Quality Manual Structure and Contents optional Documents and

















- Slides: 22

Quality Manual Structure and Contents optional Documents and Records-Writing a Quality Manual-Module 16 1

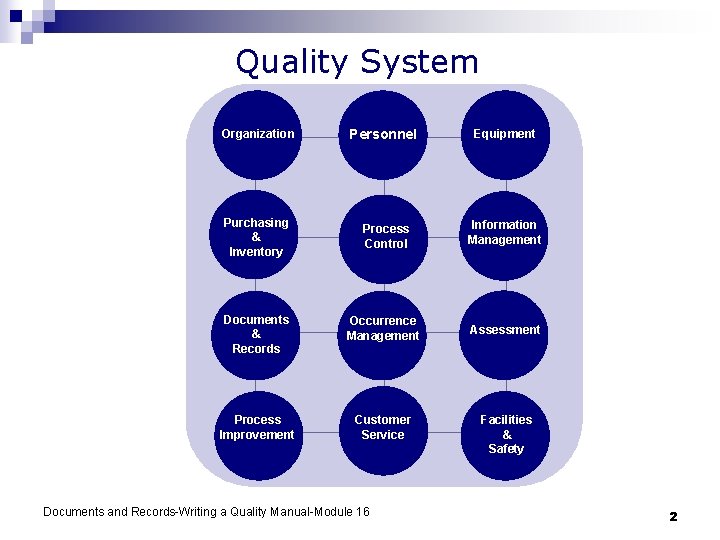
Quality System Organization Personnel Equipment Purchasing & Inventory Process Control Information Management Documents & Records Occurrence Management Assessment Process Improvement Customer Service Documents and Records-Writing a Quality Manual-Module 16 Facilities & Safety 2

Writing a Quality Manual n ISO 15189 standards requirement, but style and structure are not specified n use a steering committee n set the policies for each of the twelve elements of the quality system n for each policy, state the goals and designate responsibility n the content of the manual must include the quality policies, with reference to processes and procedures Documents and Records-Writing a Quality Manual-Module 16 3

Example 1. 2. 3. 4. 5. 6. Quality Manual Outline Introduction Organization and management Quality policy Personnel (staff education and training ) Document control, including records, maintenance and archiving Accommodation and environment Documents and Records-Writing a Quality Manual-Module 16 4

Quality Manual Outline 7. 8. 9. Instruments, reagents, consumables management Safety Research and development (optional) Preexamination procedures 11. Examination procedures 12. Postexamination procedures 10. Documents and Records-Writing a Quality Manual-Module 16 5

Quality Manual Outline Quality control 14. Laboratory information system 15. Handling of complaints– occurrence management 16. Communications and other interactions 17. Preventive and corrective action, internal audit 18. Ethics 13. Documents and Records-Writing a Quality Manual-Module 16 6

1. Introduction laboratory history n activities n manual’s field of application n manual updates: n q who q what q where q. When q. How q. Why Documents and Records-Writing a Quality Manual-Module 16 7

2. Organization and Management § description of laboratory organization § legal identity § resource requirements § assignment of responsibility /authority Documents and Records-Writing a Quality Manual-Module 16 8

3. Quality Policy official declaration of a quality policy by appropriate laboratory management n assures that the laboratory director will designate a quality manager n defines the laboratory: n ¨ missions ¨ objectives ¨ roles Documents and Records-Writing a Quality Manual-Module 16 9

4. Personnel job descriptions, including qualifications needed n personnel list n laboratory organizational chart n recruitment conditions n intern and student management n Documents and Records-Writing a Quality Manual-Module 16 10

5. Document Control n n n management approval finalizing document: verification, printing, signature, transmission confidentiality management storage, archiving producing reports list of reference documents: manuals ¨ books ¨ articles ¨ Documents and Records-Writing a Quality Manual-Module 16 11

