2 1 To C of the CTD Mod
















































































- Slides: 94

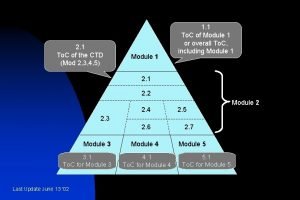
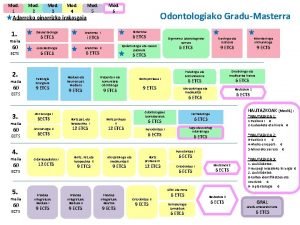
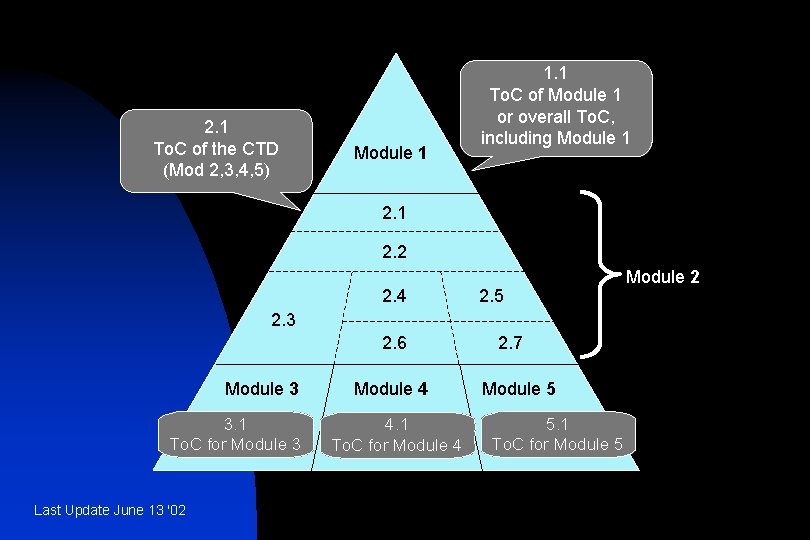
2. 1 To. C of the CTD (Mod 2, 3, 4, 5) Module 1 1. 1 To. C of Module 1 or overall To. C, including Module 1 2. 2 2. 4 2. 5 2. 3 2. 6 Module 3 3. 1 To. C for Module 3 Last Update June 13 '02 Module 4 4. 1 To. C for Module 4 2. 7 Module 5 5. 1 To. C for Module 5 Module 2

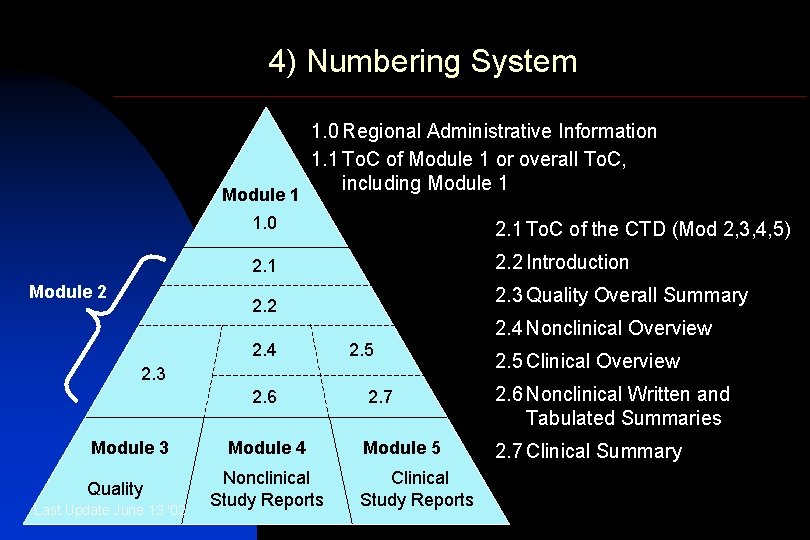
4) Numbering System Module 1 Module 2 1. 0 Regional Administrative Information 1. 1 To. C of Module 1 or overall To. C, including Module 1 1. 0 2. 1 To. C of the CTD (Mod 2, 3, 4, 5) 2. 1 2. 2 Introduction 2. 2 2. 3 Quality Overall Summary 2. 4 Nonclinical Overview 2. 4 2. 5 2. 3 2. 6 Module 3 Quality Last Update June 13 '02 Module 4 Nonclinical Study Reports 2. 5 Clinical Overview 2. 7 2. 6 Nonclinical Written and Tabulated Summaries Module 5 2. 7 Clinical Summary Clinical Study Reports

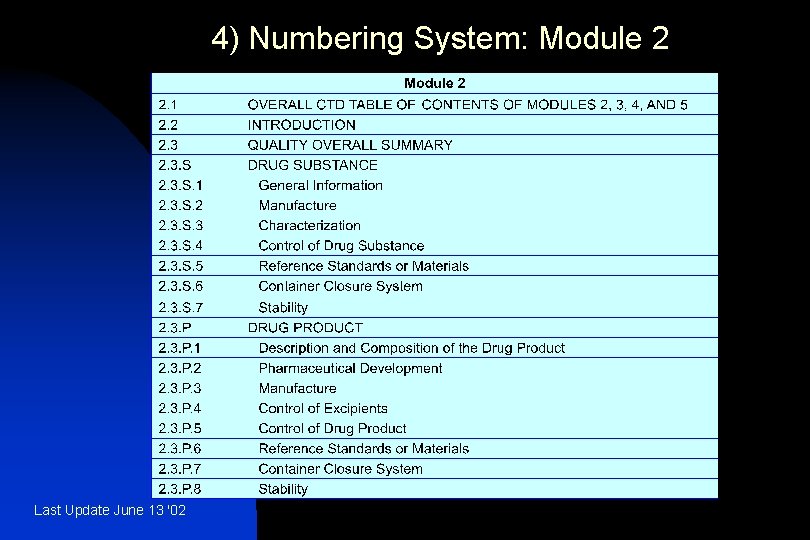
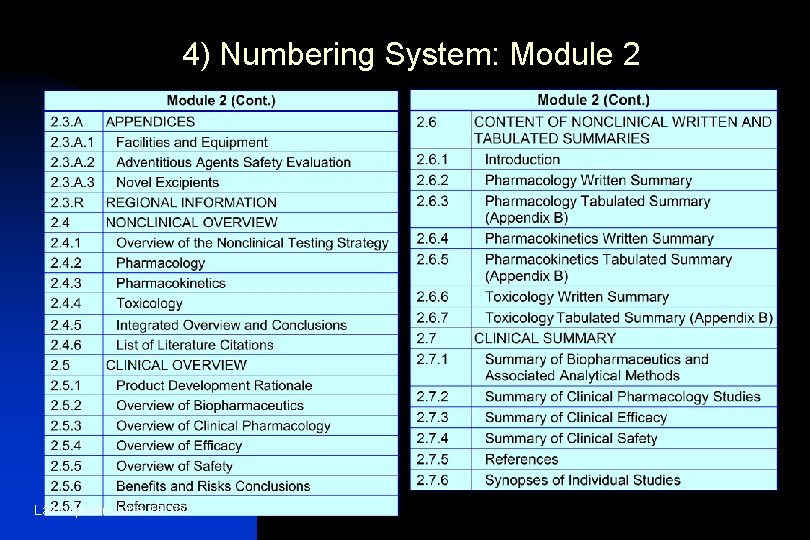
4) Numbering System: Module 2 Last Update June 13 '02

