New oral anticoagulants an update Julian Holmes HT

























- Slides: 28

New oral anticoagulants: an update Julian Holmes H+T Pharmacist NUH Julian. Holmes@nuh. nhs. uk

Problems with warfarin n Variable dose n INR affected by diet, illness etc n Drug interactions can be problematic n Narrow therapeutic index n BUT – its cheap! n AND – the INR is a good measure of compliance

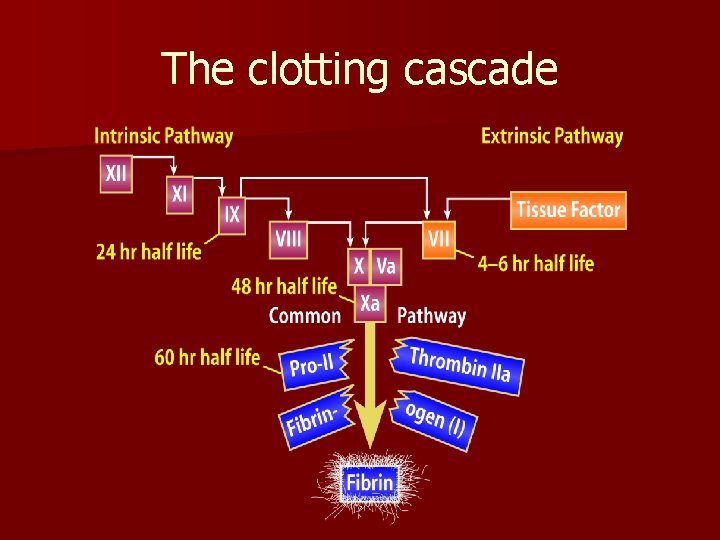
The clotting cascade

New agents Ideally need: n Once daily constant dose for all patients n Predictable kinetics and anticoagulant effect n No problematic drug interactions n Several new agents – dabigatran, rivaroxaban and apixaban are most advanced three

Dabigatran in AF n n n RELY trial Oral direct thrombin inhibitor Half life 12 -17 hours No reversal agent Contraindicated if Cr. Cl<30 ml/min Substrate of efflux transporter P-glycoprotein so levels affected by inducers (St Johns Wort) or inhibitors (amiodarone, quinidine (C/I), verapamil, clarithromycin) of this

Dabigatran in AF n n n n Mortality and haemorrhagic stroke rates favoured dabigatran Time in range ~64% for warfarin pts Dabigatran slightly more effective than warfarin at the higher dose Higher MI and GI bleed rates with dabigatran Drop out trial rate of ~10% with dyspepsia with dabigatran Now licensed in UK NICE TA 249 Mar 2012 – can be used as a treatment option

Dabigatran in AF n Dose is 150 mg twice daily reduced to 110 mg twice daily if: – – – Creatinine clearance 30 -50 ml/min Over 80 years Body weight <50 kg Increased bleeding risk On verapamil Patients with gastritis, oesophagitis or gastoesophageal reflux – Patients continuing to take aspirin to manage a chronic condition

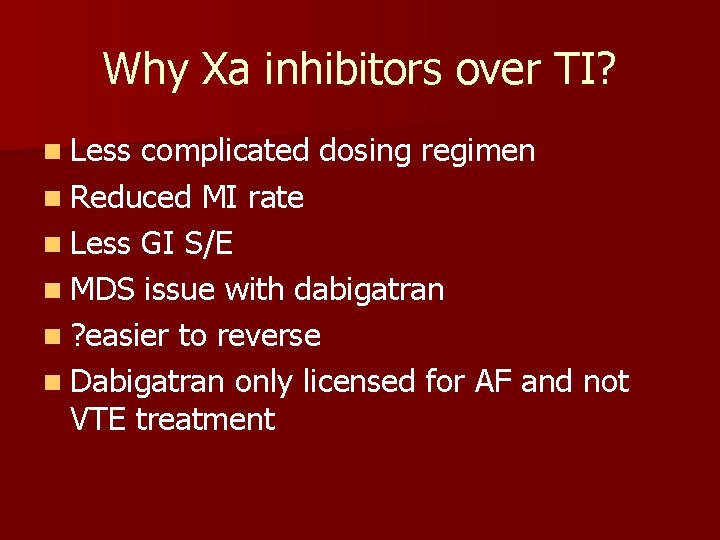
Why Xa inhibitors over TI? n Less complicated dosing regimen n Reduced MI rate n Less GI S/E n MDS issue with dabigatran n ? easier to reverse n Dabigatran only licensed for AF and not VTE treatment

Rivaroxaban in AF n n n Dose 20 mg daily (15 mg daily if Cr. Cl 30 -49 ml/min or HASBLED score over 3) in ROCKET trial vs warfarin in 14, 264 patients Primary end point of stroke or systemic embolism Rates of primary outcome 1. 7% per year rivaroxaban and 2. 2% per year warfarin Bleeding rates 14. 9% per rivaroxaban and 14. 5% per year warfarin Time in range for warfarin ~55%

