia be t es e Ag ity ic hn Et ey di se as e pe Ty dn Ki AF Th er a py 0 AP Chi-Square / Degrees of freedom 120

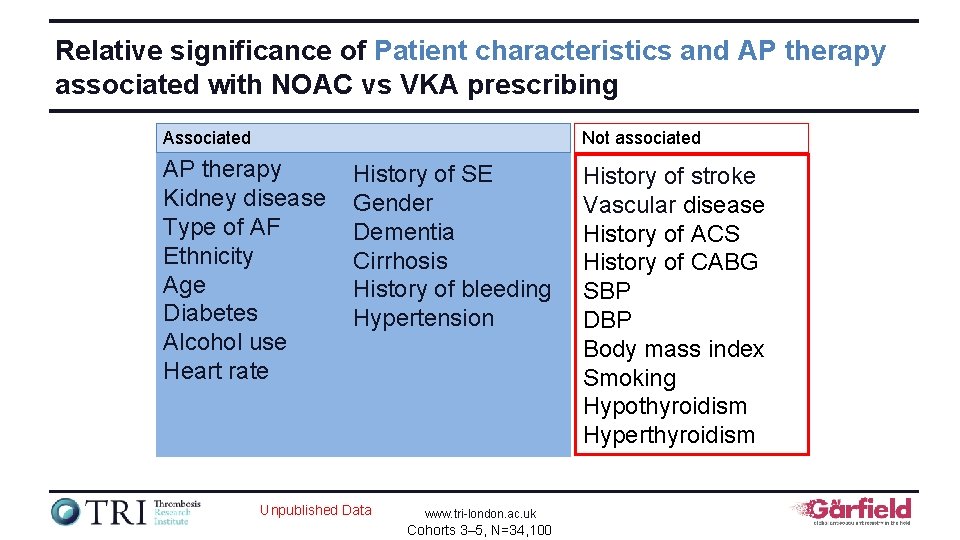
Relative significance of Patient characteristics and AP therapy associated with NOAC vs VKA prescribing Associated Not associated AP therapy Kidney disease Type of AF Ethnicity Age Diabetes Alcohol use Heart rate History of SE Gender Dementia Cirrhosis History of bleeding Hypertension Unpublished Data www. tri-london. ac. uk Cohorts 3– 5, N=34, 100 History of stroke Vascular disease History of ACS History of CABG SBP DBP Body mass index Smoking Hypothyroidism Hyperthyroidism

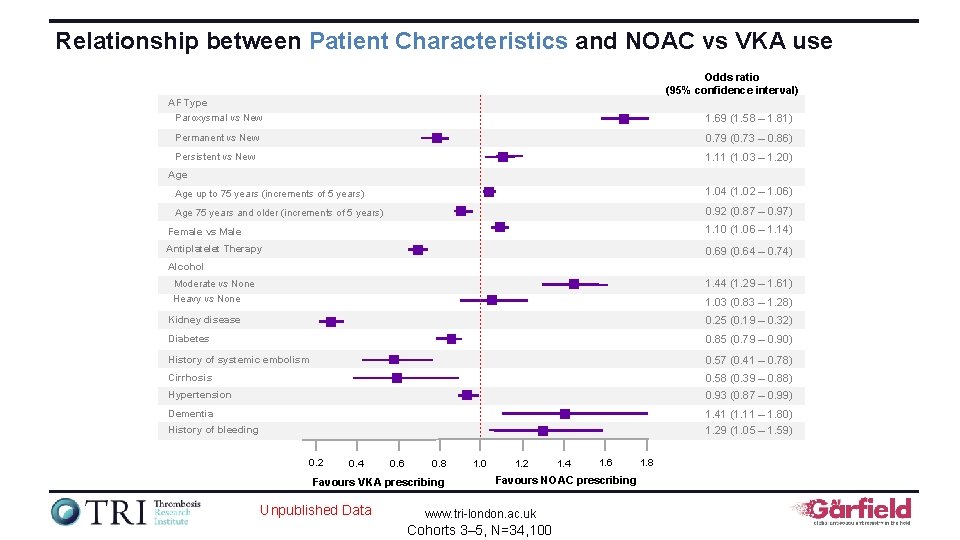
Relationship between Patient Characteristics and NOAC vs VKA use Odds ratio (95% confidence interval) AF Type Paroxysmal vs New 1. 69 (1. 58 – 1. 81) Permanent vs New 0. 79 (0. 73 – 0. 86) Persistent vs New 1. 11 (1. 03 – 1. 20) Age up to 75 years (increments of 5 years) 1. 04 (1. 02 – 1. 06) Age 75 years and older (increments of 5 years) 0. 92 (0. 87 – 0. 97) Female vs Male 1. 10 (1. 06 – 1. 14) Antiplatelet Therapy 0. 69 (0. 64 – 0. 74) Alcohol 1. 44 (1. 29 – 1. 61) Moderate vs None Heavy vs None 1. 03 (0. 83 – 1. 28) Kidney disease 0. 25 (0. 19 – 0. 32) Diabetes 0. 85 (0. 79 – 0. 90) History of systemic embolism 0. 57 (0. 41 – 0. 78) Cirrhosis 0. 58 (0. 39 – 0. 88) Hypertension 0. 93 (0. 87 – 0. 99) Dementia 1. 41 (1. 11 – 1. 80) History of bleeding 1. 29 (1. 05 – 1. 59) 0. 2 0. 4 0. 6 0. 8 Favours VKA prescribing Unpublished Data 1. 0 1. 2 1. 4 1. 6 Favours NOAC prescribing www. tri-london. ac. uk Cohorts 3– 5, N=34, 100 1. 8

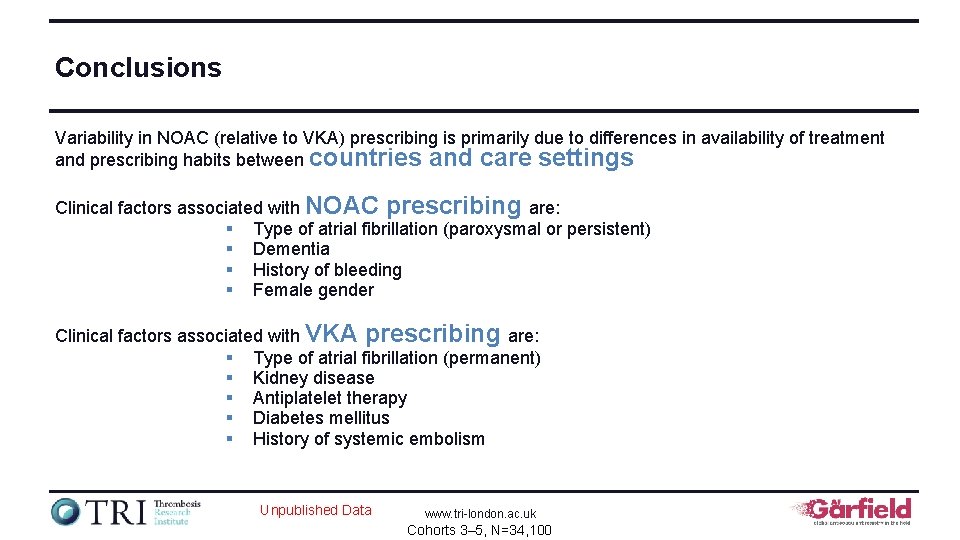
Conclusions Variability in NOAC (relative to VKA) prescribing is primarily due to differences in availability of treatment and prescribing habits between countries and care settings Clinical factors associated with NOAC prescribing are: § Type of atrial fibrillation (paroxysmal or persistent) § Dementia § History of bleeding § Female gender Clinical factors associated with VKA prescribing are: § Type of atrial fibrillation (permanent) § Kidney disease § Antiplatelet therapy § Diabetes mellitus § History of systemic embolism Unpublished Data www. tri-london. ac. uk Cohorts 3– 5, N=34, 100