4) Numbering System: Module 2 Last Update June 13 '02

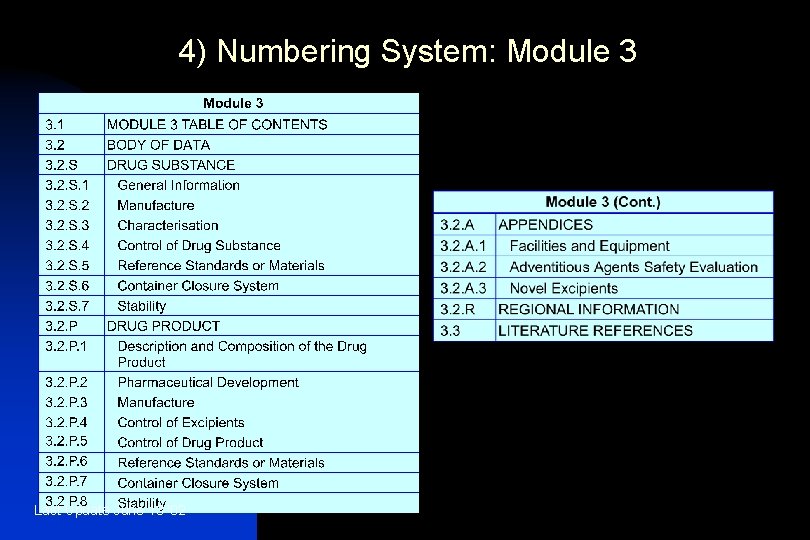
4) Numbering System: Module 3 Last Update June 13 '02

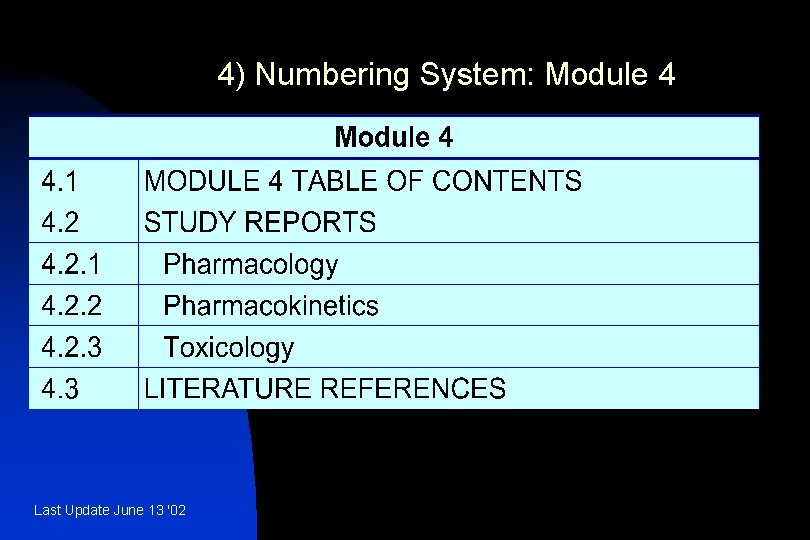
4) Numbering System: Module 4 Last Update June 13 '02

4. 1 Table of Contents of Module 4 n A Table of Contents should be provided that lists all of the nonclinical study reports and gives the location of each study report in the Common Technical Document. Last Update June 13 '02

4. 2 Study Reports n The study reports should be presented in the following order: « 4. 2. 1. 1 Pharmacology Primary Pharmacodynamics « 4. 2. 1. 2 Secondary Pharmacodynamics « 4. 2. 1. 3 Safety Pharmacology « 4. 2. 1. 4 Pharmacodynamic Drug Interactions Last Update June 13 '02

n Species should be ordered as follows: u u u u u • Mouse • Rat • Hamster • Other rodent • Rabbit • Dog • Non-human primate • Other non-rodent mammal • Non-mammals Last Update June 13 '02

n Routes of administration should be ordered as follows : u u u u u • The intended route for human use • Oral • Intravenous • Intramuscular • Intraperitoneal • Subcutaneous • Inhalation • Topical • Other Last Update June 13 '02

4. 2. 2 Pharmacokinetics u u u u 4. 2. 2. 1 Analytical Methods and Validation Reports (if separate reports are available) 4. 2. 2. 2 Absorption 4. 2. 2. 3 Distribution 4. 2. 2. 4 Metabolism 4 2. 2. 5 Excretion 4. 2. 2. 6 Pharmacokinetic Drug Interactions (nonclinical) 4. 2. 2. 7 Other Pharmacokinetic Studies Last Update June 13 '02

