Advances in ColoRectal Surgery CCRT Sphincter Saving procedures
































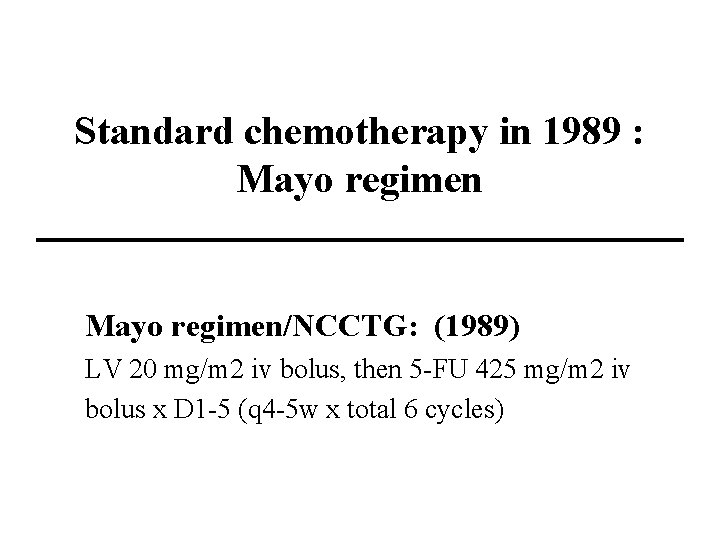













- Slides: 105




Advances in Colo-Rectal Surgery • • • CCRT Sphincter Saving procedures Laparoscopic Surgery PPH in hemorrhoid Chemotherapy in metastatic Colo-Rectal Cnacer

Preoperative Radiotherapy for Locally Advanced Rectal Cancer

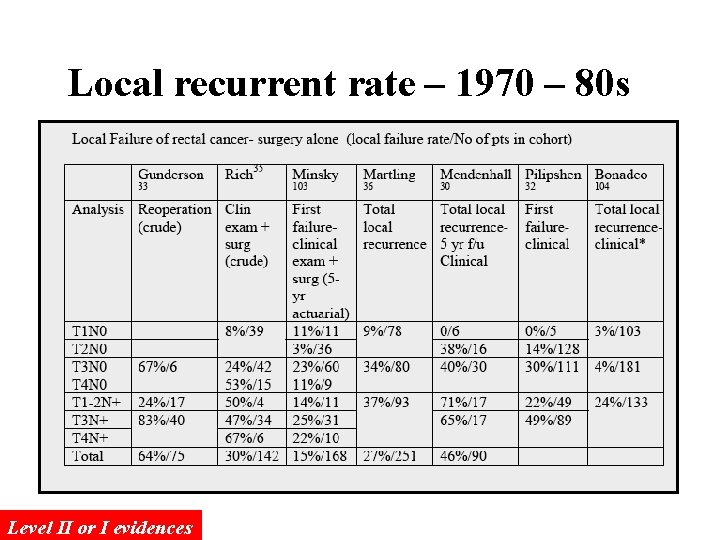
Local recurrent rate – 1970 – 80 s Level II or I evidences

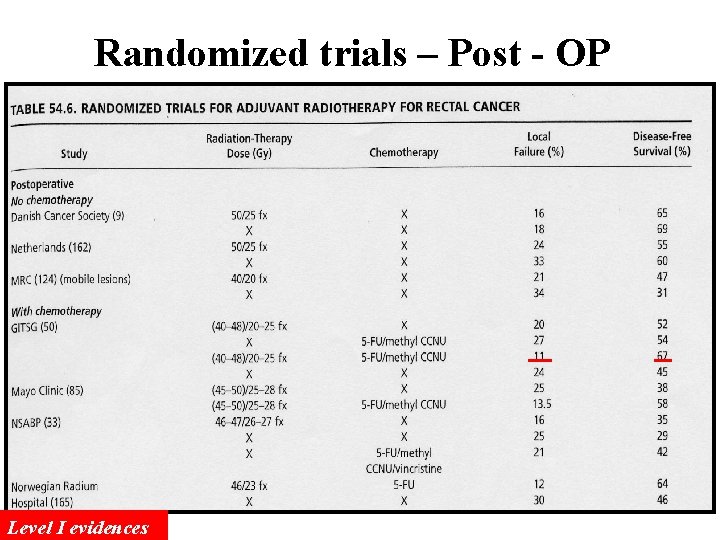
Randomized trials – Post - OP Level I evidences

1990 NIH consensus statement Combined postoperative chemotherapy and radiation therapy improves local control and survival in Stage II and III patients and is recommended. At the present time, the most effective combined modality regimen appears to be 5 -FU plus methyl. CCNU, and high-dose pelvic irradiation (45 to 55 Gy) but chronic toxicity considerations of methyl. CCNU mitigate against using this regimen outside ongoing clinical trials

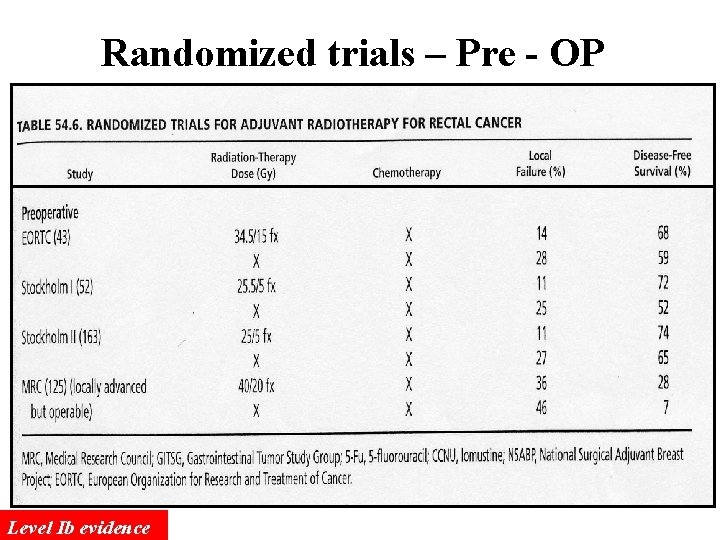
Pre-Operative Radiotherapy More easily to achieve complete tumor resection because of the shrinkage of tumor after pre-OP RT The injury to the bowels might be less since the irradiated bowel loop will be removed if the RT is delivered pre-operatively The sphincter preservation rate might be increased for low-lying rectal tumor after pre-OP CCRT.

Randomized trials – Pre - OP Level Ib evidence

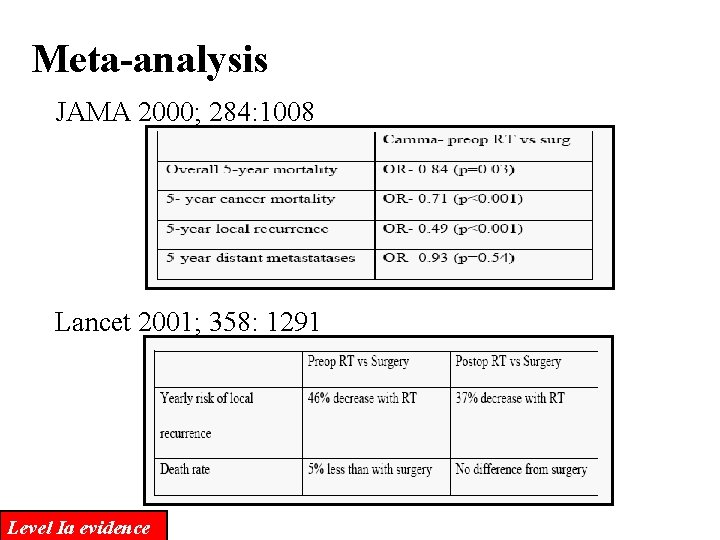
Meta-analysis JAMA 2000; 284: 1008 Lancet 2001; 358: 1291 Level Ia evidence

Long course vs Short course Pre-OP RT

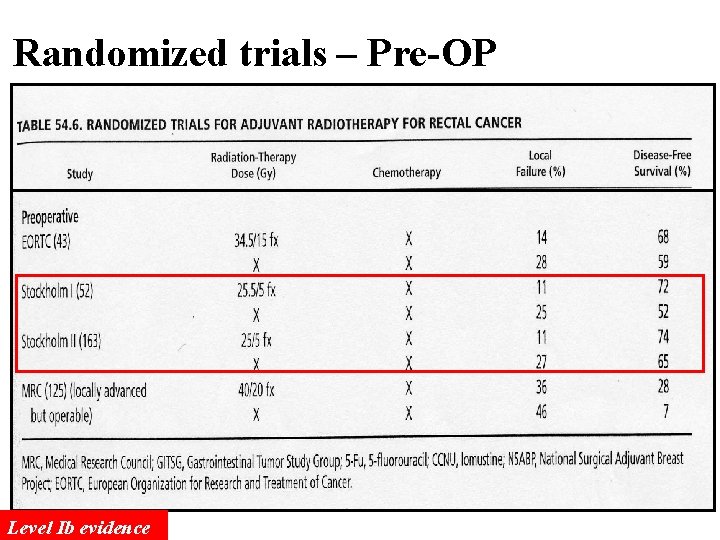
Randomized trials – Pre-OP Level Ib evidence

