COLORECTAL CANCER SCREENING Scenarios for National Colorectal Cancer







- Slides: 52

COLORECTAL CANCER SCREENING Scenarios for National Colorectal Cancer Screening Network (NCCSN) May 15 2014 1

Acknowledgements • • • Andy Coldman Anthony Miller Claude Nadeau Norm Phillips Saima Memon William Flanagan 2

Background • Feedback received from Network members: – More on FIT vs. g. FOBT – FIT cut-off thresholds – Error bands – Breakdown of males/females – Jurisdictional analysis • Report to be disseminated to Network in June 3

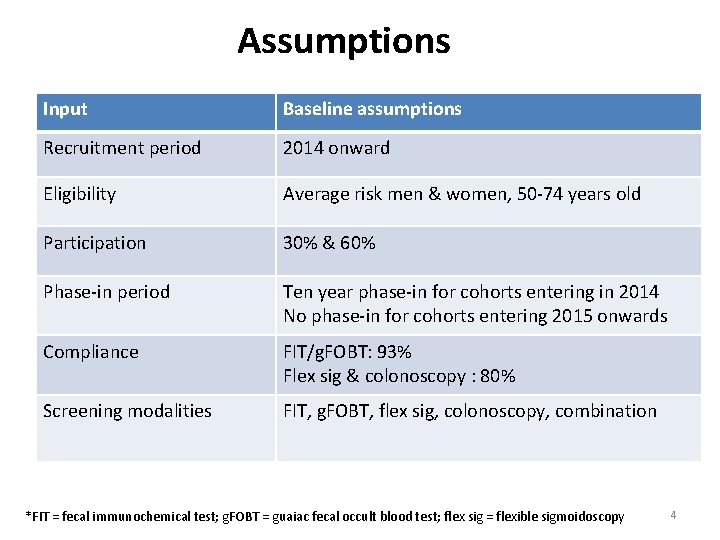
Assumptions Input Baseline assumptions Recruitment period 2014 onward Eligibility Average risk men & women, 50 -74 years old Participation 30% & 60% Phase-in period Ten year phase-in for cohorts entering in 2014 No phase-in for cohorts entering 2015 onwards Compliance FIT/g. FOBT: 93% Flex sig & colonoscopy : 80% Screening modalities FIT, g. FOBT, flex sig, colonoscopy, combination *FIT = fecal immunochemical test; g. FOBT = guaiac fecal occult blood test; flex sig = flexible sigmoidoscopy 4

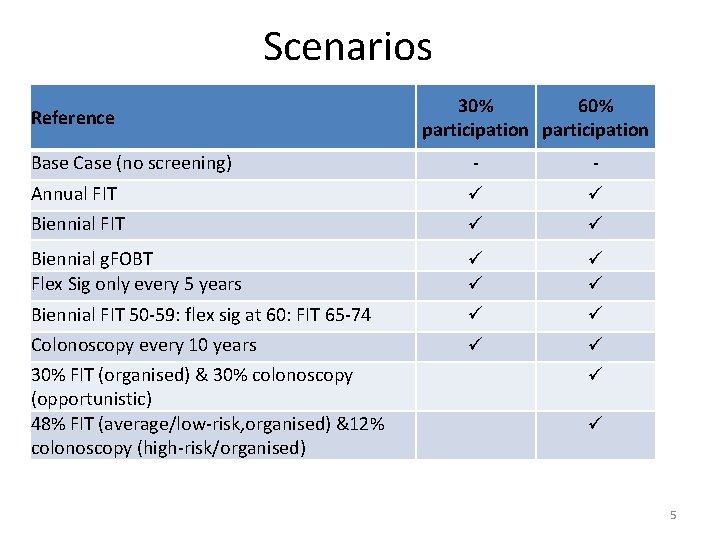
Scenarios Reference 30% 60% participation Base Case (no screening) - - Annual FIT Biennial g. FOBT Flex Sig only every 5 years Biennial FIT 50 -59: flex sig at 60: FIT 65 -74 Colonoscopy every 10 years 30% FIT (organised) & 30% colonoscopy (opportunistic) 48% FIT (average/low-risk, organised) &12% colonoscopy (high-risk/organised) 5

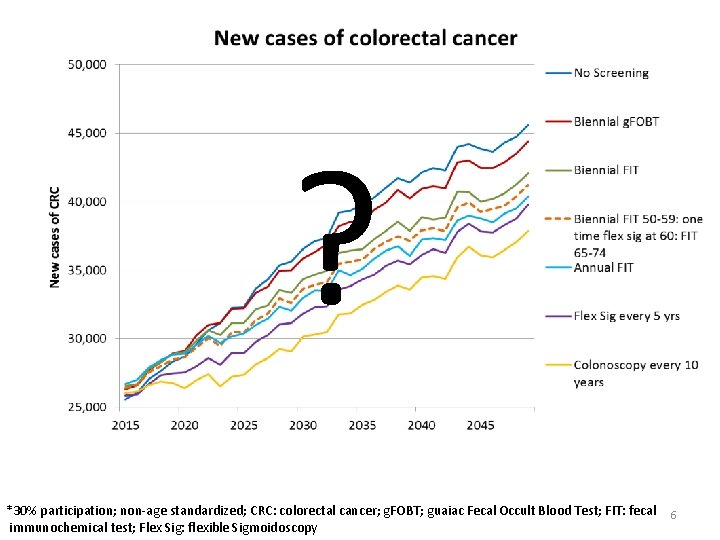
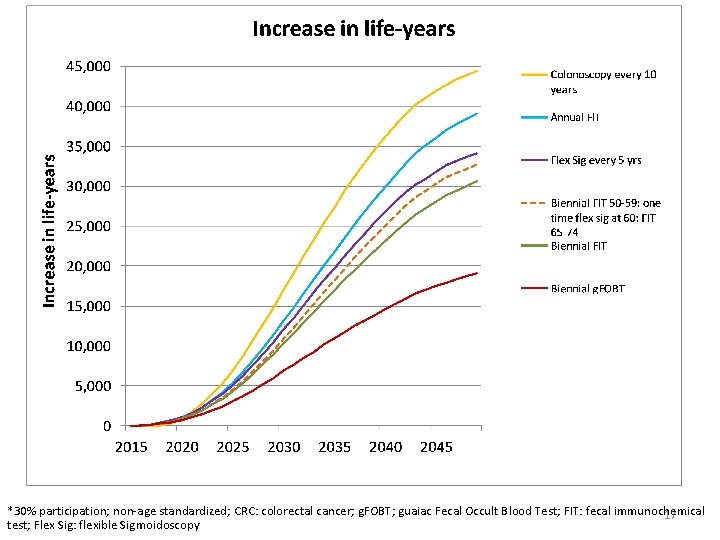
? *30% participation; non-age standardized; CRC: colorectal cancer; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test; Flex Sig: flexible Sigmoidoscopy 6

CRC Incidence not helpful. . . 7

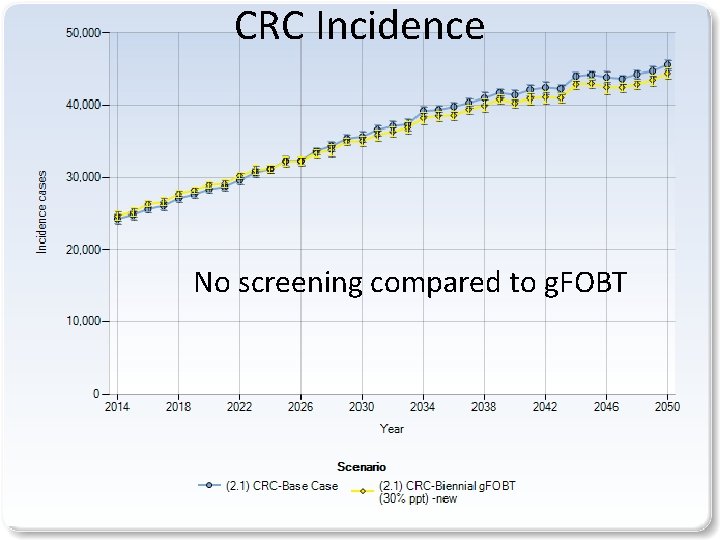
CRC Incidence No screening compared to g. FOBT 8