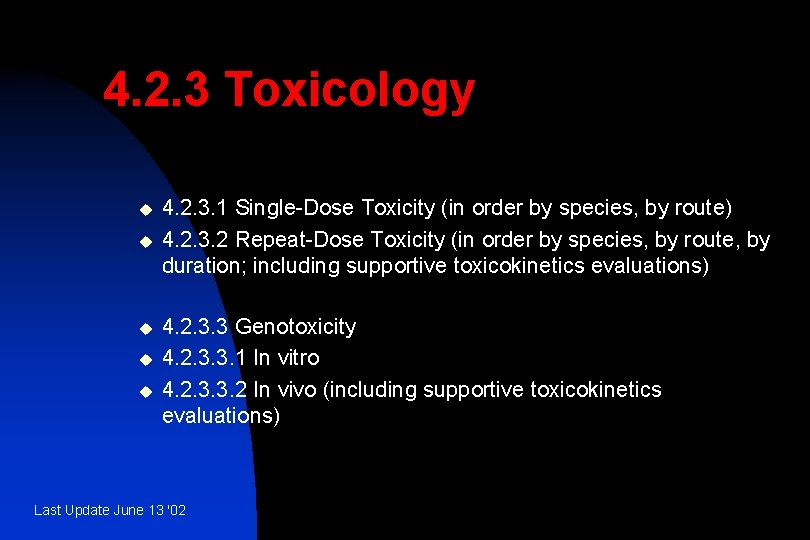
4. 2. 3 Toxicology u u u 4. 2. 3. 1 Single-Dose Toxicity (in order by species, by route) 4. 2. 3. 2 Repeat-Dose Toxicity (in order by species, by route, by duration; including supportive toxicokinetics evaluations) 4. 2. 3. 3 Genotoxicity 4. 2. 3. 3. 1 In vitro 4. 2. 3. 3. 2 In vivo (including supportive toxicokinetics evaluations) Last Update June 13 '02

4. 2. 3 Toxicology n n 4. 2. 3. 4 Carcinogenicity (including supportive toxicokinetics evaluations) u 4. 2. 3. 4. 1 Long-term studies (in order by species; including range-finding studies that cannot appropriately be included under repeat-dose toxicity or pharmacokinetics) u 4. 2. 3. 4. 2 Short- or medium-term studies (including rangefinding studies that cannot appropriately be included under repeat-dose toxicity or pharmacokinetics) u 4. 2. 3. 4. 3 Other studies Last Update June 13 '02

4. 2. 3 Toxicology n 4. 2. 3. 5 Reproductive and Developmental Toxicity (including range-finding studies and supportive toxicokinetics evaluations) (If modified study designs are used, the following sub-headings should be modified accordingly. ) u u 4. 2. 3. 5. 1 Fertility and early embryonic development 4. 2. 3. 5. 2 Embryo-fetal development 4. 2. 3. 5. 3 Prenatal and postnatal development, including maternal function 4. 2. 3. 5. 4 Studies in which the offspring (juvenile animals) are dosed and/or further evaluated. Last Update June 13 '02

4. 2. 3 Toxicology n n n n n 4. 2. 3. 6 Local Tolerance 4. 2. 3. 7 Other Toxicity Studies (if available) 4. 2. 3. 7. 1 Antigenicity 4. 2. 3. 7. 2 Immunotoxicity 4. 2. 3. 7. 3 Mechanistic studies (if not included elsewhere) 4. 2. 3. 7. 4 Dependence 4. 2. 3. 7. 5 Metabolites 4. 2. 3. 7. 6 Impurities 4. 2. 3. 7. 7 Other Last Update June 13 '02

n Last Update June 13 '02 4. 3 Literature References

4) Numbering System: Module 5 Last Update June 13 '02

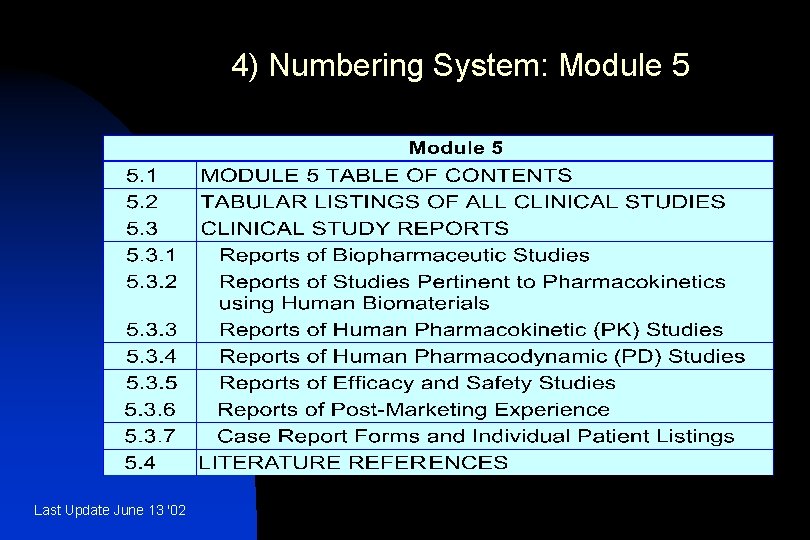
5. 1 Table of Contents of Module 5 n A Table of Contents for study reports should be provided. 5. 2 Tabular Listing of All Clinical Studies Last Update June 13 '02

5. 3 Clinical Study Reports n n n 5. 3. 1 Reports of Biopharmaceutic Studies 5. 3. 1. 1 Bioavailability (BA) Study Reports BA studies in this section should include • studies comparing the release and systemic availability of a drug substance from a solid oral dosage form to the systemic availability of the drug substance given intravenously or as an oral liquid dosage form n n • dosage form proportionality studies, and • food-effect studies. Last Update June 13 '02

5. 3. 1. 2 Comparative BA and Bioequivalence (BE) Study Reports n n Comparative BA or BE studies may include comparisons between : the drug product used in clinical studies supporting effectiveness and the to-be-marketed drug product, • the drug product used in clinical studies supporting effectiveness and the drug product used in stability batches, and • similar drug products from different manufacturers. Last Update June 13 '02

n n 5. 3. 1. 3 In vitro-In vivo Correlation Study Reports 5. 3. 1. 4 Reports of Bioanalytical and Analytical Methods for Human Studies Last Update June 13 '02

5. 3 Clinical Study Reports n n n n n 5. 3. 2 Reports of Studies Pertinent to Pharmacokinetics using Human Biomaterials 5. 3. 2. 1 Plasma Protein Binding Study Reports 5. 3. 2. 2 Reports of Hepatic Metabolism and Drug Interaction Studies 5. 3. 2. 3 Reports of Studies Using Other Human Biomaterials 5. 3. 3 Reports of Human Pharmacokinetic (PK) Studies 5. 3. 3. 1 Healthy Subject PK and Initial Tolerability Study Reports 5. 3. 3. 2 Patient PK and Initial Tolerability Study Reports 5. 3. 3. 3 Intrinsic Factor PK Study Reports 5. 3. 3. 4 Extrinsic Factor PK Study Reports 5. 3. 3. 5 Population PK Study Reports Last Update June 13 '02