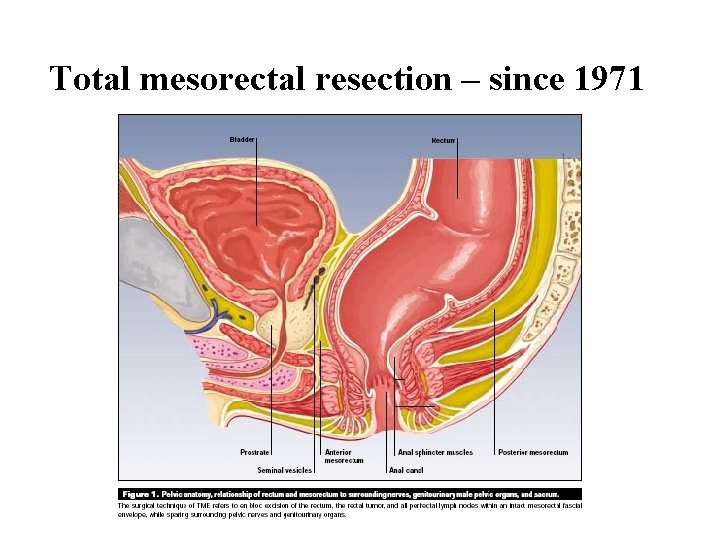
Total mesorectal resection – since 1971

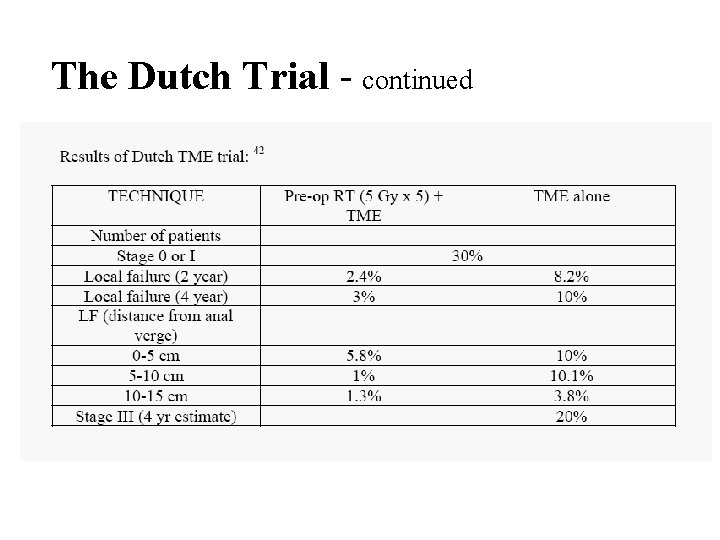
The Dutch Trial - continued

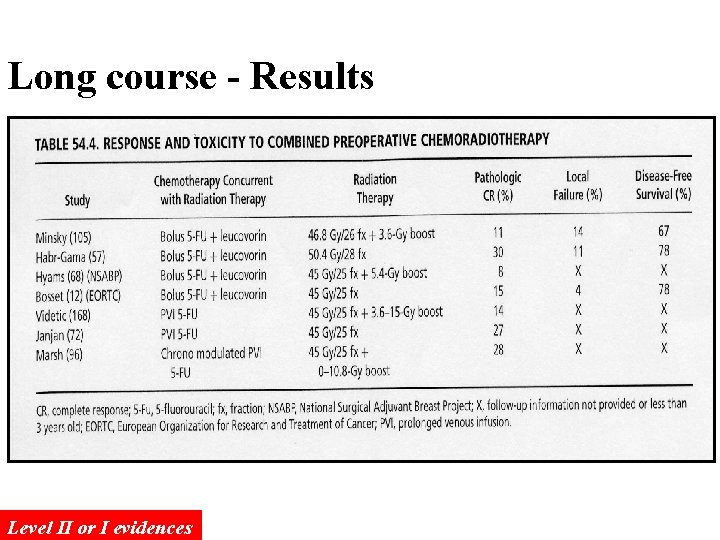
Long course - Results Level II or I evidences

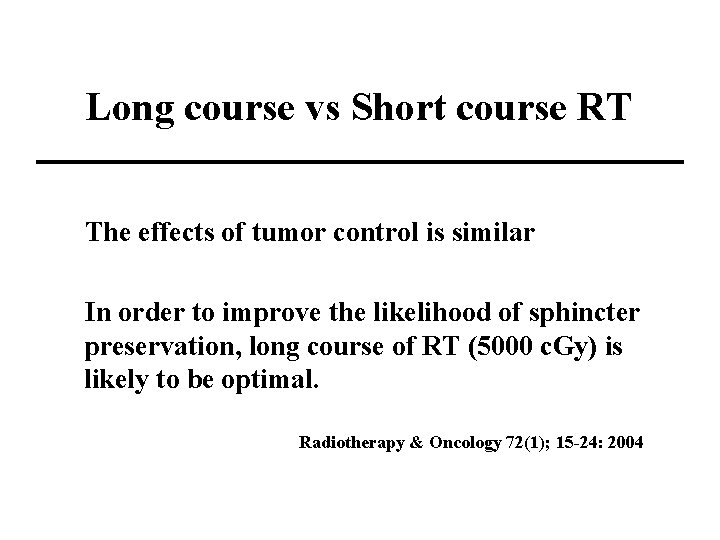
Long course vs Short course RT The effects of tumor control is similar In order to improve the likelihood of sphincter preservation, long course of RT (5000 c. Gy) is likely to be optimal. Radiotherapy & Oncology 72(1); 15 -24: 2004
ASCO news – 2006 June

Rationale of using FOLFOX regimen In a large international multicenter cooperative trial (MOSAIC), the addition of oxaliplatin to adjuvant chemotherapy with 5 -FU and LV is associated with a significant better disease-free survival at 3 -year in 2246 patients with stage II or III colon cancer (N Eng J Med 350: 2343 -51, 2004) In several phase I-II studies, oxaliplatin also shows activities in patients with rectal cancer (Br J Cancer 93: 993 -8, 2005, Ann Oncol, 16: 1898 -905, 2005, Br J Cancer 92: 655 -61, 2005) Level Ib or II evidence


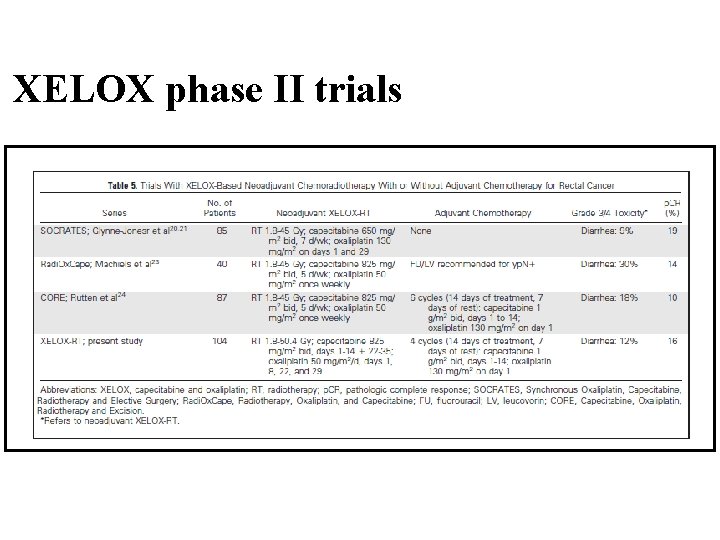
XELOX phase II trials

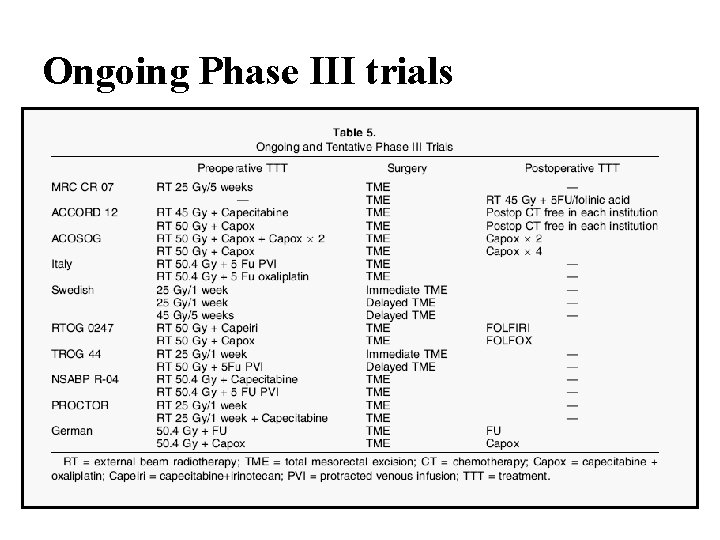
Ongoing Phase III trials

Intersphincteric Resection (ISR) for lower rectal cancer

If a distal clearance of 1 cm can be achieved, a low rectal cancer should be suitable for anterior resection ~ a recommendation in the ‘Guidelines for the Management of Colorectal Cancer’ from the Royal College of Surgeons of England the Association of Coloproctology of Great Britain and Ireland ~ Association of Coloproctology of Great Britain and Ireland. (1996) Guidelines for the management of colorectal cancer. The Royal College of Surgeons of England, London.