Rivaroxaban in AF n n n Intracranial and fatal bleeds favoured rivaroxaban Rivaroxaban non inferior to warfarin C/I if - Cr. Cl less than 30 ml/min, active bleeding, hepatic disease (Child’s Pugh B and C), pregnancy and breast feeding Adverse effects – anaemia, dizziness, headache, epistaxis, GI s/e, haemorrhage License application for Dec 2011 and NICE appraisal May 2012

Rivaroxaban n n n Half life 7 -11 hours Review use of agents affecting haemostasis (e. g. NASIDS and anti platelets) Metabolised by CYP 3 A 4 so interactions could be problematic: Inducers – barbiturates, carbamazepine, phenytoin, rifampicin, St Johns Wort – avoid use of strong inducers Inhibitors – clarithromycin, ciclosporine, danazol, dlitiazem, erythromycin, fluconazole, fluoxetine, fluvoxamine, indinavir, isoniazid, ritonavir, verapamil Avoid itraconazole, ketoconazole, voriconazole and protease inhibitors

Rivaroxaban in DVT (instead of warfarin/enoxaparin) n n n n Patients must have a recommendation from a Haematology specialist dose is 15 mg BD for 3 weeks then 20 mg daily Allergy to warfarin Unstable INR control Intravenous drug users Liver disease (N. B. All anticoagulants are contraindicated in established liver cirrhosis including rivaroxaban) Anticoagulation in malignancy – In this patient group LMWH’s are first line, warfarin and rivaroxaban should only be used if LMWH’s are contraindicated – Newly diagnosed patients with active malignancy should not be warfarinised until their treatment plan is agreed as control is often very unstable in these patients. – Patients with chronic malignant conditions eg. prostate cancer may be suitable for warfarin, but treatment should be reviewed by the specialist team if liver metastases are present. Unable to comply with variable dosing (e. g dementia but with no support in community)

Rivaroxaban for SPAF new pts n n n Patients must fit the criteria in the Nottinghamshire guidance (see http: //www. nottsapc. nhs. uk) and have a recommendation from a secondary care Consultant Haematologist, Consultant in Stroke Medicine , or Consultant Cardiologist. Excluded patient groups are those with significant valvular disease and patients suitable for cardioversion Patients requiring domiciliary phlebotomy Known unpredictable alcohol excess (N. B. All anticoagulants are contraindicated in established liver cirrhosis including rivaroxaban) Unable to comply with variable dosing schedule Contraindications to warfarin – N. B. many contraindications for warfarin will be contraindications to anticoagulation in general, and thus are likely to be contraindications for rivaroxaban also

Rivaroxaban switch in pts on warfarin n n Patients must fit the criteria in the Nottinghamshire guidance (see http: //www. nottsapc. nhs. uk) and have a recommendation from a secondary care Consultant Haematologist, Consultant in Stroke Medicine, or Consultant Cardiologist. Excluded patient groups are those with significant valvular disease and patients suitable for cardioversion TTR<60% after 4 months of VKA in presence of compliance Intolerant of VKA (e. g. side effects etc) INR>8 without any strong cause (e. g. interacting meds) History of significant bleed associated with poor warfarin control

Rivaroxaban n n Classed as amber drug for DVT and AF Secondary care supply first month then continues in primary care Rebate scheme available Can refer pts to NUH anticoag for counselling etc (as per warfarin) No INR’s needed – monitor U+E’s according to baseline renal function – see APC guideline

Rivaroxaban and elective surgery Stop rivaroxaban at least 24 hours before intervention. If procedure cannot be delayed until at least 24 hours post dose, the increased risk of bleeding should be assessed against the urgency of the intervention. This should be discussed with a haematologist. n Rivaroxaban should be re-started post procedure when risk of bleeding is judged to be low n

Rivaroxaban and emergency surgery/haemorrhage n No reversal agent n Can use charcoal if ingested within 1 hr n Emergency surgery – measure INR (neoplastin) or Xa level – if low proceed if not delay or give octaplex n Haemorrhage – as above and use haemorrhage control measures/tranexamic acid/octaplex

Monitoring rivaroxaban Rivaroxaban does not routinely require monitoring of therapeutic response (unlike warfarin). However, if a patient has an episode of bleeding or requires an invasive procedure, measurement of an anticoagulant effect may be advantageous. n A standard clotting screen has not been validated for assessing the degree of anticoagulation in a patient taking Rivaroxaban and should not be used for this purpose n A prothrombin time using a sensitive reagent such as neoplastin plus or a specific anti Xa can be used to measure the effect only after discussion with a haematologist. n

Effect of NOAC on clotting screens Dabigatran, rivaroxaban and apixaban are new oral anticoagulants that are alternatives to coumarins (e. g. warfarin) in selected groups of patients for certain indications. All drugs accumulate in renal impairment. n A standard clotting screen has not been validated for assessing the degree of anticoagulation in a patient taking these agents and should not be used for this purpose. Consult haematology for advice n

Daily costs n Dabigatran and rivaroxaban – community ~£ 2. 60, hospital ~£ 1. 60 n Warfarin - (+costs of drug, INR, monitoring, dosing etc) approx £ 0. 67 -0. 83 daily n Apixaban likely to be similar other new agents