5. 3 Clinical Study Reports n n n n 5. 3. 4 Reports of Human Pharmacodynamic (PD) Studies 5. 3. 4. 1 Healthy Subject PD and PK/PD Study Reports 5. 3. 4. 2 Patient PD and PK/PD Study Reports 5. 3. 5 Reports of Efficacy and Safety Studies 5. 3. 5. 1 Study Reports of Controlled Clinical Studies Pertinent to the Claimed Indication 5. 3. 5. 2 Study Reports of Uncontrolled Clinical Studies 5. 3 Reports of Analyses of Data from More Than One Study 5. 3. 5. 4 Other Clinical Study Reports Last Update June 13 '02

5. 3. 5. 1 Study Reports of Controlled Clinical Studies Pertinent to the Claimed Indication n The controlled clinical study reports should be sequenced by type of control: Placebo control (could include other control groups, such as an active comparator or other doses) u No-treatment control u Dose-response (without placebo) u Active control (without placebo) u External (Historical) control, regardless of the control treatment u Last Update June 13 '02

5. 3. 5. 4 Other Clinical Study Reports n n n Reports of interim analyses of studies pertinent to the claimed indications − Reports of controlled safety studies not reported elsewhere − Reports of controlled or uncontrolled studies not related to the claimed indication Published reports of clinical experiences with the medicinal product that are not included in Section 5. 3. 5. 1. However, when literature is important to the demonstration or substantiation of efficacy, it should be included in Section 5. 3. 5. 1 − Reports of ongoing studies Last Update June 13 '02

5. 3 Clinical Study Reports n n 5. 3. 6 Reports of Post-Marketing Experience 5. 3. 7 Case Report Forms and Individual Patient Listings Last Update June 13 '02

n Last Update June 13 '02 5. 4 Literature References

THE EXTENT OF POPULATION EXPOSURE n TO ASSESS CLINICAL SAFETY n FOR DRUGS INTENDED FOR LONG-TERM TREATMENT OF n NON-LIFE-THREATENING CONDITIONS n E 1 n Last Update June 13 '02

n The objective of this guideline is to present an accepted set of principles for the safety evaluation of drugs intended for the long-term treatment (chronic or repeated intermittent use for longer than 6 months) of non-life-threatening diseases. The safety evaluation during clinical drug development is expected to characterise and quantify the safety profile of a drug over a reasonable duration of time consistent with the intended longterm use of the drug. Thus, duration of drug exposure and its relationship to both time and magnitude of occurrence of adverse events are important considerations in determining the size of the data base necessary to achieve such goals. Last Update June 13 '02

n CLINICAL SAFETY DATA MANAGEMENT: n Last Update June 13 '02 DEFINITIONS AND STANDARDS FOR n EXPEDITED REPORTING n E 2 A

n n n There are two issues within the broad subject of clinical safety data management that are appropriate for harmonisation at this time: (1) the development of standard definitions and terminology for key aspects of clinical safety reporting, and (2) the appropriate mechanism for handling expedited (rapid) reporting, in the investigational (i. e. , preapproval) phase. Last Update June 13 '02

n KEY DATA ELEMENTS FOR INCLUSION IN EXPEDITED n REPORTS OF SERIOUS ADVERSE DRUG REACTIONS Last Update June 13 '02

n 1. Patient Details Initials u Other relevant identifier (clinical investigation number, for example) u Gender u Age and/or date of birth u Weight u Height u Last Update June 13 '02

n n n n n 2. Suspected Medicinal Product(s) Brand name as reported International Non-Proprietary Name (INN) Batch number Indication(s) for which suspect medicinal product was prescribed or tested Dosage form and strength Daily dose and regimen (specify units - e. g. , mg, ml, mg/kg) Route of administration Starting date and time of day Stopping date and time, or duration of treatment Last Update June 13 '02

n 3. Other Treatment (s) Last Update June 13 '02

n n n n Details of Suspected Adverse Drug Reaction(s) Full description of reaction(s) including body site and severity, as well as the criterion (or criteria) for regarding the report as serious should be given. In addition to a description of the reported signs and symptoms, whenever possible, attempts should be made to establish a specific diagnosis for the reaction. Start date (and time) of onset of reaction Stop date (and time) or duration of reaction Dechallenge and rechallenge information Setting (e. g. , hospital, out-patient clinic, home, nursing home) Outcome: Last Update June 13 '02

n n n Details on Reporter of Event (Suspected ADR) Name Address Telephone number Profession (speciality) Administrative and Sponsor/Company Details Last Update June 13 '02

n n CLINICAL SAFETY DATA MANAGEMENT: PERIODIC SAFETY UPDATE REPORTS FOR MARKETED DRUGS n E 2 C(R 1) Last Update June 13 '02

n The current situation for periodic safety reports on marketed drugs is different among the three ICH regions. For example: u u u − The US regulations require quarterly reports during the first 3 years, then annual reports. The FDA has recently published proposed rules 1 which take into account the CIOMS Working Group II proposals 2. − In the EU, Council Directive 93/39/EEC and Council Regulation 2309/93 require reports with a periodicity of 6 months for two years, annually for the three following years and then every five years, at time of renewal of registration. − In Japan, the authorities require a survey on a cohort of a few thousand patients established by a certain number of identified institutions during the 6 years following authorization. Systematic information on this cohort, taking into account a precise denominator, must be reported annually. Regarding other marketing experience, adverse reactions which are nonserious, but both mild in severity and unlabeled must be reported every 6 months for 3 years and annually thereafter. Last Update June 13 '02

SAMPLE TITLE PAGE PERIODIC SAFETY UPDATE REPORT FOR: (PRODUCT) MAHs NAME AND ADDRESS (Corporate headquarters or other company entity responsible for report preparation) n PERIOD COVERED BY THIS REPORT: (dates) n INTERNATIONAL BIRTH DATE: Date (Country of IBD) n DATE OF REPORT (Other identifying information at the option of MAH, such as report number) n n Last Update June 13 '02

n TABLE OF CONTENTS FOR MODEL PSUR u Introduction. . . . . . . World-wide market authorization status. . . . . u Update of regulatory authority or MAH actions taken for safety reasons. . . . . u Changes to Reference Product Information. . . . . u Patient exposure. . . . . . . u Presentation of individual case histories. . . . . u Studies. . . . . . . . u Other information. . . . . . . u Overall safety evaluation. . . . . . . u Conclusion. . . . . . . . u APPENDIX: COMPANY CORE DATA SHEET. . . . u Last Update June 13 '02

n n n 3. GLOSSARY OF SPECIAL TERMS Company Core Data Sheet (CCDS) A document prepared by the MAH containing, in addition to safety information, material relating to indications, dosing, pharmacology and other information concerning the product. Last Update June 13 '02

n Company Core Safety Information (CCSI) n Data Lock-Point (Data Cut-off Date) n International Birth Date IBD n Listed Adverse Drug Reaction n Spontaneous Report or Spontaneous Notification n Unlisted Adverse Drug Reaction Last Update June 13 '02