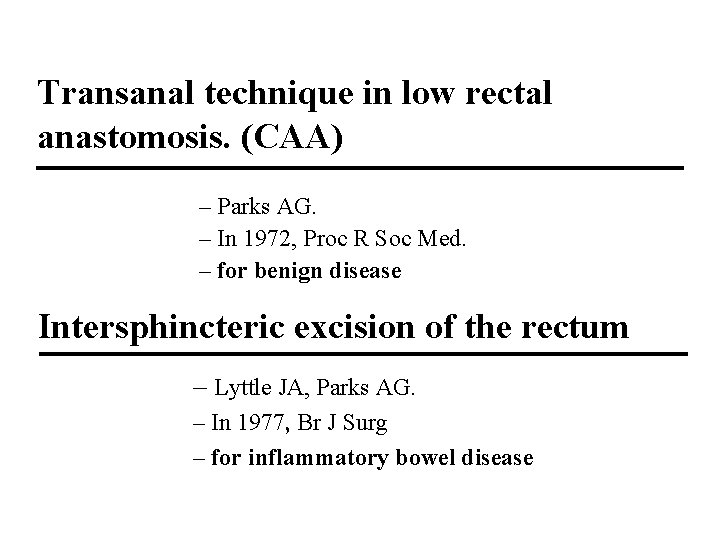
Transanal technique in low rectal anastomosis. (CAA) – Parks AG. – In 1972, Proc R Soc Med. – for benign disease Intersphincteric excision of the rectum – Lyttle JA, Parks AG. – In 1977, Br J Surg – for inflammatory bowel disease

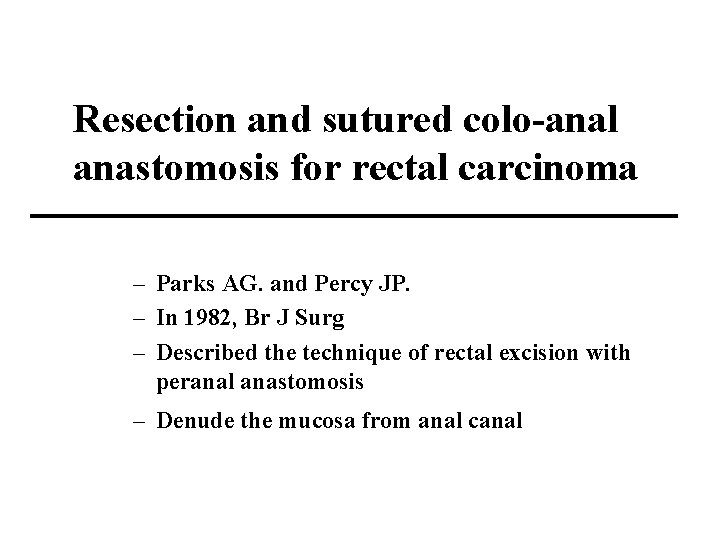
Resection and sutured colo-anal anastomosis for rectal carcinoma – Parks AG. and Percy JP. – In 1982, Br J Surg – Described the technique of rectal excision with peranal anastomosis – Denude the mucosa from anal canal



Resection and colo-anal anastomosis with colonic reservoir for rectal carcinoma – R. Parc, et al. – In 1986, Br J Surg – 31 patients, – Technique of excision as method of Parks AG. , use a J-shaped reservoir of 8 cm



Results of Intersphincteric Resection of the Rectum With Direct Coloanal Anastomosis for Rectal Carcinoma – Joseph Braun, et al. – In 1992, Am J Surg – 63 patients underwent ISR – Evaluate the oncologic results (comparing with APR) and continence

Intersphincteric resection for low rectal tumours – R. Schiessel, et al. – In 1994, Br J Surg – Complete or partial excision of the internal sphincter – 38 patients , reported the oncological follow-up, continence and bowel habit


Intersphincteric Resection in Patients with Very Low Rectal Cancer: A Review of the Japanese Experience – Norio Saito et al. – In 2006, Dis Colon Rectum – ISR, PESR (partial external sphincter resection)


Laparoscopic colorectal surgery

History Laparoscopic surgery was developed by gynecologists in the 1960 s as a diagnostic tool. The procedure was gradually extended to allow minor surgical interventions. In 1984 Reddick first applied the technique to laparoscopic cholecystectomy. By 1991 more than 10, 000 laparoscopic cholecystectomy cases were reported. In the last decades laparoscopic surgery has been extended to surgery of the appendix, colon , stomach , kidney , and liver.

Procedures for Laparoscopic Colorectal Surgery High anterior Resection Right hemicolectomy Left hemicolectomy Abdomino-perineal Resection Lower anterior Resection Subtotal colectomy Total proctocolectomy Almost No Limitation!!

The rationales for laparoscopic surgery Advantages over conventional techniques - reduced postoperative pain - earlier recovery of bowel function - shorter length of hospital stay - a decreased risk of peritoneal tumor growth with laparoscopic as compared with open procedures

Challenge in Laparoscopic colorectal surgery Different Training course and Technique It requires dissection in multiple parts of the abdomen. Need to isolate and ligate major arteries and veins. Need to divide of colonic attachments Identification and preservation of critical retroperitonealstructures Intestinal division, and reconstruction of bowel continuity.

Important Issues in Laparoscopic Surgery for Colorectal Cancer

Issue (1) Dose the laparoscopic colorectal surgery increase the Port-site (wound) recurrence ?

• Laparoscopic surgery of colon and rectum : Port-site recurrence remains a leading concern. World J Surg 1999 Apr 23; (4) 397 -405 • Port-site recurrence rate : 0. 5%~1. 1% Dis colon rectum 2000 Jan /Am Surg 2000 Mar 66(3) 245~8 • Results of a multicenter study of 1, 057 cases of rectal cancer treated by laparoscopic surgery. No port-site metastases Surg Endosc. 2008 Sep 19. • A cochrane systematic review of randomised controlled trials. Port-site metastases were rare and no differences in occurrence after laparoscopic and open surgery Cancer Treat Rev. 2008 Oct; 34(6): 498 -504

Issue (2) Could minimally invasive surgery achieve a proper oncologic resection, with the same extent of exploration and information about lymph-node staging provided by a standard open resection ?

Prospective comparison of laparoscopic vs. open resections for colorectal cancer over a ten-year period. (III) Dis Colon Rectum. 2003 May; 46(5): 601 -11 Stage-for-stage overall five-year survival rates were similar in the two groups. The statistical analysis performed for colonic vs. rectal primary adenocarcinoma confirmed that TNM stage for stage-overall survival was similar in the laparoscopic and open-resection groups.

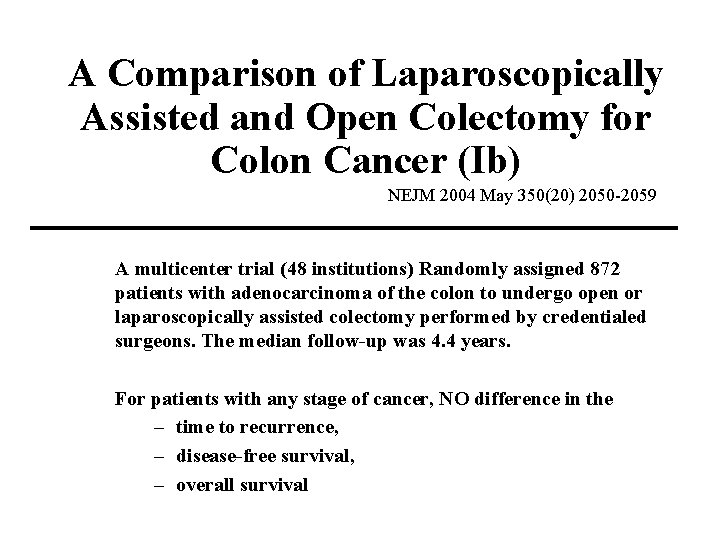
A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer (Ib) NEJM 2004 May 350(20) 2050 -2059 A multicenter trial (48 institutions) Randomly assigned 872 patients with adenocarcinoma of the colon to undergo open or laparoscopically assisted colectomy performed by credentialed surgeons. The median follow-up was 4. 4 years. For patients with any stage of cancer, NO difference in the – time to recurrence, – disease-free survival, – overall survival

Issue (3) Dose laparoscopic surgery increase the Morbidity or Mortality ?

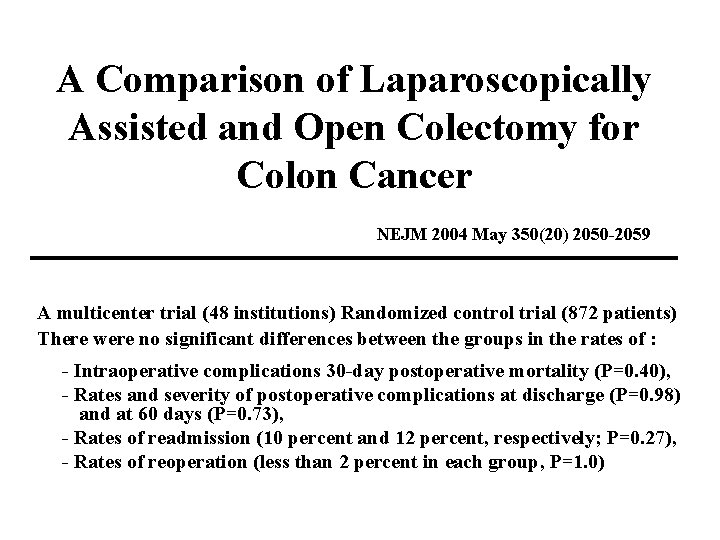
A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer NEJM 2004 May 350(20) 2050 -2059 A multicenter trial (48 institutions) Randomized control trial (872 patients) There were no significant differences between the groups in the rates of : - Intraoperative complications 30 -day postoperative mortality (P=0. 40), - Rates and severity of postoperative complications at discharge (P=0. 98) and at 60 days (P=0. 73), - Rates of readmission (10 percent and 12 percent, respectively; P=0. 27), - Rates of reoperation (less than 2 percent in each group, P=1. 0)

What is the learning curve for laparoscopic colorectal surgery ? • The gap in laparoscopic colorectal experience between colon and rectal and general surgery residency training programs Dis Colon Rectum. 2007 Dec; 50(12): 202331; • Learning curves for laparoscopic colectomy are reported in the range of 20 to 60 cases. • Does the laparoscopic colorectal surgery learning curve adversely affect the results of colorectal cancer resection? A 3 -year prospective study in a district general hospital. Colorectal Dis. 2008 May; 10(4): 363 -9. • Changing from open to laparoscopic dissection for colorectal cancer is safe even during the initial learning curve. Estabilish training Program!