6. Accommodation and Environment n map of the laboratory premises n restricted points of access n laboratory signs or other identification n environmental requirements for the laboratory (size, temperature, water, electrical, airflow) ¨ verification ¨ tolerated uncertainties Documents and Records-Writing a Quality Manual-Module 16 12

7. Instruments, Reagents, and Consumables Management n n n specify that each instrument requires written procedures, maintenance, quality control reagents ¨ ordering and receipt ¨ validation ¨ storage consumables or supplies – define management Documents and Records-Writing a Quality Manual-Module 16 13

8. Safety handling of samples and materials n disinfection n fire instructions n hazardous chemical instructions n waste disposal n sterilization n product labelling n Documents and Records-Writing a Quality Manual-Module 16 14

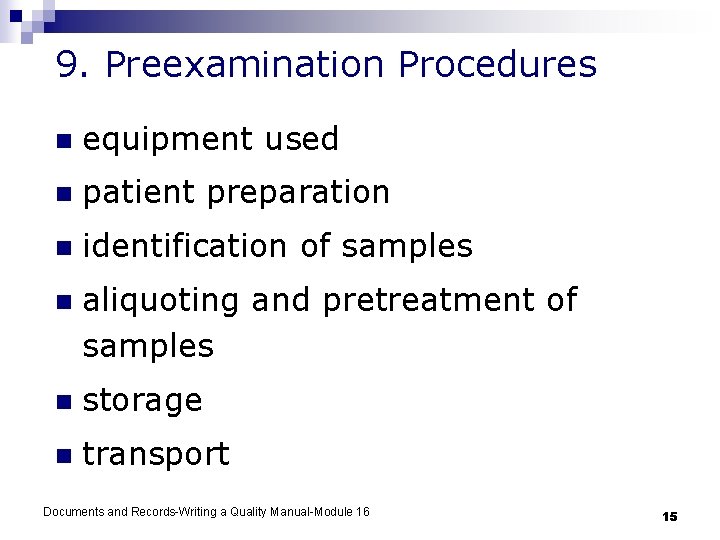
9. Preexamination Procedures n equipment used n patient preparation n identification of samples n aliquoting and pretreatment of samples n storage n transport Documents and Records-Writing a Quality Manual-Module 16 15

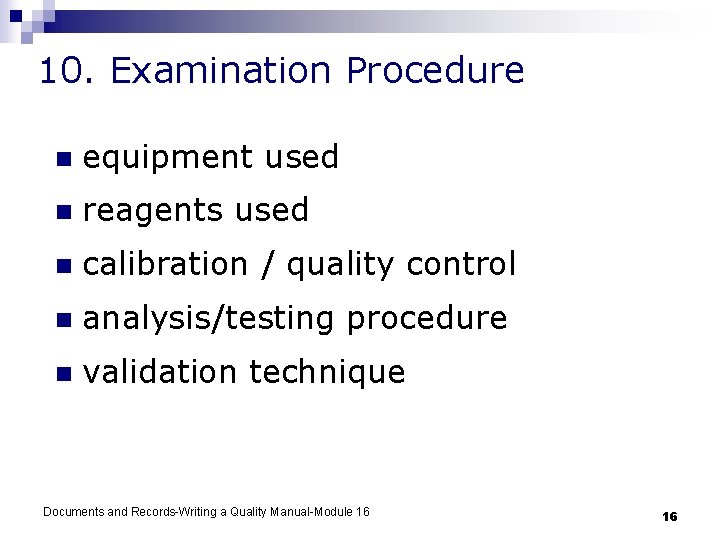
10. Examination Procedure n equipment used n reagents used n calibration / quality control n analysis/testing procedure n validation technique Documents and Records-Writing a Quality Manual-Module 16 16