ADDENDUM TO ICH E 2 C n CLINICAL SAFETY DATA MANAGEMENT PERIODIC SAFETY UPDATE REPORTS FOR MARKETED DRUGS n ICH Harmonised Tripartite Guideline n Last Update June 13 '02

n n n Summary Bridging Report Addendum Report Proprietary information Executive Summary Risk management programme Benefit-risk analysis Last Update June 13 '02

n POST-APPROVAL SAFETY DATA MANAGEMENT: DEFINITIONS AND STANDARDS FOR EXPEDITED REPORTING n E 2 D Last Update June 13 '02

PHARMACOVIGILANCE PLANNING n E 2 E This guideline is intended to aid in planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new drug The guideline describes a method for summarising the important identified risks of a drug, important potential risks, and important missing information, including the potentially at-risk populations and situations where the product is likely to be used that have not been studied pre-approval. n n n Last Update June 13 '02

n STRUCTURE AND CONTENT OF CLINICAL STUDY REPORTS n E 3 Last Update June 13 '02

n n 1. TITLE PAGE The title page should contain the following information: u u u u − study title − name of test drug/ investigational product − indication studied − if not apparent from the title, a brief (1 to 2 sentences) description giving design (parallel, cross-over, blinding, randomised) comparison (placebo, active, dose/response), duration, dose, and patient population − name of the sponsor − protocol identification (code or number) − development phase of study − study initiation date (first patient enrolled, or any other verifiable definition) − date of early study termination, if any − study completion date (last patient completed) − name and affiliation of principal or coordinating investigator(s) or sponsor’s responsible medical officer − name of company/sponsor signatory (the person responsible for the study report within the company/sponsor. The name, telephone number and fax number of the company/sponsor contact persons for questions arising during review of the study report should be indicated on this page or in the letter of application. ) − statement indicating whether the study was performed in compliance with Good Clinical Practices (GCP), including the archiving of essential documents − date of the report (identify any earlier reports from the same study by title and date). Last Update June 13 '02

n 2. SYNOPSIS Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

n n 3. TABLE OF CONTENTS FOR THE INDIVIDUAL CLINICAL STUDY REPORT − the page number or other locating information of each section, including summary tables, figures and graphs; − a list and the locations of appendices, tabulations and any case report forms provided. 4. LIST OF ABBREVIATIONS AND DEFINITION OF TERMS Last Update June 13 '02

n n 5. ETHICS 5. 1 INDEPENDENT ETHICS COMMITTEE (IEC) OR INSTITUTIONAL REVIEW BOARD (IRB) n 5. 2 ETHICAL CONDUCT OF THE STUDY n 5. 3 PATIENT INFORMATION AND CONSENT n 6. INVESTIGATORS AND STUDY ADMINISTRATIVE STRUCTURE Last Update June 13 '02

Last Update June 13 '02

n 7. INTRODUCTION n 8. STUDY OBJECTIVES n 9. INVESTIGATIONAL PLAN n 9. 1 OVERALL STUDY DESIGN AND PLAN DESCRIPTION Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

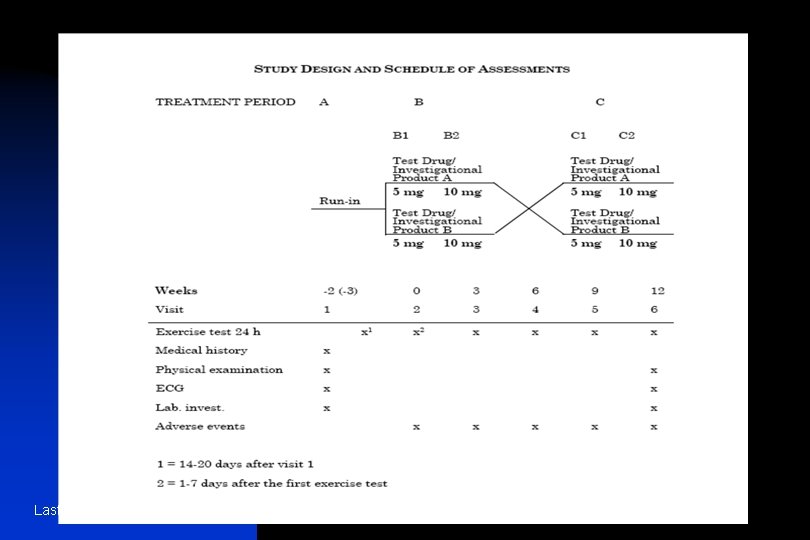
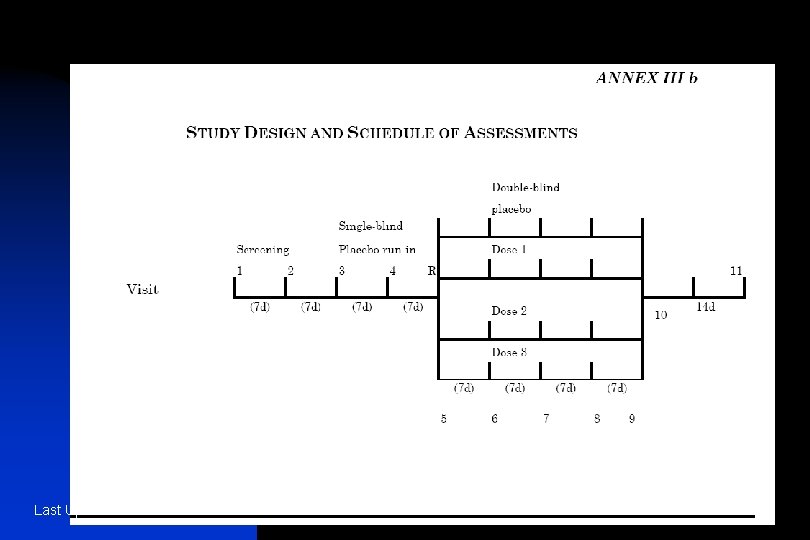
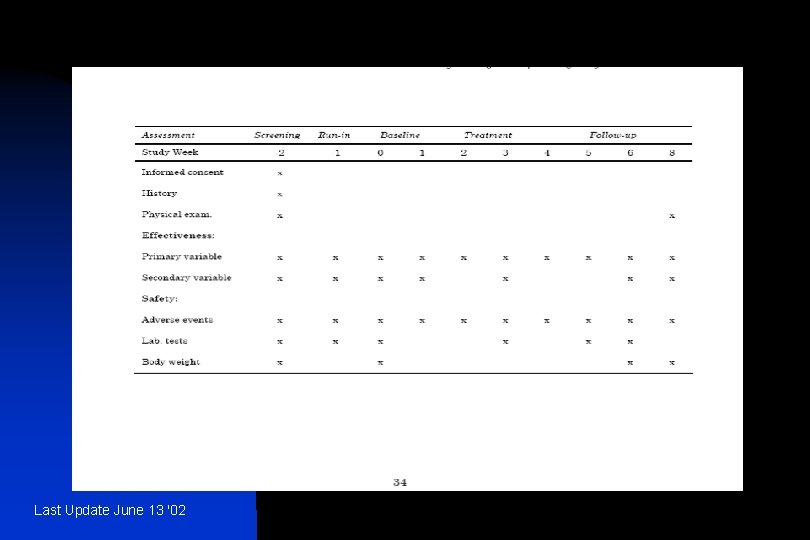
n n The information provided should include: u − treatments studied (specific drugs, doses and procedures); u − patient population studied and the number of patients to be included; u − level and method of blinding/masking (e. g. , open, double-blind, single-blind, blinded evaluators and unblinded patients and/or investigators); u − kind of control(s) (e. g. , placebo, no treatment, active drug, dose-response, historical) and study configuration (parallel, cross-over); u − method of assignment to treatment (randomisation, stratification); u − sequence and duration of all study periods, including pre-randomisation and post-treatment periods, therapy withdrawal periods and single- and double-blind treatment periods. When patients are randomised should be specified. It is usually helpful to display the design graphically with a flow chart which includes timing of assessments (see Annexes IIIa and IIIb for an example); − any safety, data monitoring or special steering or evaluation committees; − any interim analyses. Last Update June 13 '02