PPH Hemorrhoidopexy


Moving forward the optimal therapy in metastatic colorectal cancer

Optimal Therapy for m. CRC in Taiwan Chemotherapy evolution Targeted therapy evolution

Chemotherapy evolution
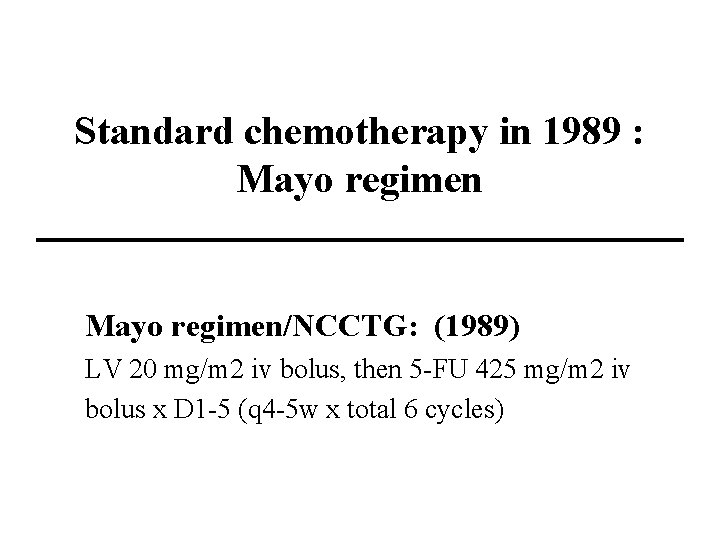
Standard chemotherapy in 1989 : Mayo regimen/NCCTG: (1989) LV 20 mg/m 2 iv bolus, then 5 -FU 425 mg/m 2 iv bolus x D 1 -5 (q 4 -5 w x total 6 cycles)

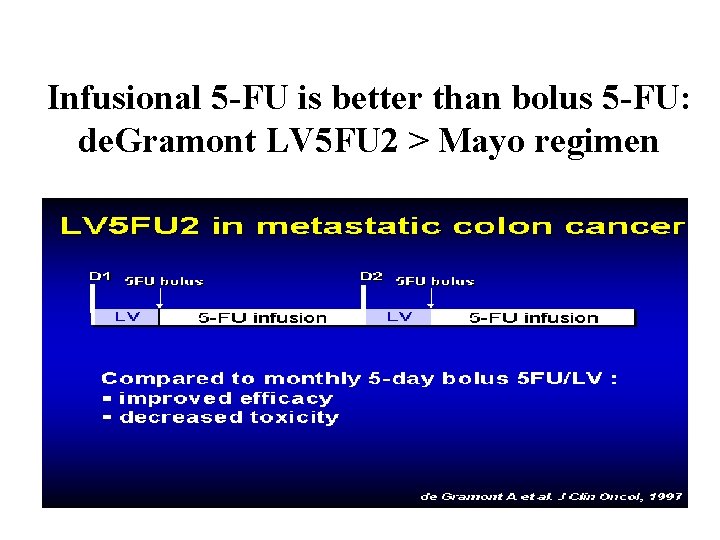
Infusional 5 -FU is better than bolus 5 -FU: de. Gramont LV 5 FU 2 > Mayo regimen

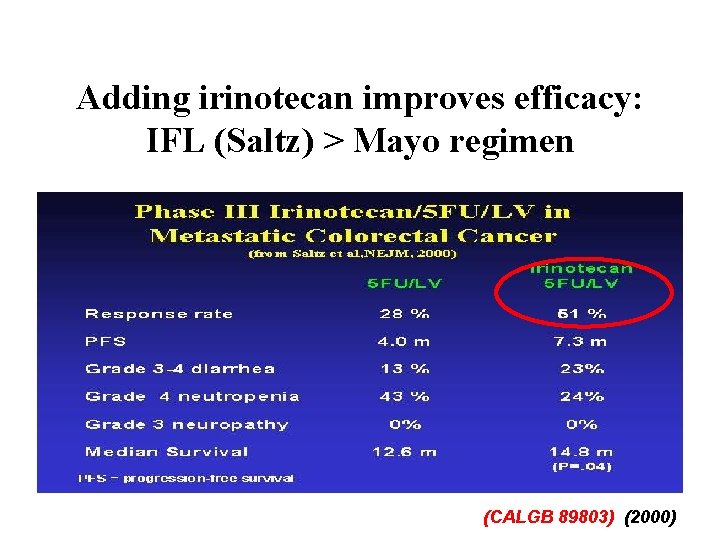
Adding irinotecan improves efficacy: IFL (Saltz) > Mayo regimen (CALGB 89803) (2000)

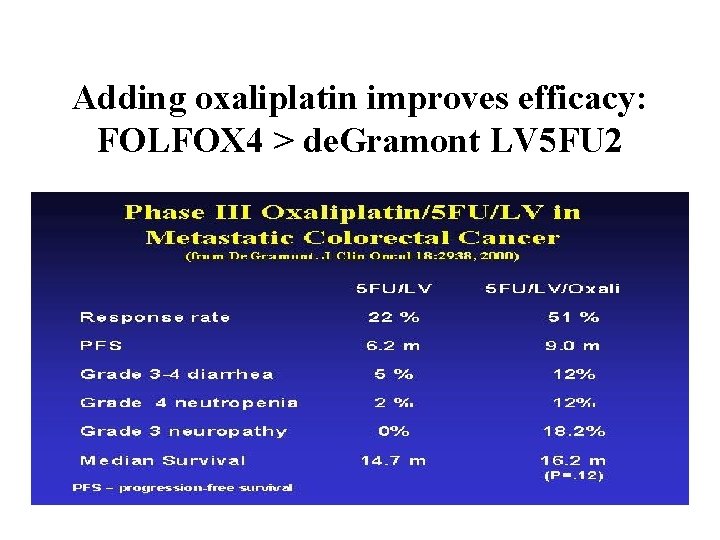
Adding oxaliplatin improves efficacy: FOLFOX 4 > de. Gramont LV 5 FU 2

FOLFOX is better than IFL (Saltz)

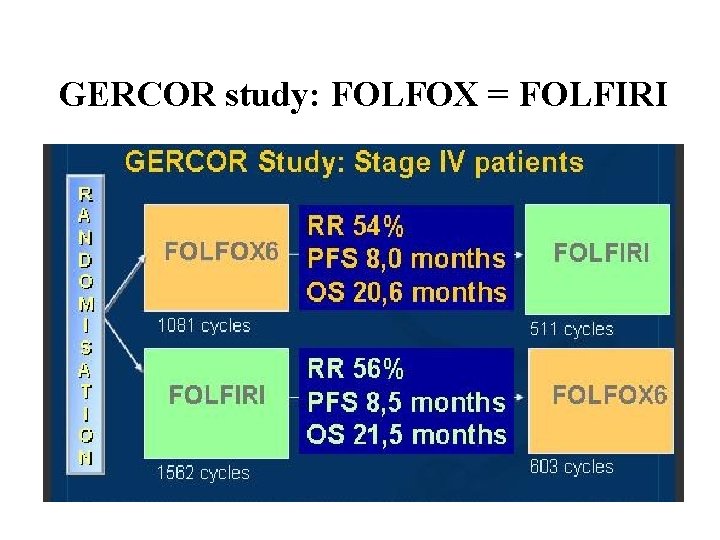
GERCOR study: FOLFOX = FOLFIRI

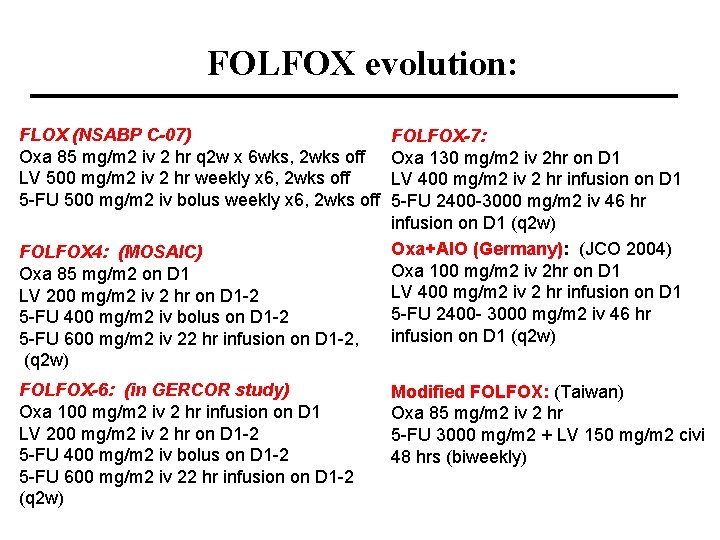
FOLFOX evolution: FLOX (NSABP C-07) Oxa 85 mg/m 2 iv 2 hr q 2 w x 6 wks, 2 wks off LV 500 mg/m 2 iv 2 hr weekly x 6, 2 wks off 5 -FU 500 mg/m 2 iv bolus weekly x 6, 2 wks off FOLFOX 4: (MOSAIC) Oxa 85 mg/m 2 on D 1 LV 200 mg/m 2 iv 2 hr on D 1 -2 5 -FU 400 mg/m 2 iv bolus on D 1 -2 5 -FU 600 mg/m 2 iv 22 hr infusion on D 1 -2, (q 2 w) FOLFOX-6: (in GERCOR study) Oxa 100 mg/m 2 iv 2 hr infusion on D 1 LV 200 mg/m 2 iv 2 hr on D 1 -2 5 -FU 400 mg/m 2 iv bolus on D 1 -2 5 -FU 600 mg/m 2 iv 22 hr infusion on D 1 -2 (q 2 w) FOLFOX-7: Oxa 130 mg/m 2 iv 2 hr on D 1 LV 400 mg/m 2 iv 2 hr infusion on D 1 5 -FU 2400 -3000 mg/m 2 iv 46 hr infusion on D 1 (q 2 w) Oxa+AIO (Germany): (JCO 2004) Oxa 100 mg/m 2 iv 2 hr on D 1 LV 400 mg/m 2 iv 2 hr infusion on D 1 5 -FU 2400 - 3000 mg/m 2 iv 46 hr infusion on D 1 (q 2 w) Modified FOLFOX: (Taiwan) Oxa 85 mg/m 2 iv 2 hr 5 -FU 3000 mg/m 2 + LV 150 mg/m 2 civi 48 hrs (biweekly)