Ongoing trials Rivaroxaban in DVT/PE licensed - as effective as warfarin and NICE TA – dose is 15 mg BD for 3 weeks and then 20 mg daily n Dabigatran now C/I in MHV after trial halted n Apixaban (10 mg BD for 5 days then 5 mg BD for 6 months then 2. 5 mg BD) and Dabigatran (150 mg BD after 5 days of LMWH) now approved for DVT/PE n Cardioversion trial riva vs warfarin n

Apixaban in AF n n n New oral Xa inhibitor Behind dabigatran and rivaroxaban in development More effective than aspirin in stroke reduction C/I if Cr. Cl less than 25 ml/min ARISTOTLE trial – apixaban 5 mg twice daily (reduced to 2. 5 mg BD if any 2 of 3 of: >80 yrs, <60 kg, Cr>133) vs warfarin in 18, 201 patients Primary endpoint of stroke and systemic embolism

Apixaban in AF n n n Warfarin time in range 62% Rates of primary outcome 1. 27% per year apixaban and 1. 6% per year warfarin – statistically significant Major bleeds 2. 13% per year apixaban and 3. 09% warfarin Apixaban superior for stroke prevention and causes less bleeding Drug interactions as per riva avoid strong inhibitors of CYP 3 A 4 or p-gp

But for all new meds n Higher costs n How do we reverse if patient is actively bleeding and/or needs emergency surgery (both companies recommend r. VIIa/PCC – no real in vivo data)? n Difficult to measure level of anticoagulation (dabigatran ECT/TT, rivaroxaban PT) which may vary according to RF

But for all new meds n n n How do we measure compliance with new agents? (given data suggests approx 43% average patient compliance with new meds – NUH coag service has approx 70% of pts in therapeutic range) Peri-operative and emergency surgery issues (cardioversion and emergency surgery) – new agents have shorter half lives so may be able to stop day before pre op Pts will need to carry alert cards and be counselled (register pts in DAWN) by coag – need to be referred Both C/I for AF treatment if Cr. Cl<30 ml/min – what do we do if AKI? Will need to go back onto warfarin if do not tolerate new agents for AF

Current situation n n n Only using rivaroxaban in selected patients (~400) and those unable to tolerate warfarin – DVT and AF Apixaban now has NICE TA and better data for AF – for use in selected patients as above only by cardiology/stroke All NOAC C/I in MHV May decide to use riva in DVT and PE if pts on for up to 6 -12 months – but ? if long term Apixaban (10 mg BD for 5 days then 5 mg BD for 6 months then 2. 5 mg BD) and Dabigatran (150 mg BD after 5 days of LMWH) now approved for DVT/PE Rivaroxaban vs warfarin in cardioversion NOAC to amber 3 shortly

What do we need to do? n n n Check pt has had correct bloods (U+E/LFT/clotting/FBC) pre starting and 3 weeks after then according to guidelines (http: //www. nottsapc. nhs. uk/attachments/article/3/APC %20 statement%20 re%20 NICE%20 CG 180%20%20 Atrial %20 fibrillation. pdf Check dose ok according to pts renal function Pt information given and alert card Counselling/DVD – side effects, missed doses, procedures etc Check interacting meds (antiplatelets)

Questions?
 Myrta z. belknap
Myrta z. belknap Deferred update and immediate update
Deferred update and immediate update Reliant coumadin clinic
Reliant coumadin clinic Pauta harmonització anticoagulants
Pauta harmonització anticoagulants Cyp450 inducers mnemonic
Cyp450 inducers mnemonic Les anticoagulants ifsi
Les anticoagulants ifsi Les anticoagulants cours ifsi
Les anticoagulants cours ifsi Anticoagulants mechanism of action
Anticoagulants mechanism of action Fall creators update whats new
Fall creators update whats new Jak wielka jest warszawa
Jak wielka jest warszawa Julian martinez victoria ocampo
Julian martinez victoria ocampo Sherlock holmes looking for clues
Sherlock holmes looking for clues Vptsd
Vptsd Csc los angeles canvas
Csc los angeles canvas Who killed dr dedman answer
Who killed dr dedman answer Rotter and mischel
Rotter and mischel Holmes rahe stress test
Holmes rahe stress test Gran asinergia de babinski
Gran asinergia de babinski The blue diamond sherlock holmes
The blue diamond sherlock holmes Fictional detective created by arthur conan doyle
Fictional detective created by arthur conan doyle Sherlock holmes 2009
Sherlock holmes 2009 Fictional character
Fictional character 1887 sherlock holmes
1887 sherlock holmes First appearance of sherlock holmes
First appearance of sherlock holmes Hermaphroditus aussehen
Hermaphroditus aussehen Rzepka julian tuwim
Rzepka julian tuwim Sherlock holmes when you have eliminated the impossible
Sherlock holmes when you have eliminated the impossible Dr julian tomkinson
Dr julian tomkinson Tp castt poetry analysis answers
Tp castt poetry analysis answers