n 9. 2 DISCUSSION OF STUDY DESIGN, INCLUDING THE CHOICE OF CONTROL GROUPS n 9. 3 SELECTION OF STUDY POPULATION n 9. 3. 1 Inclusion Criteria n 9. 3. 2 Exclusion Criteria n 9. 3. 3 Removal of Patients from Therapy or Assessment Last Update June 13 '02

n 9. 4 TREATMENTS 9. 4. 1 Treatments Administered n 9. 4. 2 Identity of Investigational Product(s) n 9. 4. 3 Method of Assigning Patients to Treatment Groups n 9. 4. 4 Selection of Doses in the Study n 9. 4. 5 Selection and Timing of Dose for each Patient n 9. 4. 6 Blinding n 9. 4. 7 Prior and Concomitant Therapy n 9. 4. 8 Treatment Compliance n Last Update June 13 '02

n n 9. 5 EFFICACY AND SAFETY VARIABLES 9. 5. 1 Efficacy and Safety Measurements Assessed and Flow Chart n 9. 5. 2 Appropriateness of Measurements n 9. 5. 3 Primary Efficacy Variable(s) n 9. 5. 4 Drug Concentration Measurements n 9. 6 DATA QUALITY ASSURANCE Last Update June 13 '02

n 9. 7 STATISTICAL METHODS PLANNED IN THE PROTOCOL AND DETERMINATION OF SAMPLE SIZE n 9. 7. 1 Statistical and Analytical Plans n 9. 7. 2 Determination of Sample Size n 9. 8 CHANGES IN THE CONDUCT OF THE STUDY OR PLANNED ANALYSES Last Update June 13 '02

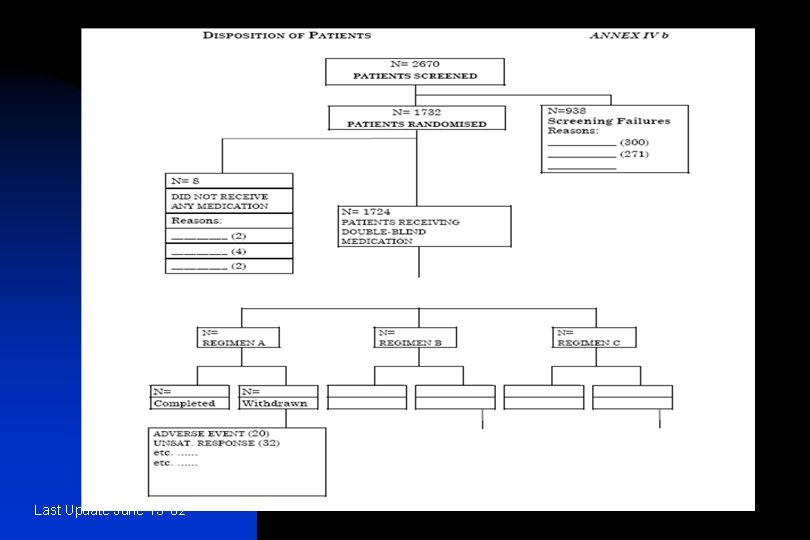
n 10. STUDY PATIENTS n 10. 1 DISPOSITION OF PATIENTS n n n 10. 2 PROTOCOL DEVIATIONS − those who entered the study even though they did criteria; − those who developed withdrawal criteria during the withdrawn; − those who received the wrong treatment or incorrect dose; − those who received an excluded concomitant treatment. Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

n 11. EFFICACY EVALUATION n 11. 1 DATA SETS ANALYSED n n 11. 2 DEMOGRAPHIC AND OTHER BASELINE CHARACTERISTICS demographic variables − age u − sex u − race u Last Update June 13 '02