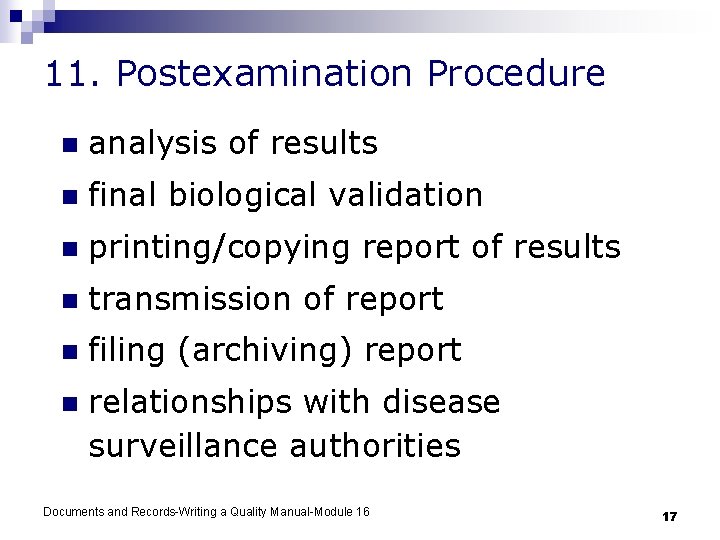
11. Postexamination Procedure n analysis of results n final biological validation n printing/copying report of results n transmission of report n filing (archiving) report n relationships with disease surveillance authorities Documents and Records-Writing a Quality Manual-Module 16 17

12. Quality Control n reminder of commitment to quality n link to control procedures: ¨ equipment ¨ reagents ¨ personnel n competencies. summary of all QC procedures and links to the appropriate sections in quality manual Documents and Records-Writing a Quality Manual-Module 16 18

13. Corrective/Preventive Actions, Internal Audits n continuous improvement n reviewing and understanding all problems and errors n internal audits are required under the ISO 15189 scheme Documents and Records-Writing a Quality Manual-Module 16 19

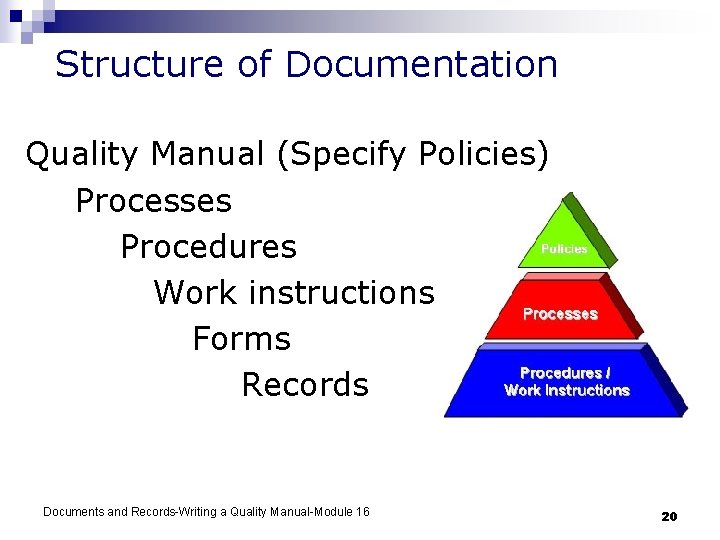
Structure of Documentation Quality Manual (Specify Policies) Processes Procedures Work instructions Forms Records Documents and Records-Writing a Quality Manual-Module 16 20

Key Points n There is only ONE official version of the Quality Manual. n The quality manual is never finished; it is always being improved. n It should be read, understood, and accepted by everyone. n It should be written in clear, easily-understood language. n The quality manual should be dated and signed by management. n Standardized page-headers should be used, and the version of each procedure should be noted. n Developing a quality manual is a very big job, but it is also very rewarding and useful for the laboratory. Documents and Records-Writing a Quality Manual-Module 16 22

Organization Purchasing & Inventory Personnel Equipment Process Control Information Management Questions? Comments? Documents & Records Occurrence Management Process Improvement Customer Service Assessment Facilities & Safety Documents and Records-Writing a Quality Manual-Module 16 23