 Cyp450 inducers mnemonic
Cyp450 inducers mnemonic Les anticoagulants ifsi
Les anticoagulants ifsi Types of anticoagulant
Types of anticoagulant Embolie pulmonaire ifsi
Embolie pulmonaire ifsi Pauta harmonització anticoagulants
Pauta harmonització anticoagulants Anticoagulants mechanism of action
Anticoagulants mechanism of action Front
Front Importance of entrepreneurship development programme
Importance of entrepreneurship development programme Sas contents
Sas contents Table of contents science
Table of contents science Table of contents science
Table of contents science Company profile table of contents
Company profile table of contents Table of contents error
Table of contents error Medial aspect of antecubital fossa
Medial aspect of antecubital fossa Contents
Contents Solar system contents
Solar system contents Fresh frozen plasma transfusion time
Fresh frozen plasma transfusion time Table of contents annex
Table of contents annex Para 10 of annexure to cost audit report
Para 10 of annexure to cost audit report Swot event management
Swot event management Mickey signature
Mickey signature Muscles of the upper arm
Muscles of the upper arm Clovis unified school district preschool
Clovis unified school district preschool Science table of contents
Science table of contents The immortal life of henrietta lacks table of contents
The immortal life of henrietta lacks table of contents Dlginit
Dlginit Ocr epq examples
Ocr epq examples Bovie pad
Bovie pad Sound contents
Sound contents Occupied
Occupied Electronic table of contents
Electronic table of contents Introduction of portfolio
Introduction of portfolio Ip contents
Ip contents Contents of a dead man's pocket conflict
Contents of a dead man's pocket conflict Omental tuberosity of liver
Omental tuberosity of liver What is pass 1 assembler
What is pass 1 assembler Pretracheal fascia
Pretracheal fascia Cell contents assignment to a non-cell array object.