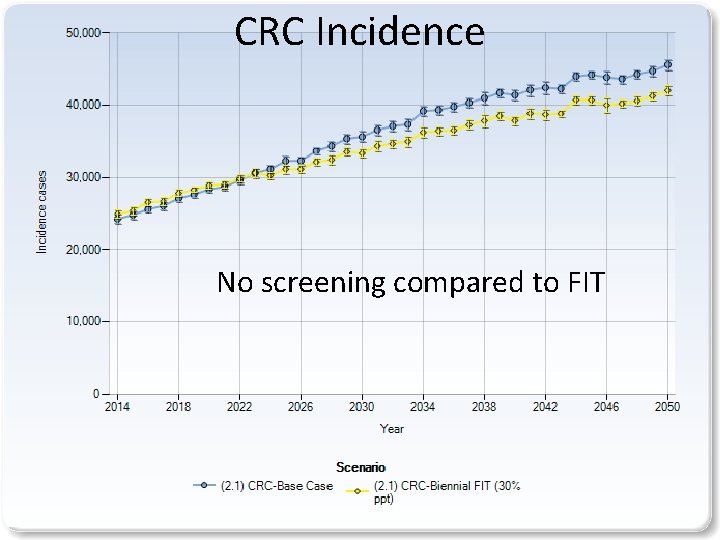
CRC Incidence No screening compared to FIT 9

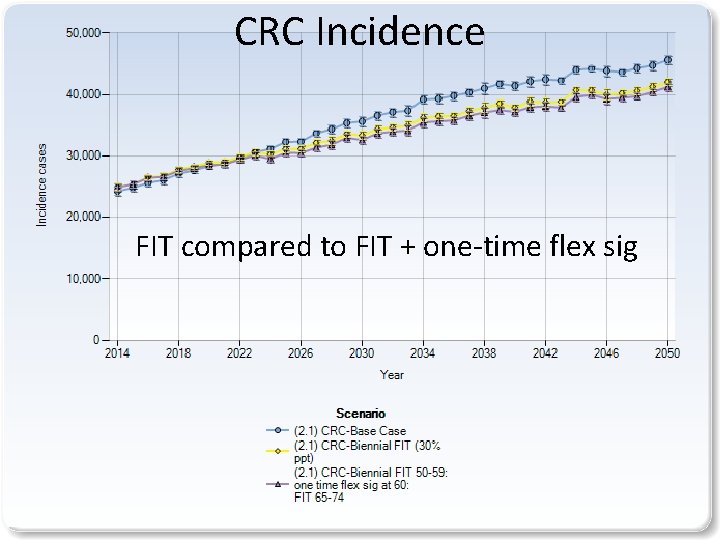
CRC Incidence FIT compared to FIT + one-time flex sig 10

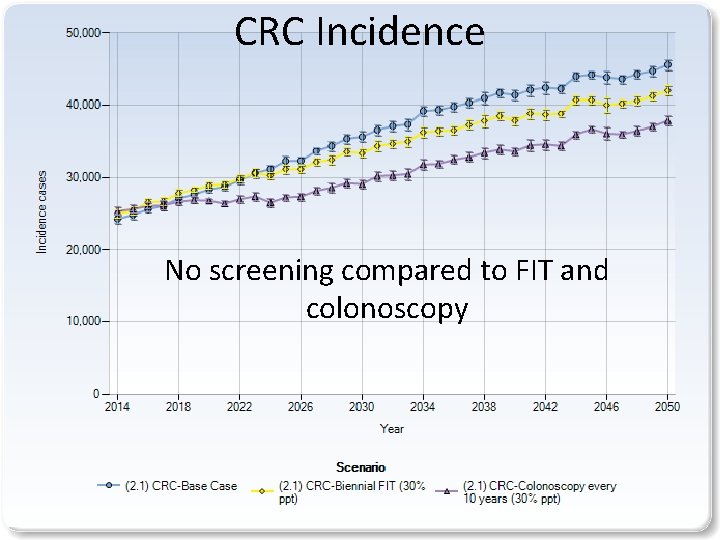
CRC Incidence No screening compared to FIT and colonoscopy 11

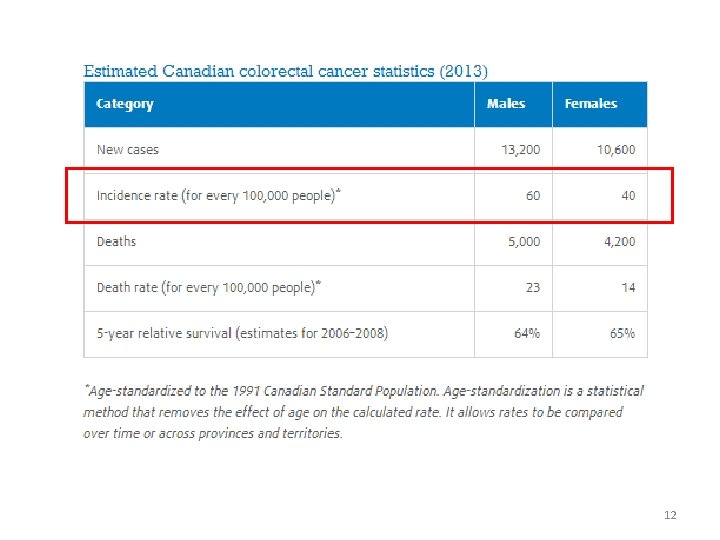
12

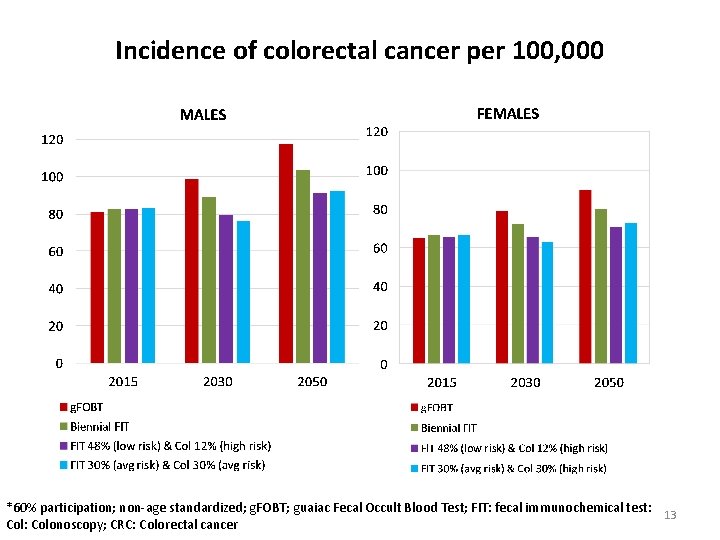
Incidence of colorectal cancer per 100, 000 *60% participation; non-age standardized; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test: 13 Col: Colonoscopy; CRC: Colorectal cancer

*30% participation; non-age standardized; CRC: colorectal cancer: g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal 14 immunochemical test; Flex Sig: flexible Sigmoidoscopy

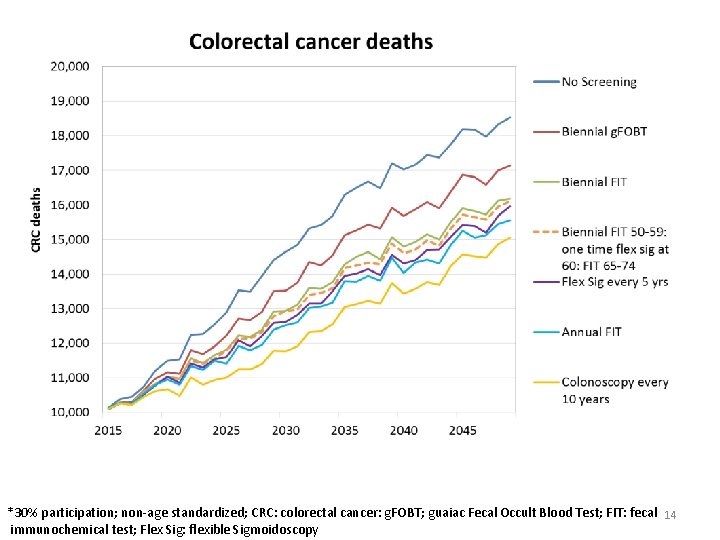
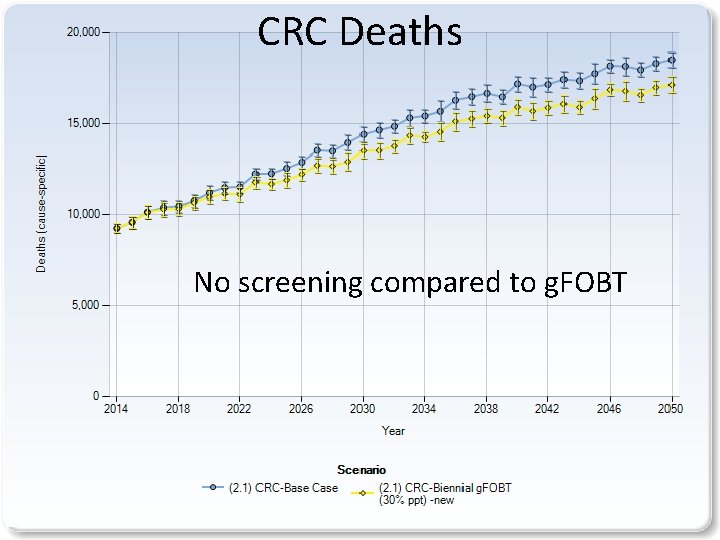
CRC Deaths No screening compared to g. FOBT 15