Targeted therapy evolution

Anti-VEGF Bevacizumab (Avastin; Roche/Genentech)

AVF 2107

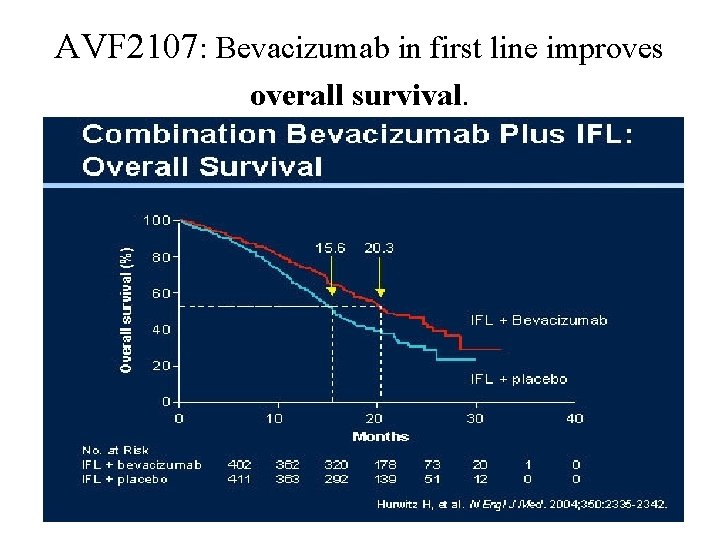
AVF 2107: Bevacizumab in first line improves overall survival.

AVF 2107 retrospective analysis Avastin improves survival irrespective of K-Ras gene mutations In patients with normal K-Ras gene, avastin increases 82% in PFS and 57% in OS versus chemotherapy alone. In patients with K-Ras gene mutations, avastin increases 69% in PFS for Avastin compared with chemotherapy alone. World Congress on Gastrointestinal Cancer , 2008

AVF 2107: summary IFL + BV > IFL (in RR, PFS, OS) PFS advantage 4. 4 months OS advantage 4. 7 months However, IFL is no longer standard treatment for m. CRC.

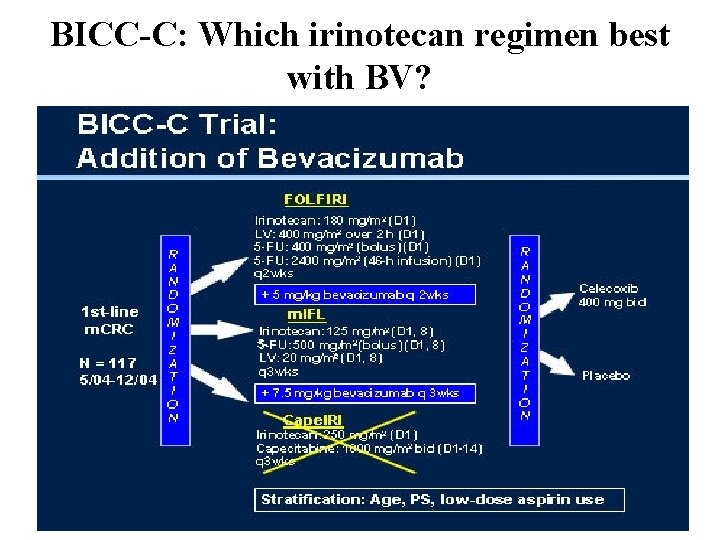
BICC-C: Which irinotecan regimen best with BV?

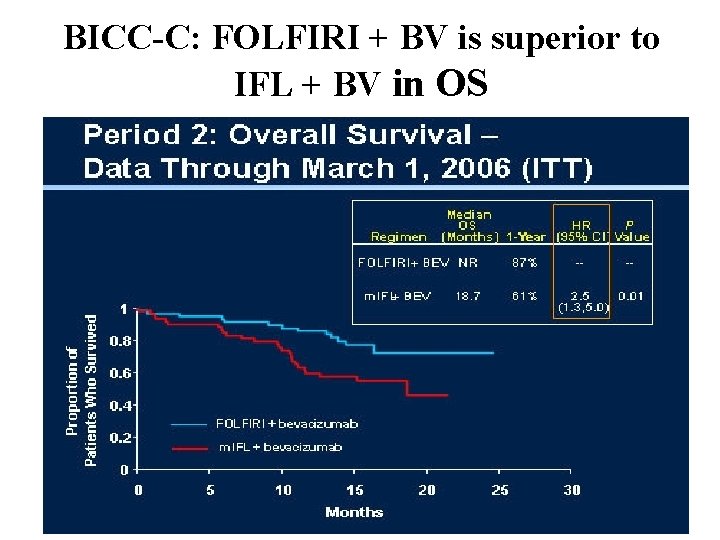
BICC-C: FOLFIRI + BV is superior to IFL + BV in OS

BICC-C These data support the use of FOLFIRI as a reasonable first-line chemotherapy platform when combined with bevacizumab.

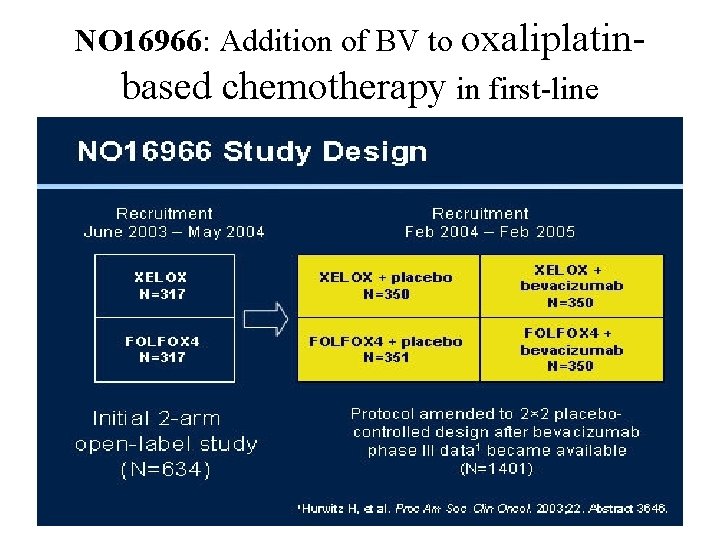
NO 16966: Addition of BV to oxaliplatinbased chemotherapy in first-line

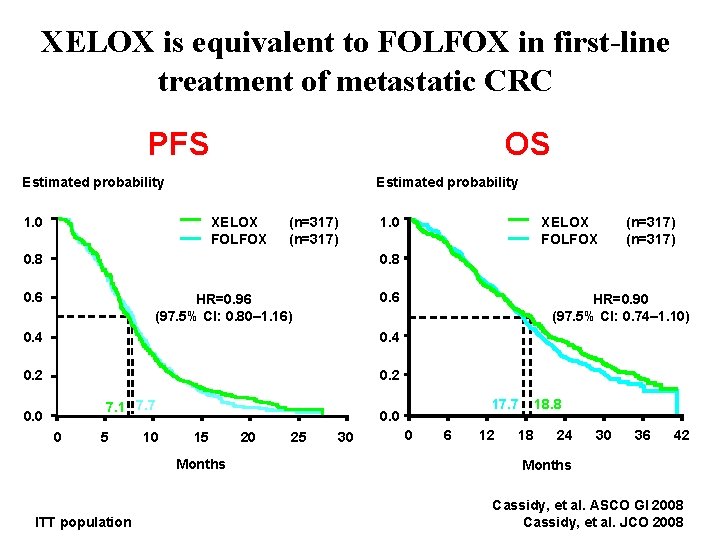
XELOX is equivalent to FOLFOX in first-line treatment of metastatic CRC PFS OS Estimated probability XELOX FOLFOX 1. 0 (n=317) 0. 8 0. 6 HR=0. 96 (97. 5% CI: 0. 80– 1. 16) 0. 4 0. 2 7. 1 7. 7 0. 0 0 5 10 17. 7 0. 0 15 Months ITT population HR=0. 90 (97. 5% CI: 0. 74– 1. 10) 20 25 30 0 6 12 18. 8 18 24 30 36 42 Months Cassidy, et al. ASCO GI 2008 Cassidy, et al. JCO 2008

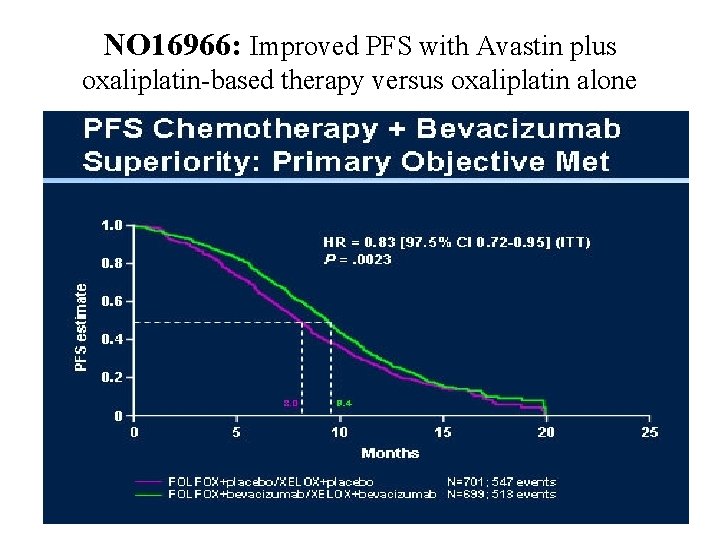
NO 16966: Improved PFS with Avastin plus oxaliplatin-based therapy versus oxaliplatin alone