Cell contents assignment to a non-cell array object. Olecrannon fossa
Olecrannon fossa C d m v t
C d m v t Femeral vein
Femeral vein Nature table of contents
Nature table of contents Triangular space contents
Triangular space contents Content of spermatic cord (mnemonic)
Content of spermatic cord (mnemonic) Persepolis table
Persepolis table Marketing plan
Marketing plan Superior mediastinum contents
Superior mediastinum contents Funiculus separans
Funiculus separans Pterygomaxillary fascia
Pterygomaxillary fascia Health educator definition
Health educator definition Continuous variable
Continuous variable Contents background
Contents background All the following accurately describe hadoop except
All the following accurately describe hadoop except Styloid apparatus
Styloid apparatus Oxygen table of contents
Oxygen table of contents Ism familiarization
Ism familiarization Infraspinatus insertion and origin
Infraspinatus insertion and origin Anterior
Anterior Turbid dentin
Turbid dentin Engineering notebook table of contents
Engineering notebook table of contents Interactive notebook table of contents
Interactive notebook table of contents Ob kit contents
Ob kit contents Air pollution contents
Air pollution contents Quality manual contents
Quality manual contents Contents of internal capsule
Contents of internal capsule Contents of the middle mediastinum
Contents of the middle mediastinum Company profile chart
Company profile chart Contents of a dead man's pocket conflict
Contents of a dead man's pocket conflict Sample of audit planning memorandum
Sample of audit planning memorandum Femoral lymph nodes
Femoral lymph nodes Deep perineal pouch contents
Deep perineal pouch contents Elagse
Elagse Science fair logbook table of contents
Science fair logbook table of contents Structures piercing perineal membrane in male
Structures piercing perineal membrane in male Pericardium and mediastinum
Pericardium and mediastinum Zheng he images
Zheng he images Ctd modules
Ctd modules Prophylaxis
Prophylaxis Blood plasma contents
Blood plasma contents Table of contents scrapbook
Table of contents scrapbook Contents introduction
Contents introduction Mla table of contents
Mla table of contents Sternocleidomastoid
Sternocleidomastoid Front
Front Geometry table of contents
Geometry table of contents Feedback contents
Feedback contents Mediastinum contents
Mediastinum contents Contents of audit planning memorandum
Contents of audit planning memorandum Curriculum vitaem
Curriculum vitaem Where is the superior cervical ganglion located
Where is the superior cervical ganglion located Module 3 ctd table contents
Module 3 ctd table contents Ttbiz
Ttbiz Branches of common femoral artery
Branches of common femoral artery Spermatic cord
Spermatic cord Femoral triangle applied anatomy
Femoral triangle applied anatomy Science fair logbook
Science fair logbook Table of contents case study
Table of contents case study Appendices in report
Appendices in report A graph made of connected lines or curves.
A graph made of connected lines or curves. Nature of prospectus
Nature of prospectus Table of contents literature review
Table of contents literature review Table of contents design
Table of contents design Inguinal line
Inguinal line Ark
Ark Glenohumeral ligament attachment
Glenohumeral ligament attachment Content of spermatic cord mnemonic
Content of spermatic cord mnemonic The city of ember summary
The city of ember summary Memorandum clause
Memorandum clause Magazine contents page analysis
Magazine contents page analysis Table of contents for project proposal
Table of contents for project proposal ü
ü Mediastinum anatomy
Mediastinum anatomy Contents provider
Contents provider What does key characteristics mean
What does key characteristics mean Fresh frozen plasma contents
Fresh frozen plasma contents Interactive notebook table of contents
Interactive notebook table of contents How to write a logbook for a science project
How to write a logbook for a science project High palate
High palate Types of abstract
Types of abstract Triangle urogénital
Triangle urogénital Science notebook table of contents
Science notebook table of contents Table of contents interactive notebook
Table of contents interactive notebook