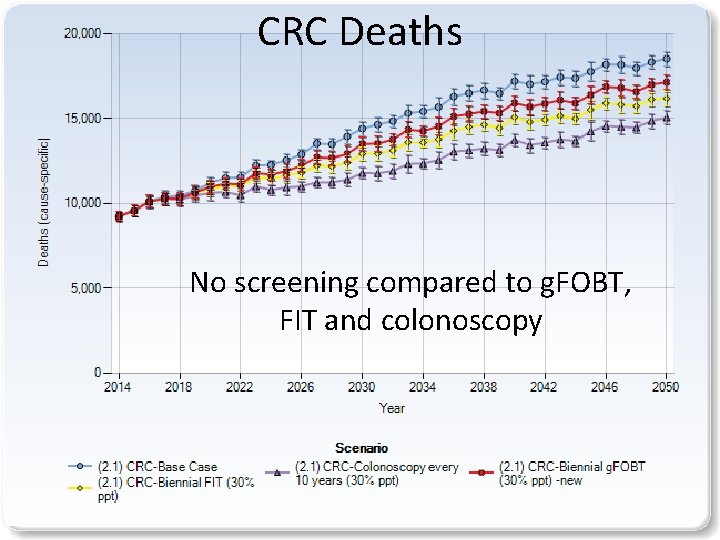
CRC Deaths No screening compared to g. FOBT, FIT and colonoscopy 16

*30% participation; non-age standardized; CRC: colorectal cancer; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical 17 test; Flex Sig: flexible Sigmoidoscopy

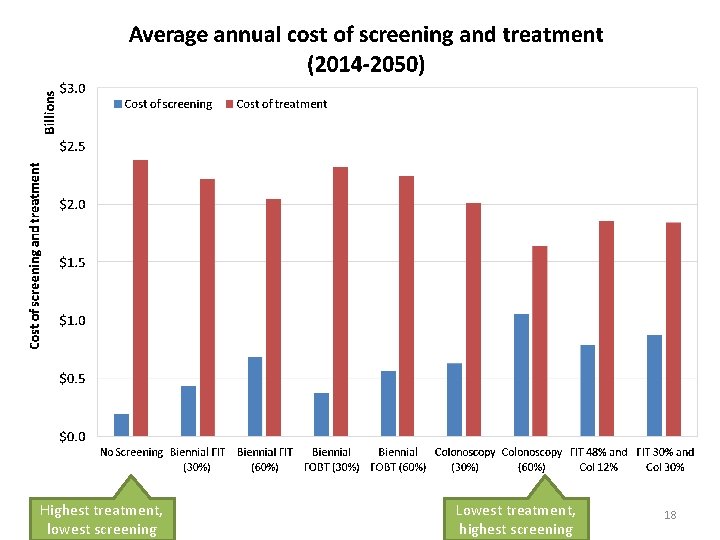
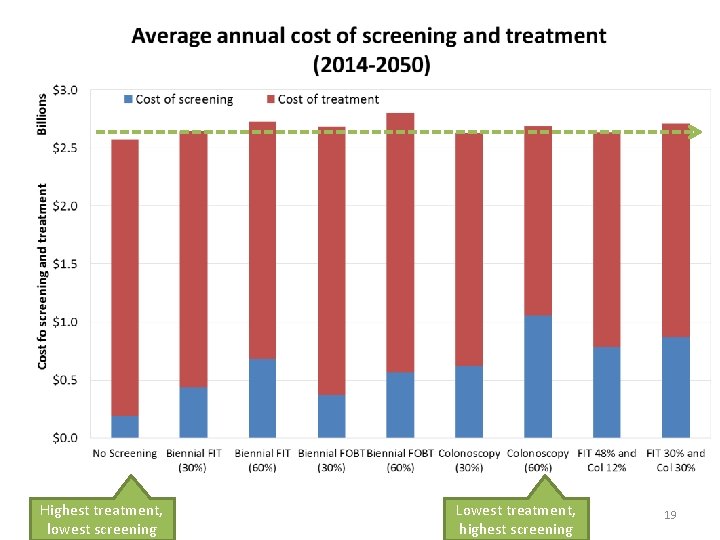
Highest treatment, lowest screening Lowest treatment, highest screening 18

Highest treatment, lowest screening Lowest treatment, highest screening 19

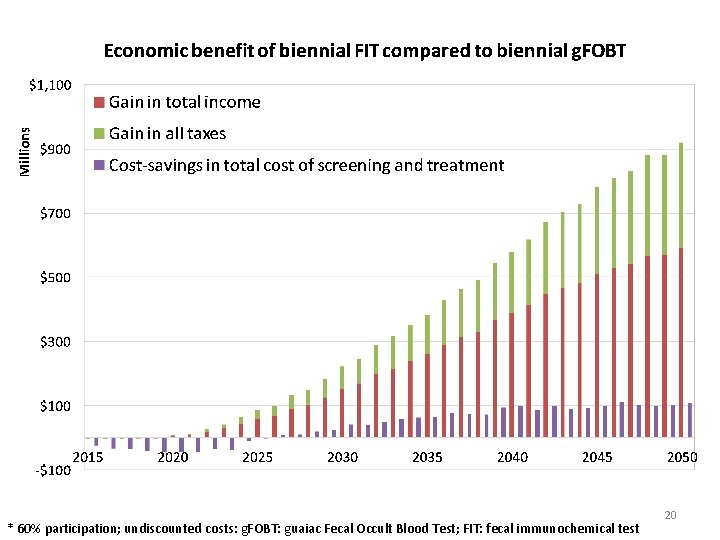
* 60% participation; undiscounted costs: g. FOBT: guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test 20

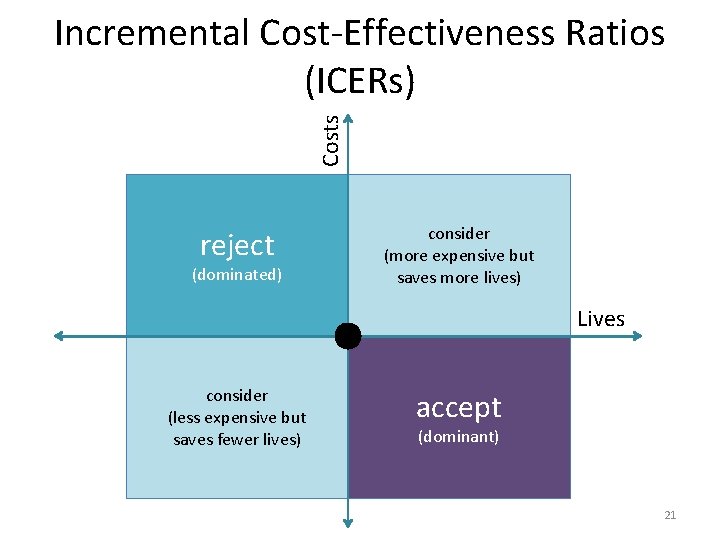
Costs Incremental Cost-Effectiveness Ratios (ICERs) reject (dominated) consider (more expensive but saves more lives) Lives consider (less expensive but saves fewer lives) accept (dominant) 21