Last Update June 13 '02

Last Update June 13 '02

n disease factors u u u − specific entry criteria (if not uniform), duration, stage and severity of disease and other clinical classifications and sub-groupings in common usage or of known prognostic significance − baseline values for critical clinical measurements carried out during the study or identified as important indicators of prognosis or response to therapy − concomitant illness at trial initiation, such as renal disease, diabetes, heart failure − relevant previous illness − relevant previous treatment for illness treated in the study − concomitant treatment maintained, even if the dose was changed during the study, including oral contraceptive and hormone replacement therapy; treatments stopped at entry into the study period (or changed at study initiation) Last Update June 13 '02

n n other factors that might affect response to therapy (e. g. , weight, renin status, antibody levels, metabolic status) other possibly relevant variables (e. g. , smoking, alcohol intake, special diets) and, for women, menstrual status and date of last menstrual period, if pertinent for the study. Last Update June 13 '02

n n 11. 3 MEASUREMENTS OF TREATMENT COMPLIANCE 11. 4 EFFICACY RESULTS AND TABULATIONS OF INDIVIDUAL PATIENT DATA n 11. 4. 1 Analysis of Efficacy n 11. 4. 2 Statistical/Analytical Issues n 11. 4. 2. 1 Adjustments for Covariates n 11. 4. 2. 2 Handling of Dropouts or Missing Data n 11. 4. 2. 3 Interim Analyses and Data Monitoring n 11. 4. 2. 4 Multicentre Studies n 11. 4. 2. 5 Multiple Comparison/Multiplicity Last Update June 13 '02

n n n n 11. 4. 2. 6 Use of an "Efficacy Subset" of Patients 11. 4. 2. 7 Active-Control Studies Intended to Show Equivalence 11. 4. 2. 8 Examination of Subgroups 11. 4. 3 Tabulation of Individual Response Data 11. 4. 4 Drug Dose, Drug Concentration, and Relationships to Response 11. 4. 5 Drug-Drug and Drug-Disease Interactions 11. 4. 6 By-Patient Displays 11. 4. 7 Efficacy Conclusions Last Update June 13 '02

n n n 12. SAFETY EVALUATION 12. 1 EXTENT OF EXPOSURE 12. 2 ADVERSE EVENTS (AEs) 12. 2. 1 Brief Summary of Adverse Events 12. 2. 2 Display of Adverse Events ( should be displayed in summary tables (section 14. 3. 1)) n n n 12. 2. 3 Analysis of Adverse Events 12. 2. 4 Listing of Adverse Events by Patient (should be listed in appendix 16. 2. 7 ) Last Update June 13 '02

n n n 12. 3 DEATHS, OTHER SERIOUS ADVERSE EVENTS, AND OTHER SIGNIFICANT ADVERSE EVENTS 12. 3. 1 Listing of Deaths, other Serious Adverse Events and Other Significant Adverse Events 12. 3. 1. 1 Deaths ( should be listed by patient in section 14. 3. 2. ) 12. 3. 1. 2 Other Serious Adverse Events (should be listed in section 14. 3. 2. ) 12. 3. 1. 3 Other Significant Adverse Events (should be listed in section 14. 3. 2. ) n Last Update June 13 '02

n n 12. 3. 2 Narratives of Deaths, Other Serious Adverse Events and Certain Other Significant Adverse Events( These narratives can be placed either in the text of the report or in section 14. 3. 3, depending on their number. ) 12. 3. 3 Analysis and Discussion of Deaths, Other Serious Adverse Events and Other Significant Adverse Events Last Update June 13 '02

n n 12. 4 CLINICAL LABORATORY EVALUATION 12. 4. 1 Listing of Individual Laboratory Measurements by Patient (16. 2. 8) and Each Abnormal Laboratory Value (14. 3. 4) n 12. 4. 2 Evaluation of Each Laboratory Parameter n 12. 4. 2. 1 Laboratory Values Over Time n 12. 4. 2. 2 Individual Patient Changes n 12. 4. 2. 3 Individual Clinically Significant Abnormalities Last Update June 13 '02

n 12. 5 VITAL SIGNS, PHYSICAL FINDINGS AND OTHER OBSERVATIONS RELATED TO SAFETY n 12. 6 SAFETY CONCLUSIONS n 13. DISCUSSION AND OVERALL CONCLUSIONS Last Update June 13 '02

n 14. TABLES, FIGURES AND GRAPHS REFERRED TO BUT NOT INCLUDED IN THE TEXT n 14. 1 DEMOGRAPHIC DATA n 14. 2 EFFICACY DATA n 14. 3 SAFETY DATA n 14. 3. 1 Displays of Adverse Events n 14. 3. 2 Listings of Deaths, Other Serious and Significant Adverse Events n n 14. 3. 3 Narratives of Deaths, Other Serious and Certain Other Significant Adverse Events 14. 3. 4 Abnormal Laboratory Value Listing (Each Patient) Last Update June 13 '02

n n 15. REFERENCE LIST A list of articles from the literature pertinent to the evaluation of the study should be provided. Copies of important publications should be attached in an appendix (16. 1. 11 and 16. 1. 12). References should be given in accordance with the internationally accepted standards of the 1979 Vancouver Declaration on "Uniform Requirements for Manuscripts Submitted to Biomedical Journals" or the system used in "Chemical Abstracts". Last Update June 13 '02

n 16. APPENDICES n 16. 1 STUDY INFORMATION n 16. 1. 1 Protocol and protocol amendments n 16. 1. 2 Sample case report form (unique pages only) n n 16. 1. 3 List of IECs or IRBs (plus the name of the committee Chair if required by the regulatory authority) - Representative written information for patient and sample consent forms 16. 1. 4 List and description of investigators and other important participants in the study, including brief (1 page) CVs or equivalent summaries of training and experience relevant to the performance of the clinical study 16. 1. 5 Signatures of principal or coordinating investigator(s) or sponsor’s responsible medical officer, depending on the regulatory authority's requirement 16. 1. 6 Listing of patients receiving test drug(s)/investigational product(s) from specific batches, where more than one batch was used Last Update June 13 '02

n n 16. 1. 7 Randomisation scheme and codes (patient identification and treatment assigned) 16. 1. 8 Audit certificates (if available) (see Annex IVa and IVb of the guideline) 16. 1. 9 Documentation of statistical methods 16. 1. 10 Documentation of inter-laboratory standardisation methods and quality assurance procedures if used n 16. 1. 11 Publications based on the study n 16. 1. 12 Important publications referenced in the report Last Update June 13 '02