Bevacizumab in First-line m. CRC : Taiwan EAP study 7 hospitals, total 40 patients T MMH 馬偕紀念醫院 T VGH-TC 台中榮民總醫院 T CMUH 中國醫藥大學附設醫院 T CGMH-CY 嘉義長庚紀念醫院 T CMH 奇美醫院 T NCKUH 成功大學附設醫院 T CGMH-KS 高雄長庚紀念醫院

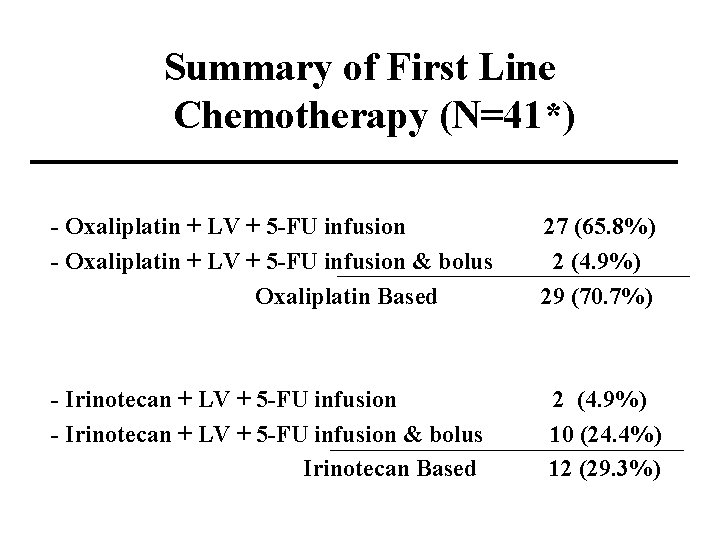
Summary of First Line Chemotherapy (N=41*) - Oxaliplatin + LV + 5 -FU infusion & bolus Oxaliplatin Based 27 (65. 8%) 2 (4. 9%) 29 (70. 7%) - Irinotecan + LV + 5 -FU infusion & bolus Irinotecan Based 2 (4. 9%) 10 (24. 4%) 12 (29. 3%)

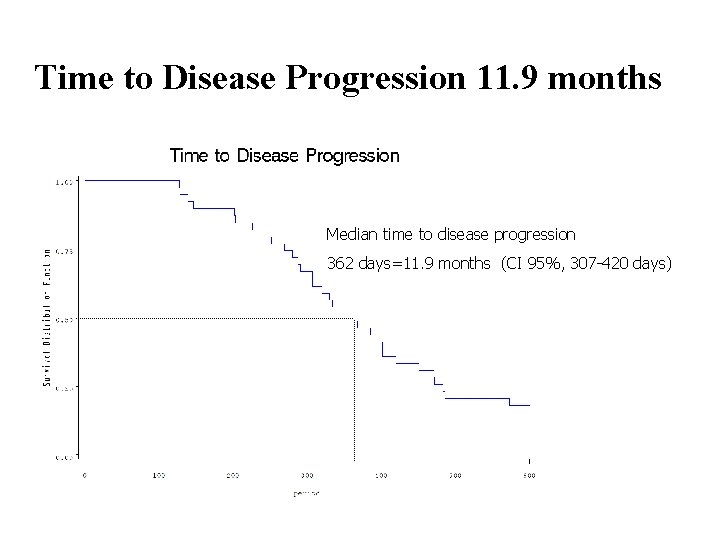
Time to Disease Progression 11. 9 months Median time to disease progression 362 days=11. 9 months (CI 95%, 307 -420 days)

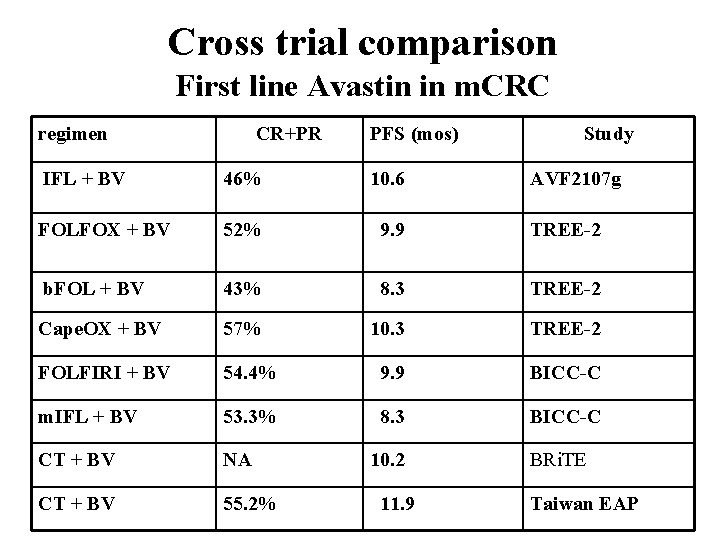
Cross trial comparison First line Avastin in m. CRC regimen CR+PR PFS (mos) Study IFL + BV 46% 10. 6 FOLFOX + BV 52% 9. 9 TREE-2 b. FOL + BV 43% 8. 3 TREE-2 Cape. OX + BV 57% 10. 3 TREE-2 FOLFIRI + BV 54. 4% 9. 9 BICC-C m. IFL + BV 53. 3% 8. 3 BICC-C CT + BV NA CT + BV 55. 2% 10. 2 11. 9 AVF 2107 g BRi. TE Taiwan EAP

Anti-EGFR Cetuximab (Erbitux; Bristol-Myers Squibb/Im. Clone and Merck) Panitumumab (ABX-EGF; Vectibix, Amgen)

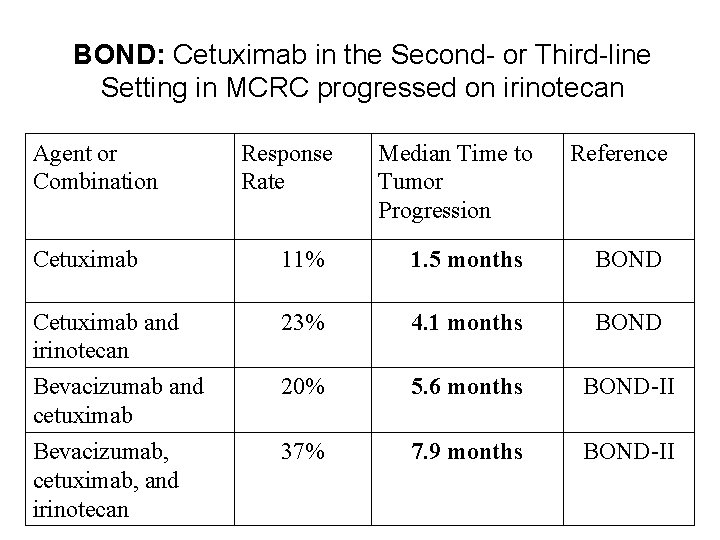
BOND: Cetuximab in the Second- or Third-line Setting in MCRC progressed on irinotecan Agent or Combination Response Rate Median Time to Tumor Progression Reference Cetuximab 11% 1. 5 months BOND Cetuximab and irinotecan Bevacizumab and cetuximab 23% 4. 1 months BOND 20% 5. 6 months BOND-II Bevacizumab, cetuximab, and irinotecan 37% 7. 9 months BOND-II

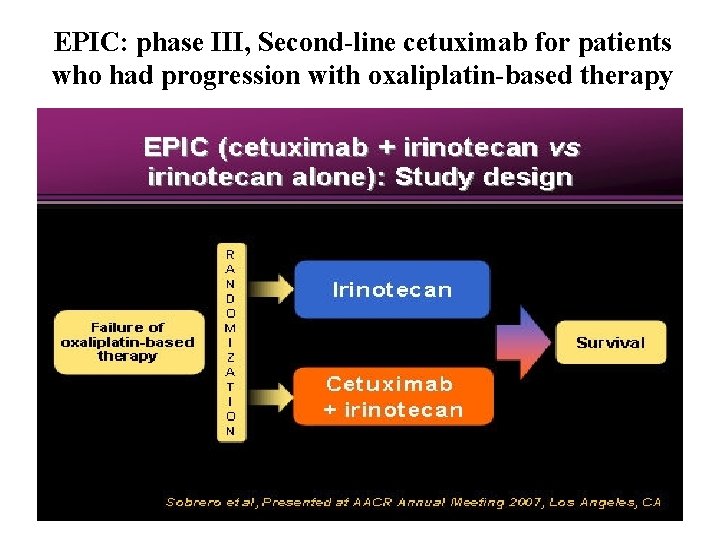
EPIC: phase III, Second-line cetuximab for patients who had progression with oxaliplatin-based therapy

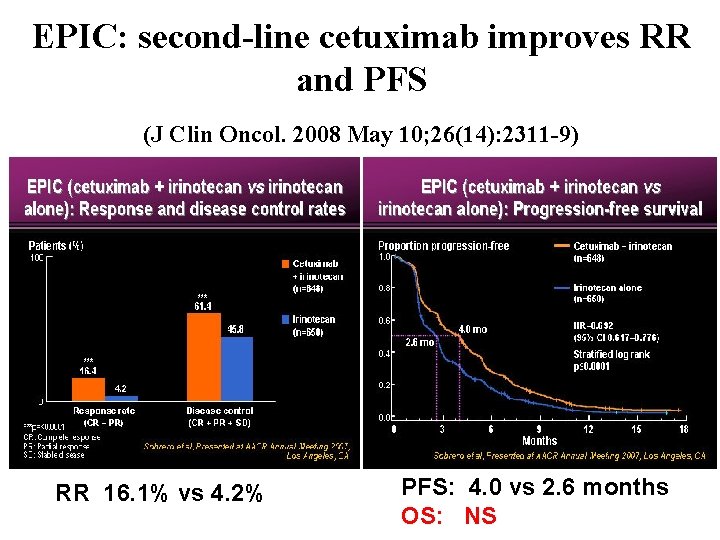
EPIC: second-line cetuximab improves RR and PFS (J Clin Oncol. 2008 May 10; 26(14): 2311 -9) RR 16. 1% vs 4. 2% PFS: 4. 0 vs 2. 6 months OS: NS