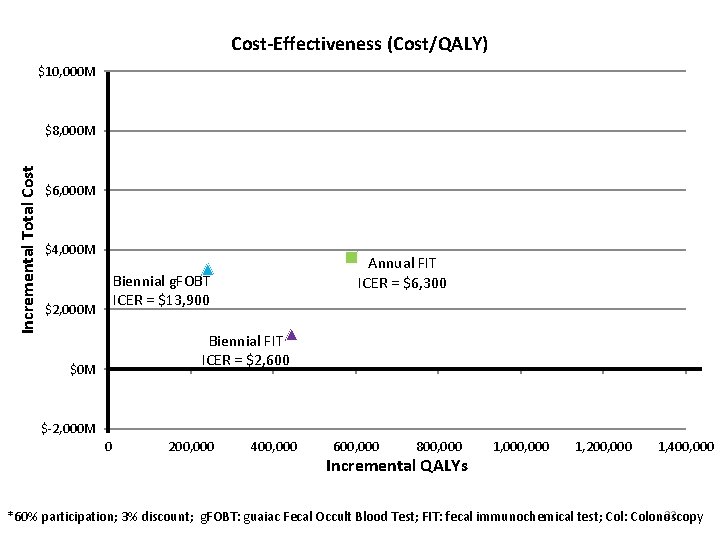
Cost-Effectiveness (Cost/QALY) $10, 000 M Incremental Total Cost $8, 000 M $6, 000 M $4, 000 M Annual FIT ICER = $6, 300 Biennial g. FOBT ICER = $13, 900 $2, 000 M Biennial FIT ICER = $2, 600 $0 M $-2, 000 M 0 200, 000 400, 000 600, 000 800, 000 Incremental QALYs 1, 000 1, 200, 000 1, 400, 000 22 *60% participation; 3% discount; g. FOBT: guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test; Col: Colonoscopy

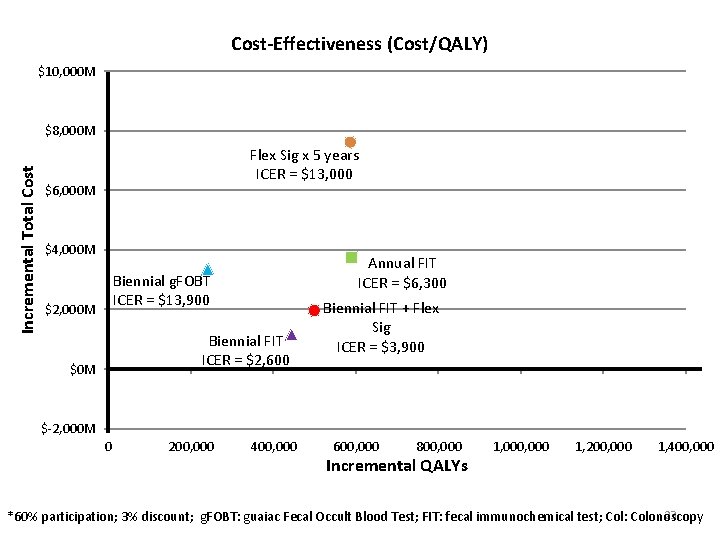
Cost-Effectiveness (Cost/QALY) $10, 000 M Incremental Total Cost $8, 000 M Flex Sig x 5 years ICER = $13, 000 $6, 000 M $4, 000 M Annual FIT ICER = $6, 300 Biennial g. FOBT ICER = $13, 900 $2, 000 M Biennial FIT ICER = $2, 600 $0 M Biennial FIT + Flex Sig ICER = $3, 900 $-2, 000 M 0 200, 000 400, 000 600, 000 800, 000 Incremental QALYs 1, 000 1, 200, 000 1, 400, 000 23 *60% participation; 3% discount; g. FOBT: guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test; Col: Colonoscopy

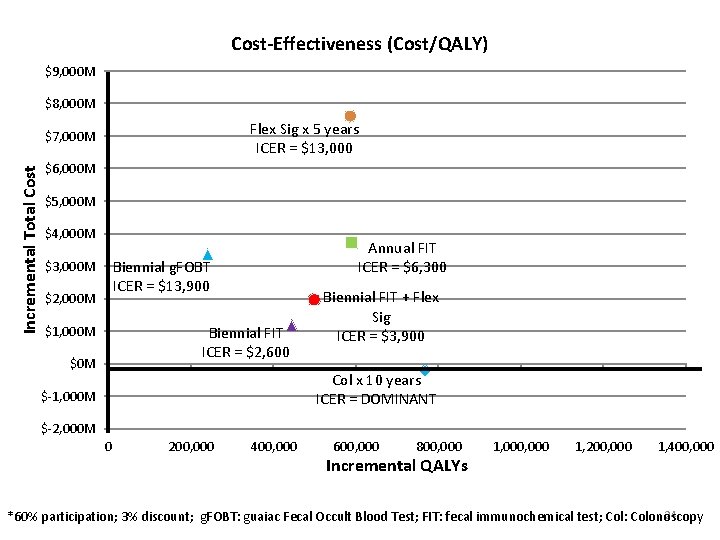
Cost-Effectiveness (Cost/QALY) $9, 000 M $8, 000 M Flex Sig x 5 years ICER = $13, 000 Incremental Total Cost $7, 000 M $6, 000 M $5, 000 M $4, 000 M Annual FIT ICER = $6, 300 Biennial g. FOBT ICER = $13, 900 $3, 000 M $2, 000 M Biennial FIT ICER = $2, 600 $1, 000 M $0 M Biennial FIT + Flex Sig ICER = $3, 900 Col x 10 years ICER = DOMINANT $-1, 000 M $-2, 000 M 0 200, 000 400, 000 600, 000 800, 000 Incremental QALYs 1, 000 1, 200, 000 1, 400, 000 24 *60% participation; 3% discount; g. FOBT: guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test; Col: Colonoscopy

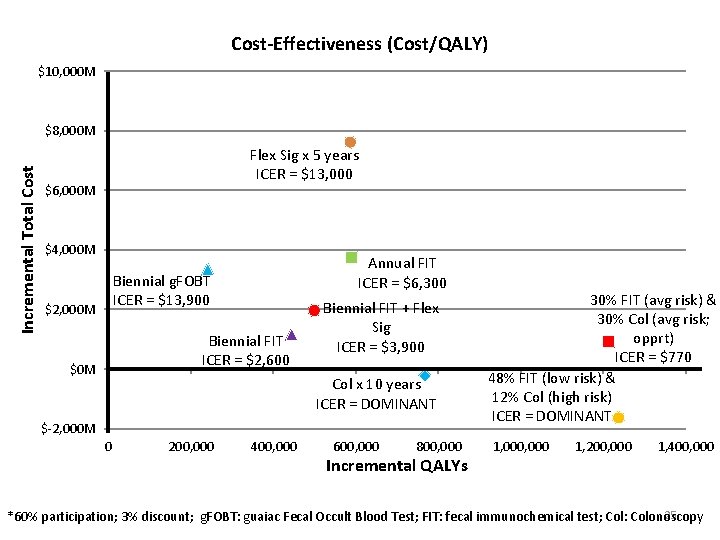
Cost-Effectiveness (Cost/QALY) $10, 000 M Incremental Total Cost $8, 000 M Flex Sig x 5 years ICER = $13, 000 $6, 000 M $4, 000 M Annual FIT ICER = $6, 300 Biennial g. FOBT ICER = $13, 900 $2, 000 M Biennial FIT ICER = $2, 600 $0 M Biennial FIT + Flex Sig ICER = $3, 900 Col x 10 years ICER = DOMINANT $-2, 000 M 0 200, 000 400, 000 600, 000 800, 000 Incremental QALYs 30% FIT (avg risk) & 30% Col (avg risk; opprt) ICER = $770 48% FIT (low risk) & 12% Col (high risk) ICER = DOMINANT 1, 000 1, 200, 000 1, 400, 000 25 *60% participation; 3% discount; g. FOBT: guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test; Col: Colonoscopy