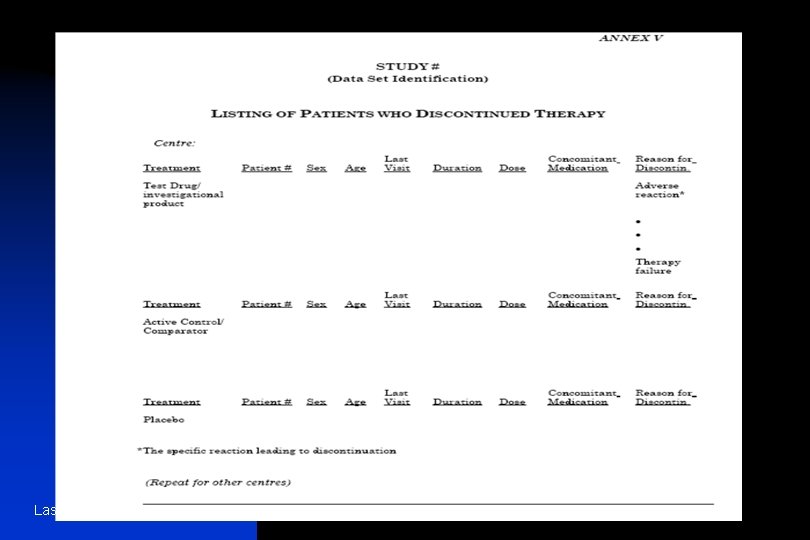
n 16. 2. PATIENT DATA LISTINGS n 16. 2. 1 Discontinued patients n 16. 2. 2 Protocol deviations n 16. 2. 3 Patients excluded from the efficacy analysis n 16. 2. 4 Demographic data n 16. 2. 5 Compliance and/or drug concentration data (if available) n 16. 2. 6 Individual efficacy response data n 16. 2. 7 Adverse event listings (each patient) n 16. 2. 8. Listing of individual laboratory measurements by patient, when required by regulatory authorities Last Update June 13 '02

n n n 16. 3 CASE REPORT FORMS 16. 3. 1 CRFs for deaths, other serious adverse events and withdrawals for AE 16. 3. 2 Other CRFs submitted Last Update June 13 '02

n 16. 4. INDIVIDUAL PATIENT DATA LISTINGS (US ARCHIVAL LISTINGS) Last Update June 13 '02

n DOSE-RESPONSE INFORMATION TO SUPPORT DRUG REGISTRATION n Last Update June 13 '02 E 4

n n ETHNIC FACTORS IN THE ACCEPTABILITY OF FOREIGN CLINICAL DATA n E 5(R 1) ASSESSMENT OF THE CLINICAL DATA PACKAGE INCLUDING FOREIGN CLINICAL DATA FOR ITS FULFILMENT OF REGULATORY REQUIREMENTS IN THE NEW REGION Last Update June 13 '02

n Additional Studies to Meet the New Region’s Regulatory Requirements Last Update June 13 '02

n n ASSESSMENT OF THE FOREIGN CLINICAL DATA FOR EXTRAPOLATION TO THE NEW REGION Characterization of the Medicine’s Sensitivity to Ethnic Factors n Bridging Data Package n Bridging Study Last Update June 13 '02

n GUIDELINE FOR GOOD CLINICAL PRACTICE n E 6(R 1) n n Last Update June 13 '02 STUDIES IN SUPPORT OF SPECIAL POPULATIONS: n GERIATRICS n E 7

GENERAL CONSIDERATIONS FOR CLINICAL TRIALS n E 8 n n STATISTICAL PRINCIPLES FOR CLINICAL TRIALS n E 9 n CHOICE OF CONTROL GROUP AND RELATED n ISSUES IN CLINICAL TRIALS n E 10 Last Update June 13 '02

n CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN THE PEDIATRIC POPULATION n E 11 n PRINCIPLES FOR CLINICAL EVALUATION OF NEW ANTIHYPERTENSIVE DRUGS n E 12 A n THE CLINICAL EVALUATION OF QT/QTC INTERVAL PROLONGATION AND PROARRHYTHMIC POTENTIAL FOR NONANTIARRHYTHMIC DRUGS n E 14 n Last Update June 13 '02
 Time delay procedures
Time delay procedures Ctd modules
Ctd modules Ctd triangle
Ctd triangle Ctd
Ctd Civ 6 ctd
Civ 6 ctd Prophet ctd
Prophet ctd Ctd sections
Ctd sections Module 4 ctd
Module 4 ctd Module 3 ctd table contents
Module 3 ctd table contents Gesfarma
Gesfarma Map plc/slc messages
Map plc/slc messages Dosal
Dosal Ctd modules
Ctd modules Ctd module 1
Ctd module 1 Ctd royston
Ctd royston Ctd triangle
Ctd triangle Ctd ich guidelines
Ctd ich guidelines Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Phối cảnh
Phối cảnh Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Lp html
Lp html Sơ đồ cơ thể người
Sơ đồ cơ thể người Số nguyên tố là số gì
Số nguyên tố là số gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp tư thế worms-breton
Chụp tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế
Cái miệng nó xinh thế Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết Giọng cùng tên là
Giọng cùng tên là Thể thơ truyền thống
Thể thơ truyền thống Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con đại từ thay thế
đại từ thay thế Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Công thức tiính động năng
Công thức tiính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ độ dài liên kết
độ dài liên kết 1 mod 4
1 mod 4 Sulfametizol blærebetændelse
Sulfametizol blærebetændelse Xerodent mod tør mund
Xerodent mod tør mund Anyaggyűjtési mód
Anyaggyűjtési mód Nitrox mod table
Nitrox mod table Mod npdr
Mod npdr 580625685 mod 701
580625685 mod 701 Moment basıklık katsayısı
Moment basıklık katsayısı 819 mod 26
819 mod 26 Pbb matematika
Pbb matematika 3^644 mod 645
3^644 mod 645 Head.php?mod=
Head.php?mod= Csco mod
Csco mod 31415 mod 13
31415 mod 13 Natural nubers
Natural nubers Mod-025-2
Mod-025-2 Use algorithm 5 to find 11^644 mod 645
Use algorithm 5 to find 11^644 mod 645 425 mod 26
425 mod 26 Mod 6 asynchronous counter
Mod 6 asynchronous counter Animal cell mod
Animal cell mod Hoyt model
Hoyt model Plænekalk
Plænekalk Ostatak cjelobrojnog dijeljenja
Ostatak cjelobrojnog dijeljenja Normal dağılım eğrisi
Normal dağılım eğrisi 391 mod 26
391 mod 26 The x mod
The x mod Euler's theorem
Euler's theorem Apv simv
Apv simv Imprejur mod de formare
Imprejur mod de formare Bockis mod
Bockis mod Orosz felszolgálási mód
Orosz felszolgálási mód Fptan
Fptan The value of 52003 mod 7 is?
The value of 52003 mod 7 is? Mod 1 course
Mod 1 course Mod 11 check digit
Mod 11 check digit Symfyse fundus mål
Symfyse fundus mål