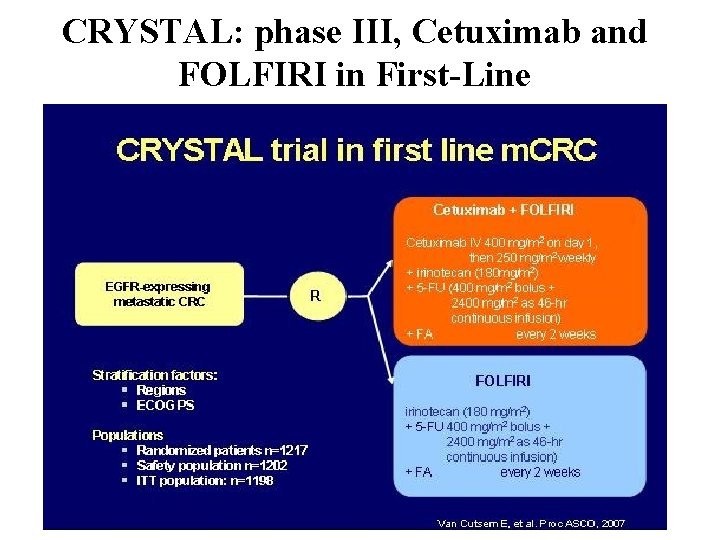
CRYSTAL: phase III, Cetuximab and FOLFIRI in First-Line

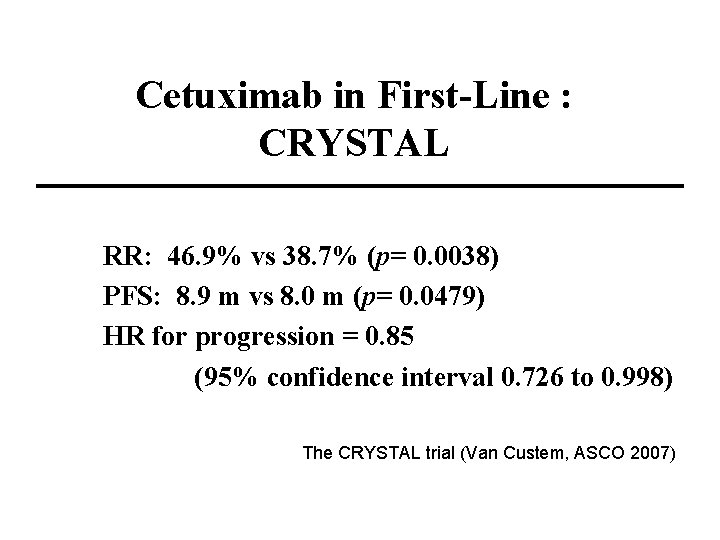
Cetuximab in First-Line : CRYSTAL RR: 46. 9% vs 38. 7% (p= 0. 0038) PFS: 8. 9 m vs 8. 0 m (p= 0. 0479) HR for progression = 0. 85 (95% confidence interval 0. 726 to 0. 998) The CRYSTAL trial (Van Custem, ASCO 2007)

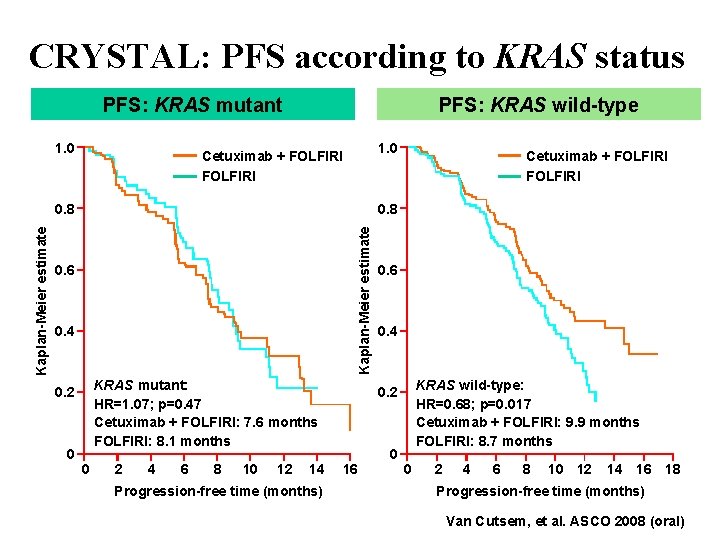
CRYSTAL: PFS according to KRAS status PFS: KRAS mutant 1. 0 PFS: KRAS wild-type 1. 0 Cetuximab + FOLFIRI 0. 8 Kaplan-Meier estimate 0. 8 0. 6 0. 4 KRAS mutant: HR=1. 07; p=0. 47 Cetuximab + FOLFIRI: 7. 6 months FOLFIRI: 8. 1 months 0. 2 0 Cetuximab + FOLFIRI 0 2 4 6 8 10 12 14 Progression-free time (months) 0. 6 0. 4 KRAS wild-type: HR=0. 68; p=0. 017 Cetuximab + FOLFIRI: 9. 9 months FOLFIRI: 8. 7 months 0. 2 16 0 0 2 4 6 8 10 12 14 16 18 Progression-free time (months) Van Cutsem, et al. ASCO 2008 (oral)

KRAS changes everything in Cetuximab !

CELIM-study Neo-adjuvant therapy for non-resectable colorectal liver metastases. Cetuximab + FOLFOX 6 versus Cetuximab + FOLFIRI ESMO 2008

Definition of non-resectability • >= 5 liver metastases and/or • Technically non-resectable (insufficient functional liver tissue remaining, infiltration of both hepatic arteries, both hepatic veins, both portal branches, both bile ducts) • No extra-hepatic disease

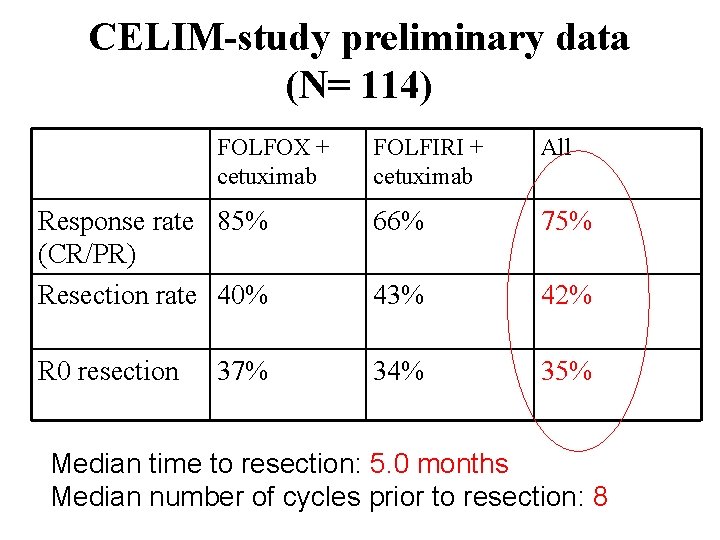
CELIM-study preliminary data (N= 114) FOLFOX + cetuximab FOLFIRI + cetuximab All Response rate 85% (CR/PR) Resection rate 40% 66% 75% 43% 42% R 0 resection 34% 35% 37% Median time to resection: 5. 0 months Median number of cycles prior to resection: 8

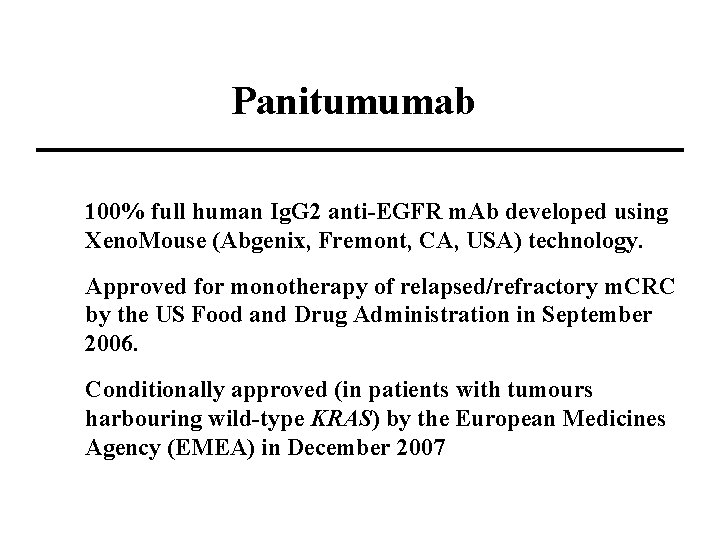
Panitumumab 100% full human Ig. G 2 anti-EGFR m. Ab developed using Xeno. Mouse (Abgenix, Fremont, CA, USA) technology. Approved for monotherapy of relapsed/refractory m. CRC by the US Food and Drug Administration in September 2006. Conditionally approved (in patients with tumours harbouring wild-type KRAS) by the European Medicines Agency (EMEA) in December 2007