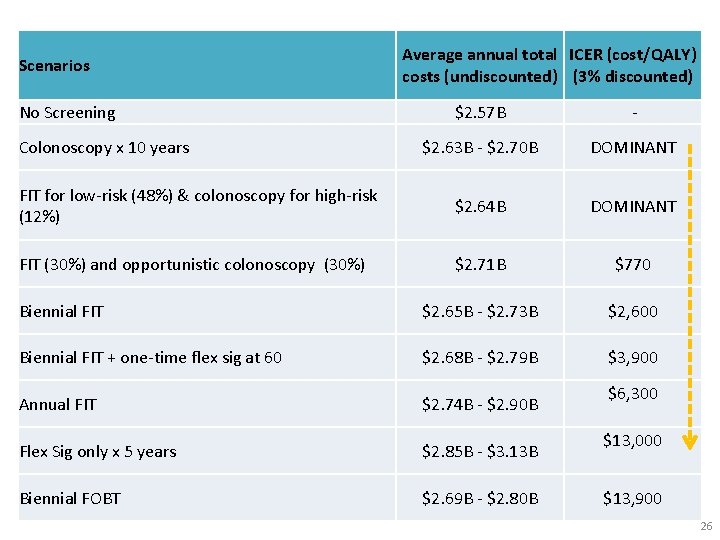
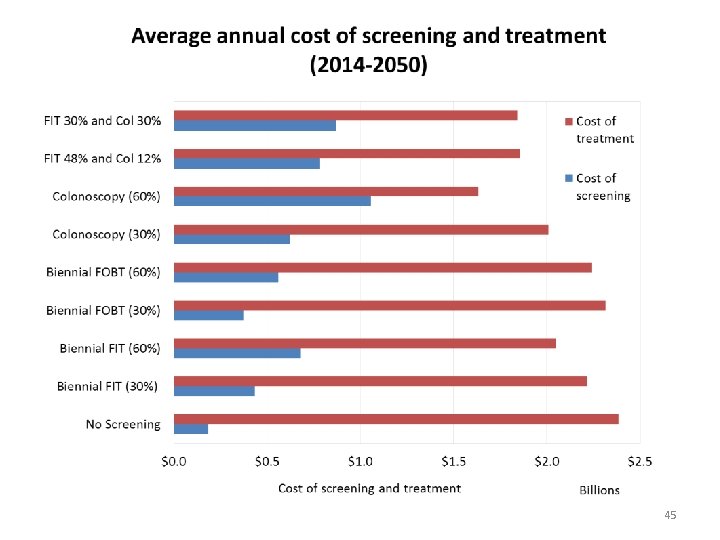
Scenarios No Screening Average annual total ICER (cost/QALY) costs (undiscounted) (3% discounted) $2. 57 B - $2. 63 B - $2. 70 B DOMINANT FIT for low-risk (48%) & colonoscopy for high-risk (12%) $2. 64 B DOMINANT FIT (30%) and opportunistic colonoscopy (30%) $2. 71 B $770 Biennial FIT $2. 65 B - $2. 73 B $2, 600 Biennial FIT + one-time flex sig at 60 $2. 68 B - $2. 79 B $3, 900 Annual FIT $2. 74 B - $2. 90 B Flex Sig only x 5 years $2. 85 B - $3. 13 B Biennial FOBT $2. 69 B - $2. 80 B Colonoscopy x 10 years $6, 300 $13, 000 $13, 900 26

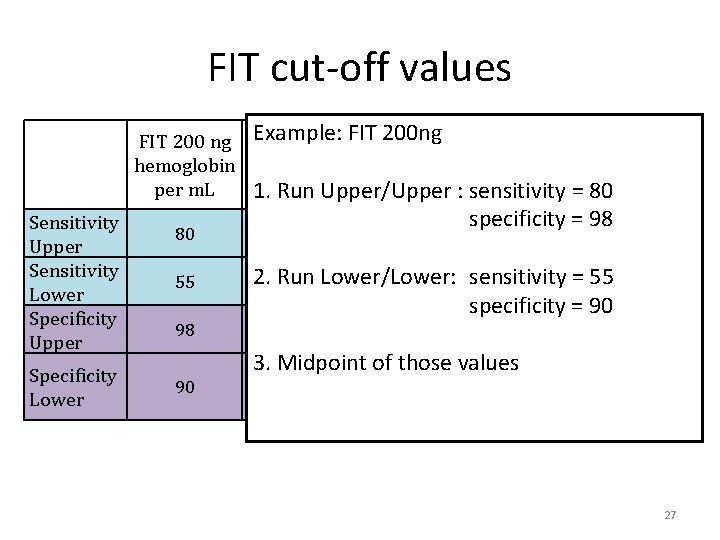
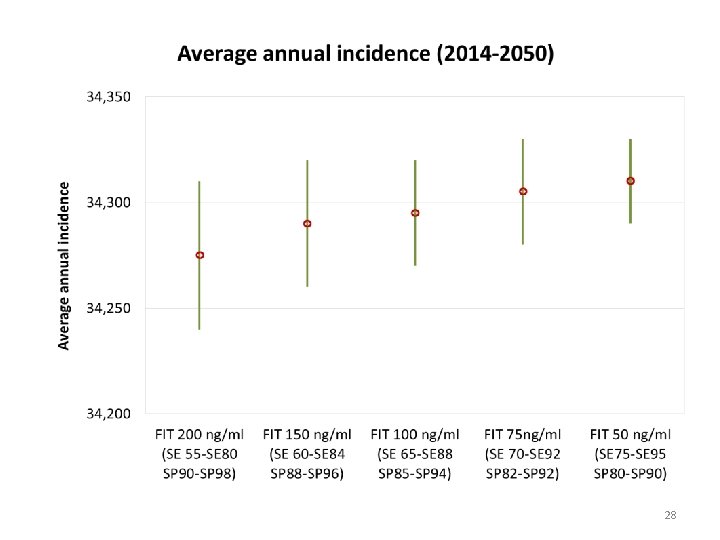
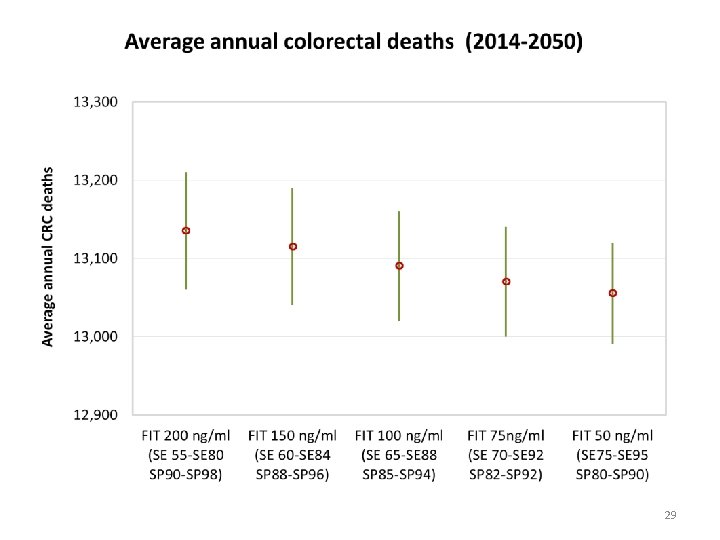
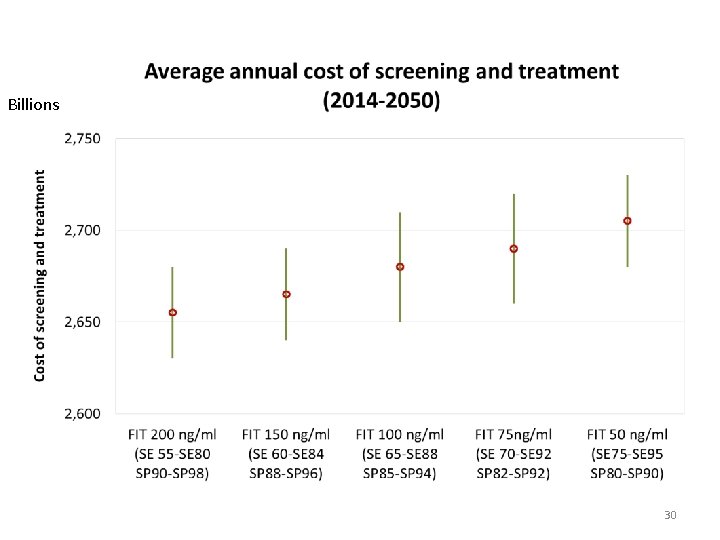
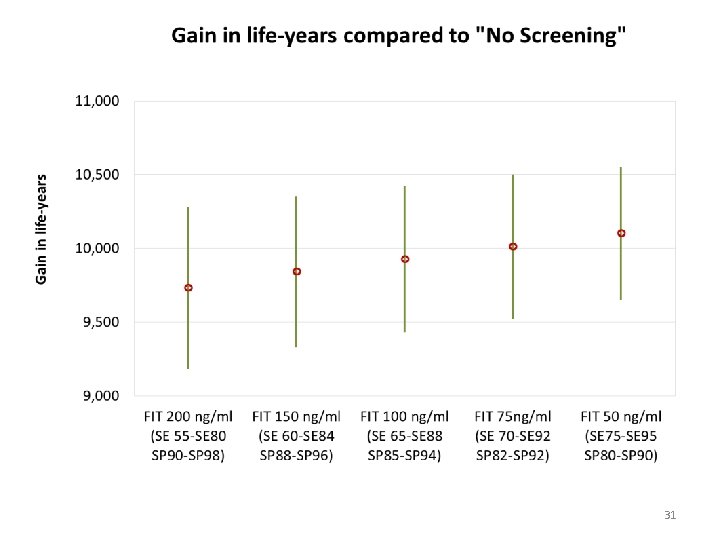
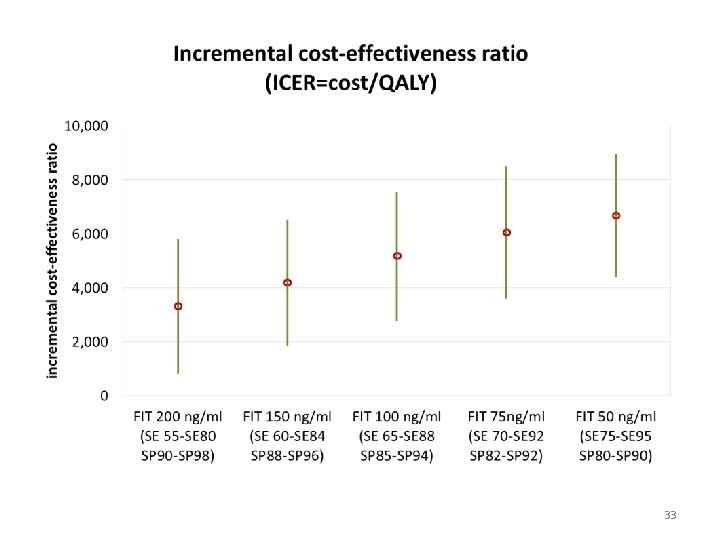
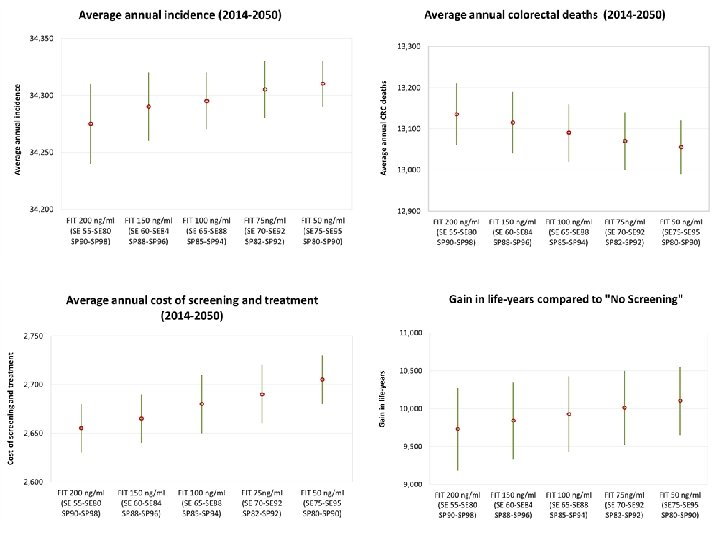
FIT cut-off values FIT 200 ng Example: FIT 200 ng FIT 150 ng FIT 100 ng FIT 75 ng FIT 50 ng hemoglobin hemoglobin per m. L 1. Run Upper/Upper : sensitivity = 80 Sensitivity Upper Sensitivity Lower Specificity Upper Specificity Lower 80 55 98 90 84 88 specificity = 98 92 2. Run Lower/Lower: sensitivity = 55 60 65 70 specificity = 90 96 94 3. Midpoint of those values 88 85 95 75 92 90 82 80 27

28

29

Billions 30

31

32

33

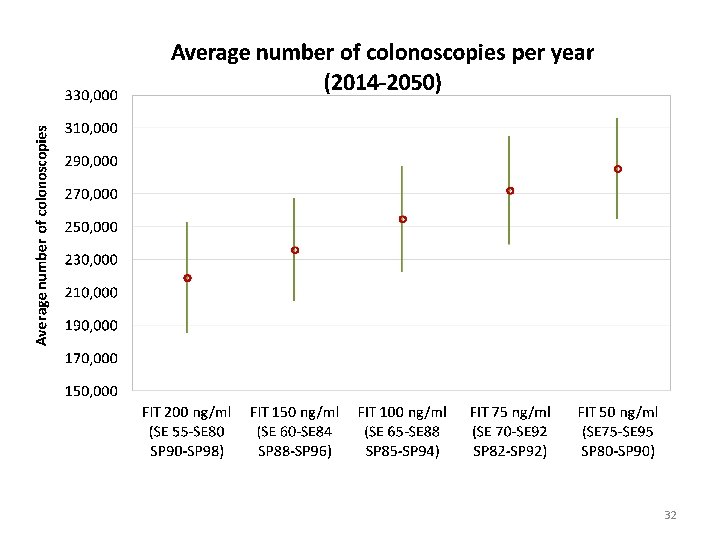
Conclusions • Colonoscopy most cost-effective, g. FOBT least • FIT reasonably cost-effective, especially when high-risk patients are directed to colonoscopy • FIT cut-offs have small impact on health outcomes or total overall cost, but significant implications on # colonoscopies 34

Limitations • Basecase scenario assumes no screening, but we know some opportunistic screening occurred in some provinces • Programmatic screening needs to be incorporated into basecase • Overhead costs of increasing colonoscopy capacity not incorporated 35

Additional work • Continue exploring FIT cut-off thresholds • Explore impact of # samples collected • Report to be disseminated with jurisdictional results 36

https: //cancerview. ca/cancerriskmanagement Natalie Fitzgerald, Program Manager, Economics, CRM natalie. fitzgerald@partnershipagainstcancer. ca 416 -619 -5780 37

APPENDIX 38

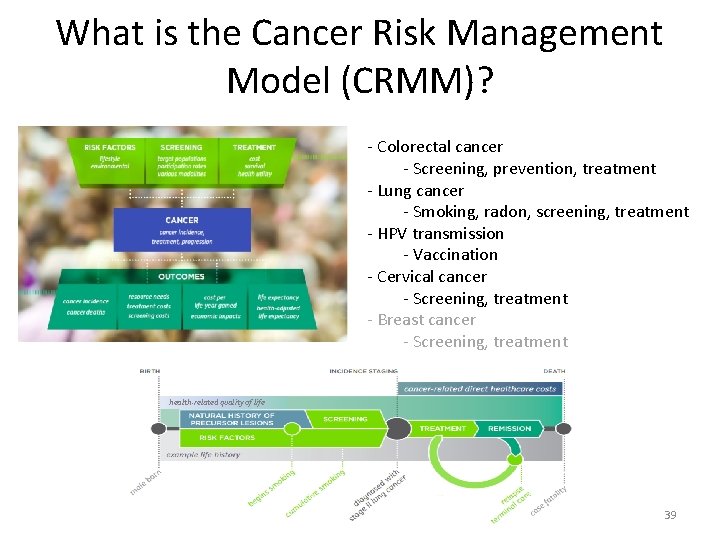
What is the Cancer Risk Management Model (CRMM)? - Colorectal cancer - Screening, prevention, treatment - Lung cancer - Smoking, radon, screening, treatment - HPV transmission - Vaccination - Cervical cancer - Screening, treatment - Breast cancer - Screening, treatment health-related quality of life 39

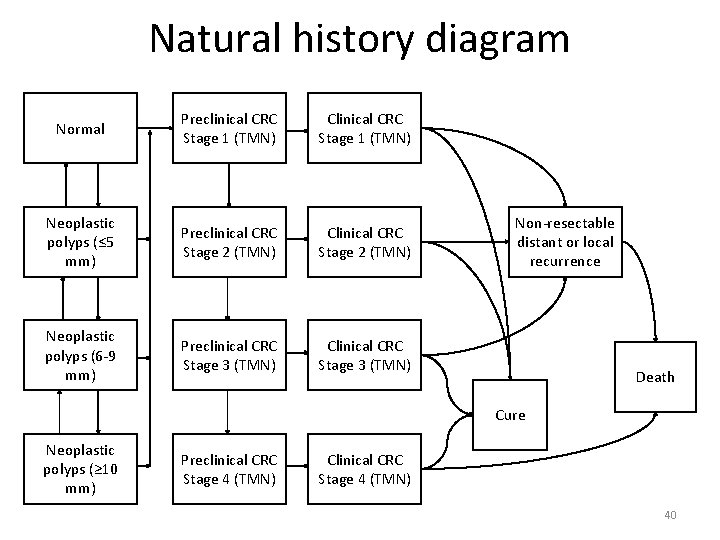
Natural history diagram Normal Preclinical CRC Stage 1 (TMN) Clinical CRC Stage 1 (TMN) Neoplastic polyps (≤ 5 mm) Preclinical CRC Stage 2 (TMN) Clinical CRC Stage 2 (TMN) Neoplastic polyps (6 -9 mm) Preclinical CRC Stage 3 (TMN) Clinical CRC Stage 3 (TMN) Non-resectable distant or local recurrence Death Cure Neoplastic polyps (≥ 10 mm) Preclinical CRC Stage 4 (TMN) Clinical CRC Stage 4 (TMN) 40