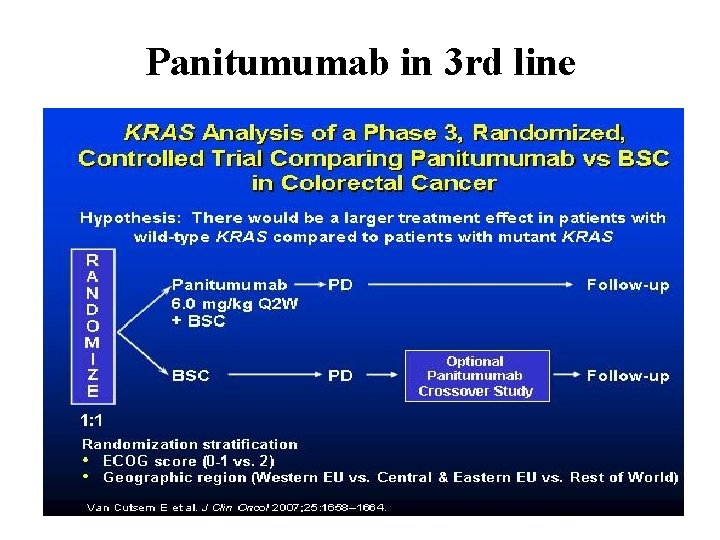
Panitumumab in 3 rd line

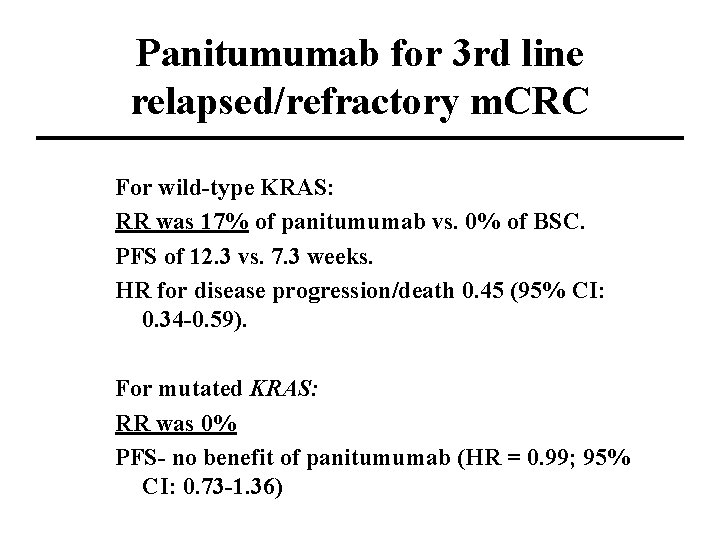
Panitumumab for 3 rd line relapsed/refractory m. CRC For wild-type KRAS: RR was 17% of panitumumab vs. 0% of BSC. PFS of 12. 3 vs. 7. 3 weeks. HR for disease progression/death 0. 45 (95% CI: 0. 34 -0. 59). For mutated KRAS: RR was 0% PFS- no benefit of panitumumab (HR = 0. 99; 95% CI: 0. 73 -1. 36)

VEGFR inhibitor Sunitinib (Sutent; Pfizer)

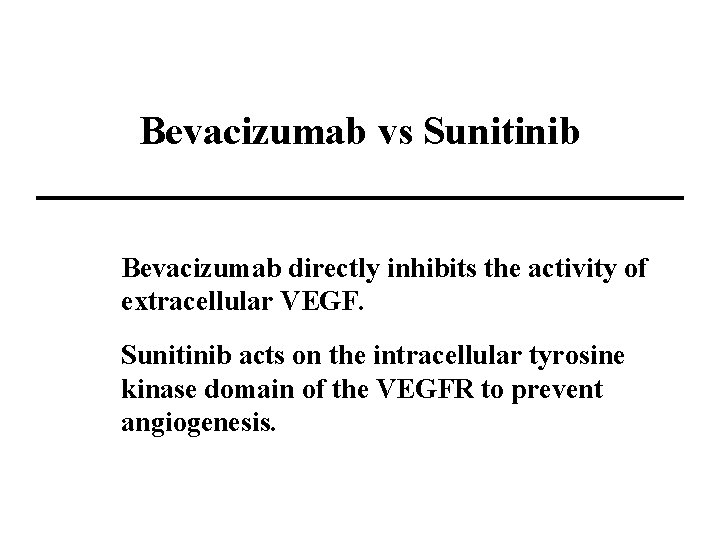
Bevacizumab vs Sunitinib Bevacizumab directly inhibits the activity of extracellular VEGF. Sunitinib acts on the intracellular tyrosine kinase domain of the VEGFR to prevent angiogenesis.

Sunitinib : a multiple target inhibitor Sunitinib is a small-molecule tyrosine kinase inhibitor with multiple targets on tumor cells. Sunitinib has been shown to be a potent inhibitor of VEGF receptors, FLT 3, c-KIT, and PDGF receptors. The half-life of sunitinib and its active metabolite ranges from 40 to 60 hours and 80 to 110 hours, respectively

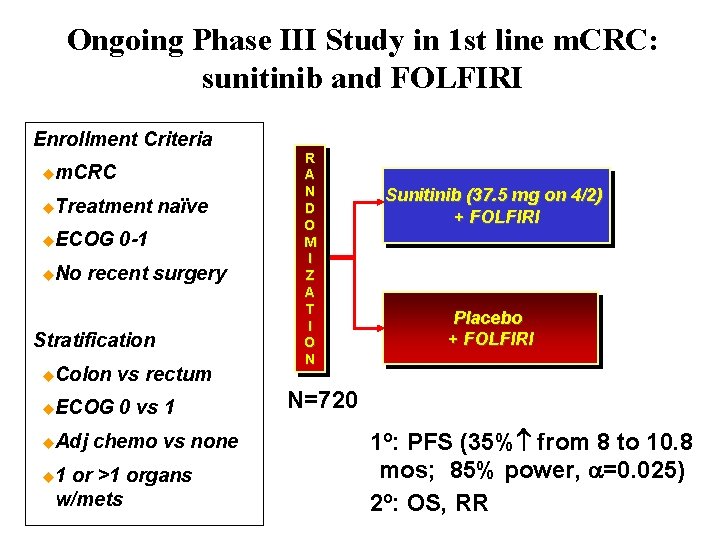
Ongoing Phase III Study in 1 st line m. CRC: sunitinib and FOLFIRI Enrollment Criteria um. CRC u. Treatment u. ECOG u. No naïve 0 -1 recent surgery Stratification u. Colon vs rectum u. ECOG 0 vs 1 u. Adj u 1 chemo vs none or >1 organs w/mets R A N D O M I Z A T I O N Sunitinib (37. 5 mg on 4/2) + FOLFIRI Placebo + FOLFIRI N=720 1º: PFS (35% from 8 to 10. 8 mos; 85% power, a=0. 025) 2º: OS, RR

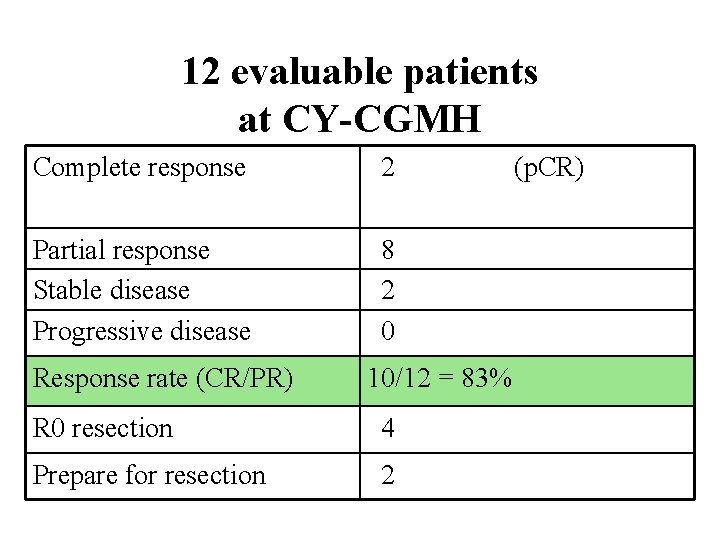
12 evaluable patients at CY-CGMH Complete response 2 Partial response Stable disease Progressive disease 8 2 0 Response rate (CR/PR) 10/12 = 83% R 0 resection 4 Prepare for resection 2 (p. CR)

Prospective Anti-IGF-1 R : MK 0646 (MSD) AMG 479 (Amgen) Anti-VEGFR : Aflibercept (Aventis) Anti-TRAIL receptor 2 (TR-2/DR 5) : AMG 655 (Amgen)

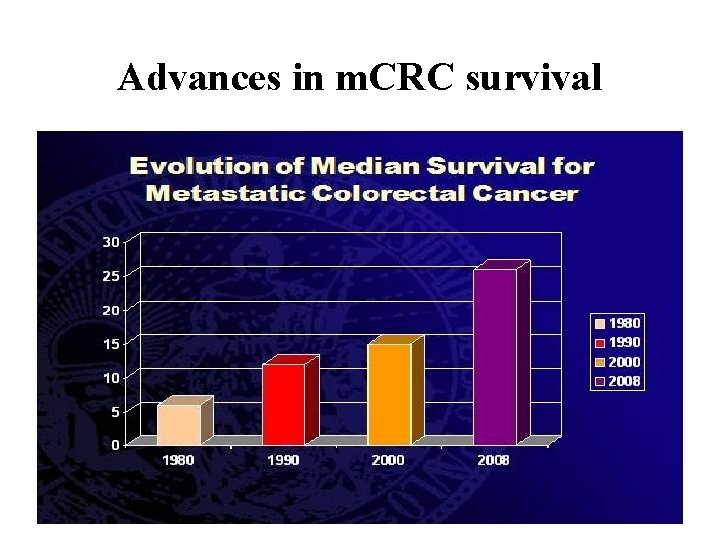
Advances in m. CRC survival

Future aspect

Translational Medicine 轉譯醫學 To integrate the basic scientific discoveries to clinical investigation, and translate the results of clinical trials into changes in clinical practice. (Lean et al 2008)


Example K-ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer: Patients with a colorectal tumor bearing mutated K-ras did not benefit from cetuximab, whereas patients with a tumor bearing wild-type K-ras did benefit from cetuximab NEJM 359: 1757 -1765, 2008 Oct

As a Colo-Rectal Surgeon • Commitment to patients and good patient care • Professionalism and ambition • Life Style

Thanks for your attention