Publications • William K. Evans, Michael C. Wolfson, William M. Flanagan, Janey Shin, John Goffin, Anthony B. Miller, Keiko Asakawa, Craig Earle, Nicole Mittmann, Lee Fairclough, Jillian Oderkirk, Philippe Finès, Stephen Gribble, Jeffrey Hoch, Chantal Hicks, D. Walter R. Omariba and Edward Ng (2013). Canadian Cancer Risk Management Model: evaluation of cancer control. International Journal of Technology Assessment in Health Care, 29, pp 131 -139. • William K. Evans, Michael Wolfson, William M. Flanagan, Janey Shin, John R. Goffin, Keiko Asakawa, Craig Earle, Nicole Mittmann, Lee Fairclough, Philippe Finès, Steve Gribble, Jeffrey Hoch, Chantal Hicks, Walter D. R. Omariba & Edward Ng (2012). The evaluation of cancer control interventions in lung cancer using the Canadian Cancer Risk Management Model. Lung Cancer Management, 1: 1 pp 25 -33. 41

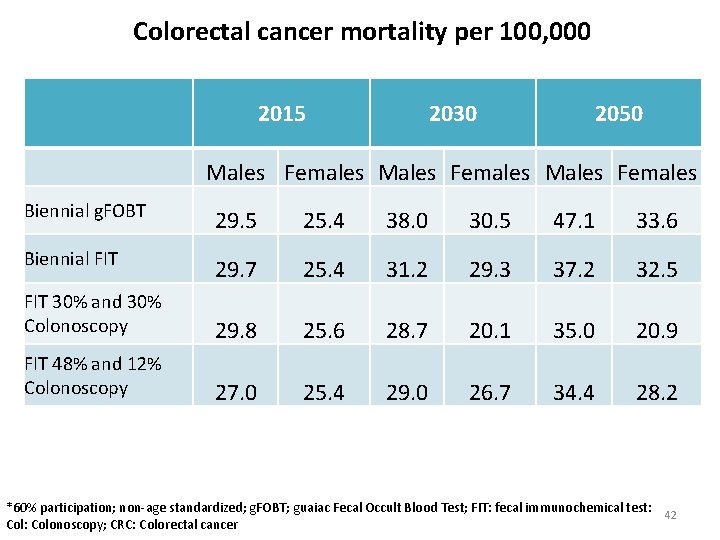
Colorectal cancer mortality per 100, 000 2015 2030 2050 Males Females Biennial g. FOBT 29. 5 25. 4 38. 0 30. 5 47. 1 33. 6 Biennial FIT 29. 7 25. 4 31. 2 29. 3 37. 2 32. 5 FIT 30% and 30% Colonoscopy 29. 8 25. 6 28. 7 20. 1 35. 0 20. 9 FIT 48% and 12% Colonoscopy 27. 0 25. 4 29. 0 26. 7 34. 4 28. 2 *60% participation; non-age standardized; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test: 42 Col: Colonoscopy; CRC: Colorectal cancer

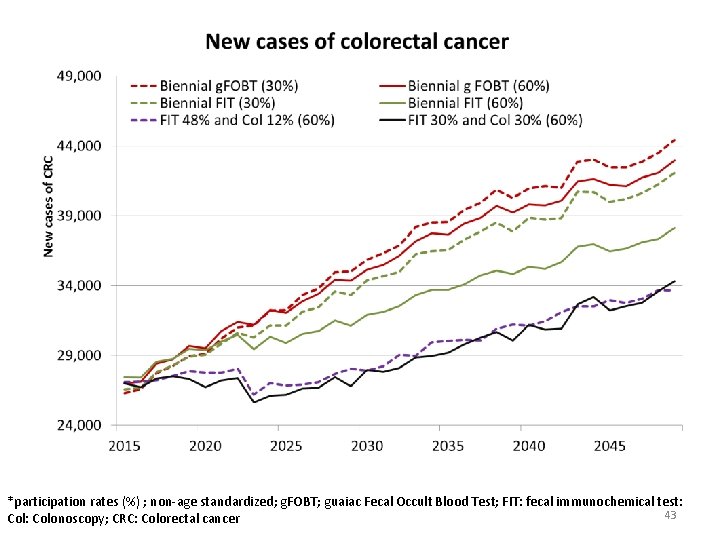
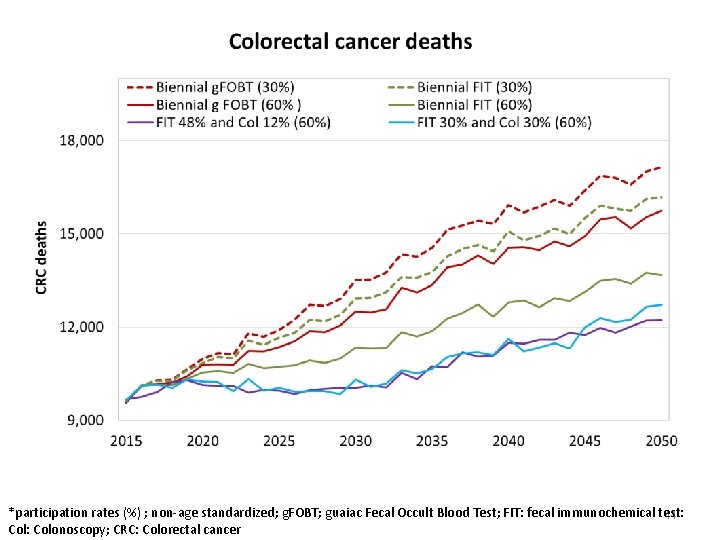
*participation rates (%) ; non-age standardized; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test: 43 Col: Colonoscopy; CRC: Colorectal cancer

*participation rates (%) ; non-age standardized; g. FOBT; guaiac Fecal Occult Blood Test; FIT: fecal immunochemical test: 44 Col: Colonoscopy; CRC: Colorectal cancer

45

46

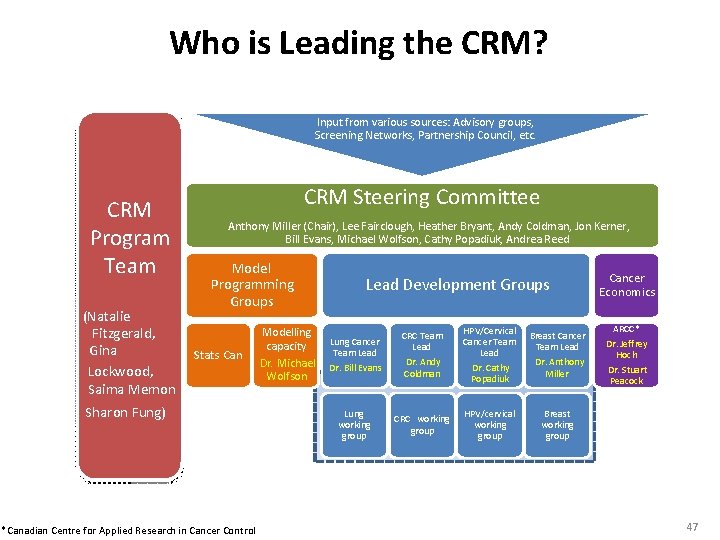
Who is Leading the CRM? Input from various sources: Advisory groups, Screening Networks, Partnership Council, etc. CRM Program Team (Natalie Fitzgerald, Gina Lockwood, Saima Memon CRM Steering Committee Anthony Miller (Chair), Lee Fairclough, Heather Bryant, Andy Coldman, Jon Kerner, Bill Evans, Michael Wolfson, Cathy Popadiuk, Andrea Reed Model Programming Groups Stats Can Sharon Fung) *Canadian Centre for Applied Research in Cancer Control Lead Development Groups Modelling Lung Cancer capacity Team Lead Dr. Michael Dr. Bill Evans Wolfson Lung working group CRC Team Lead Dr. Andy Coldman CRC working group HPV/Cervical Cancer Team Lead Dr. Cathy Popadiuk HPV/cervical working group Breast Cancer Team Lead Dr. Anthony Miller Cancer Economics ARCC* Dr. Jeffrey Hoch Dr. Stuart Peacock Breast working group 47

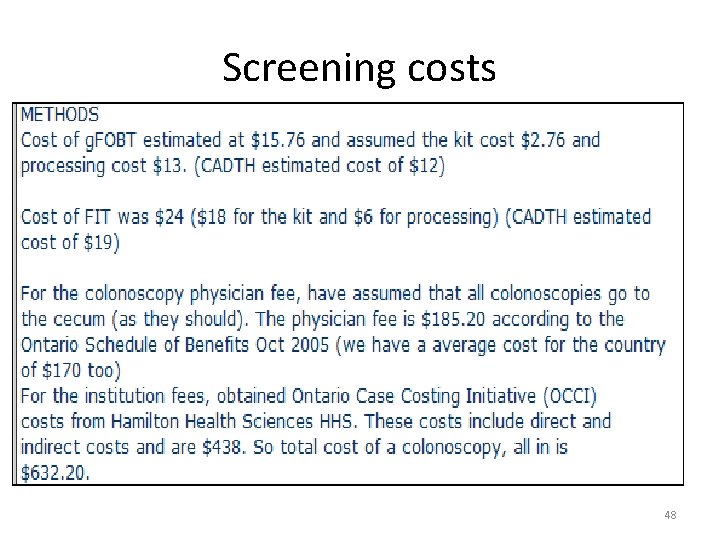
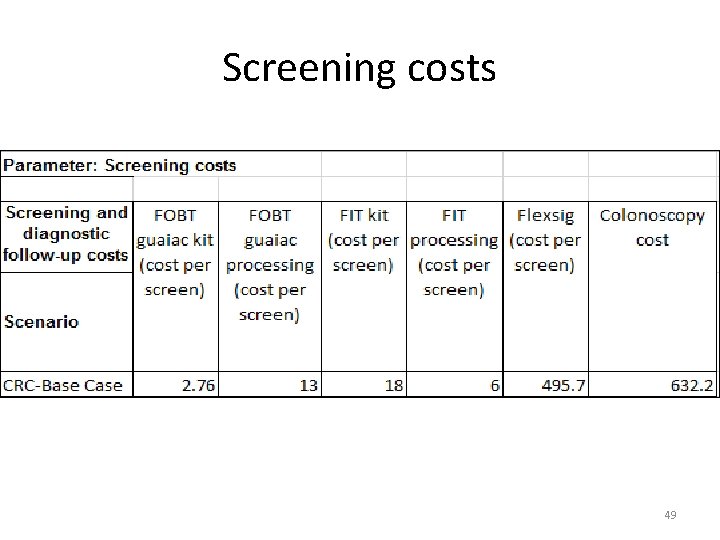
Screening costs 48

Screening costs 49

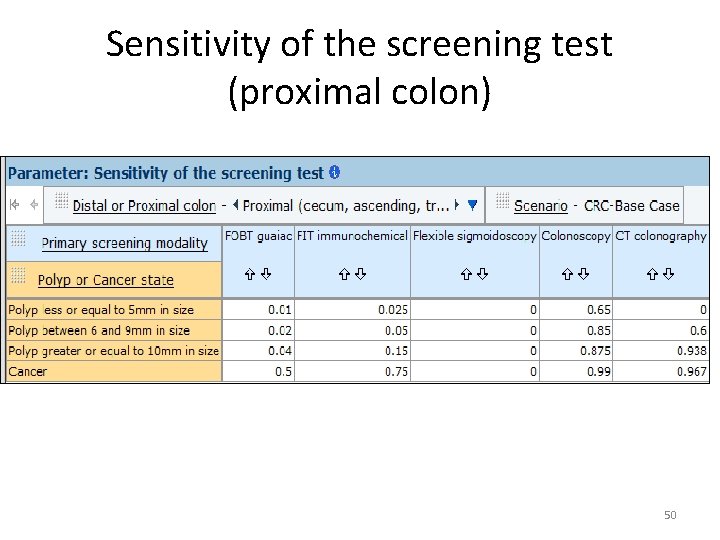
Sensitivity of the screening test (proximal colon) 50

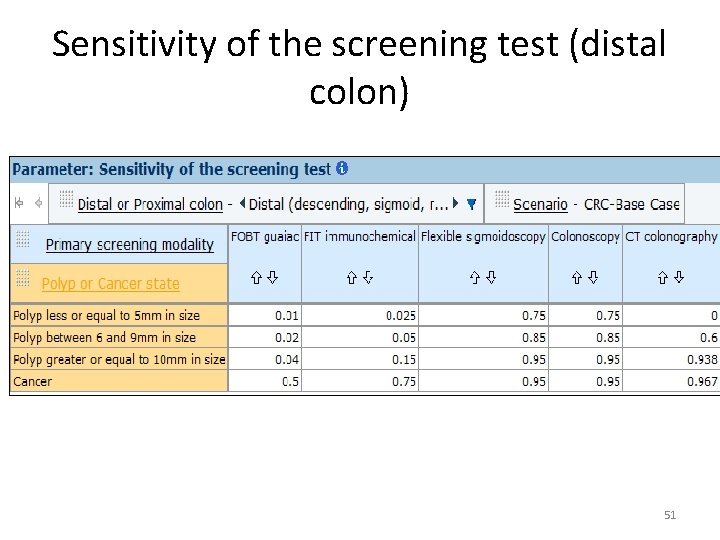
Sensitivity of the screening test (distal colon) 51

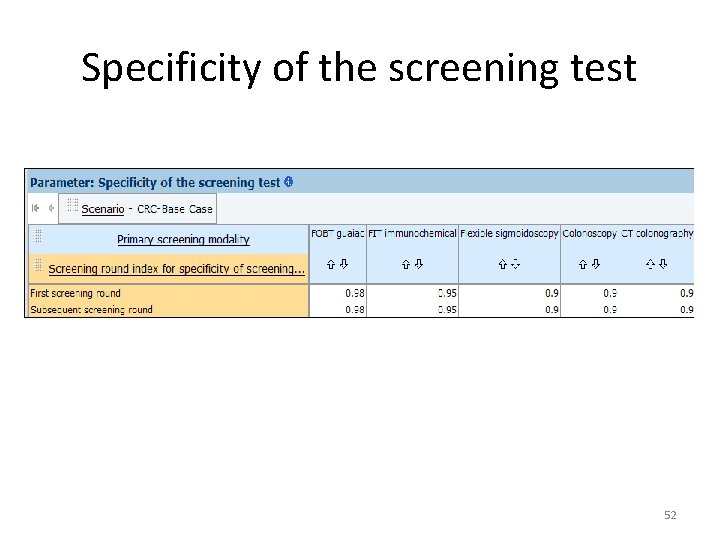
Specificity of the screening test 52
 Colorectal cancer labs
Colorectal cancer labs Colorectal cancer drug trial
Colorectal cancer drug trial Colorectal cancer
Colorectal cancer Omprov cellprov
Omprov cellprov Ann lyons colorectal surgeon
Ann lyons colorectal surgeon Neoplasia
Neoplasia Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool National breast and cervical cancer early detection program
National breast and cervical cancer early detection program National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Recompetition
Recompetition Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Sju för caesar
Sju för caesar Stig kerman
Stig kerman Informationskartläggning
Informationskartläggning Matematisk modellering eksempel
Matematisk modellering eksempel Tack för att ni har lyssnat
Tack för att ni har lyssnat Mitos steg
Mitos steg Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Cks
Cks Karttecken brant
Karttecken brant Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Påbyggnader för flakfordon
Påbyggnader för flakfordon A gastrica
A gastrica Egg för emanuel
Egg för emanuel Formel gruplar
Formel gruplar Rutin för avvikelsehantering
Rutin för avvikelsehantering Formuö
Formuö Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Frgar
Frgar Presentera för publik crossboss
Presentera för publik crossboss Tack för att ni lyssnade
Tack för att ni lyssnade Debattartikel struktur
Debattartikel struktur Tobinskatten för och nackdelar
Tobinskatten för och nackdelar En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Tack för att ni har lyssnat
Tack för att ni har lyssnat Vad är referatmarkeringar
Vad är referatmarkeringar Skapa med geometriska former
Skapa med geometriska former Bris för vuxna
Bris för vuxna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? Byggprocessen steg för steg
Byggprocessen steg för steg Fuktmätningar i betong enlig rbk
Fuktmätningar i betong enlig rbk Kraft per area
Kraft per area Kung som dog 1611
Kung som dog 1611 Densitet vatten
Densitet vatten Elektronik för barn
Elektronik för barn Tack för att ni har lyssnat
Tack för att ni har lyssnat Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Typiska novell drag
Typiska novell drag Fimbrietratt
Fimbrietratt Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Delegerande ledarskap
Delegerande ledarskap Tallinjen
Tallinjen Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader