3 FUNDAMENTALS OF GASPHASE REACTIONS AND PHOTOCHEMISTRY 1












- Slides: 75

3) FUNDAMENTALS OF GAS-PHASE REACTIONS AND PHOTOCHEMISTRY 1

3) FUNDAMENTALS OF GAS-PHASE REACTIONS AND PHOTOCHEMISTRY What do we know so far? LOTS OF REACTIVE TRACE GASES !! Natural sources – NO from soil and lightning, many hydrocarbons (isoprene) from plants, sulfur species from oceans, … Anthropogenic sources – hydrocarbons, NO, … YIPPEE!! Chemistry! The atmosphere needs a way to remove these species. 2

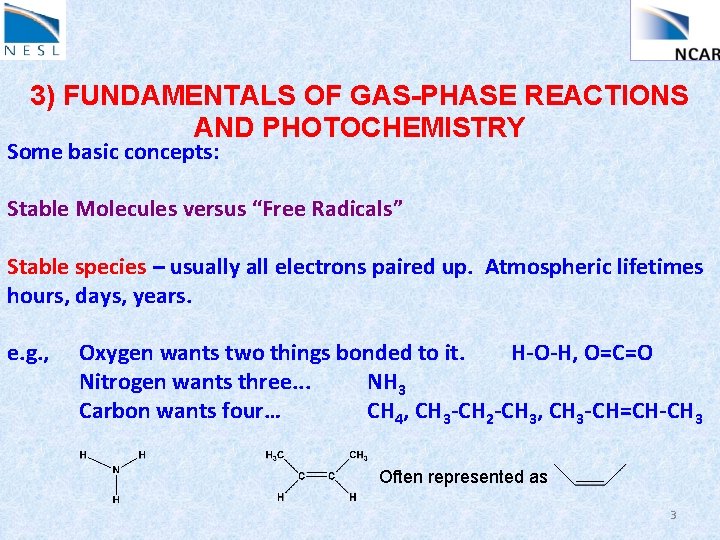
3) FUNDAMENTALS OF GAS-PHASE REACTIONS AND PHOTOCHEMISTRY Some basic concepts: Stable Molecules versus “Free Radicals” Stable species – usually all electrons paired up. Atmospheric lifetimes hours, days, years. e. g. , Oxygen wants two things bonded to it. H-O-H, O=C=O Nitrogen wants three. . . NH 3 Carbon wants four… CH 4, CH 3 -CH 2 -CH 3, CH 3 -CH=CH-CH 3 Often represented as 3

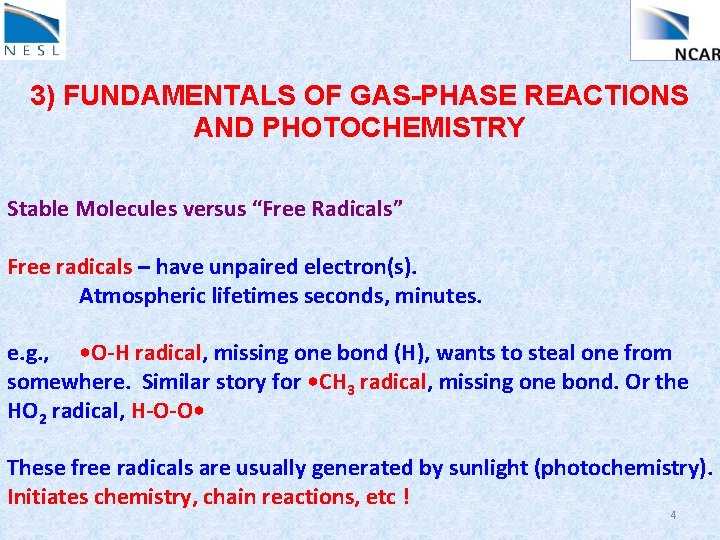
3) FUNDAMENTALS OF GAS-PHASE REACTIONS AND PHOTOCHEMISTRY Stable Molecules versus “Free Radicals” Free radicals – have unpaired electron(s). Atmospheric lifetimes seconds, minutes. e. g. , • O-H radical, missing one bond (H), wants to steal one from somewhere. Similar story for • CH 3 radical, missing one bond. Or the HO 2 radical, H-O-O • These free radicals are usually generated by sunlight (photochemistry). Initiates chemistry, chain reactions, etc ! 4

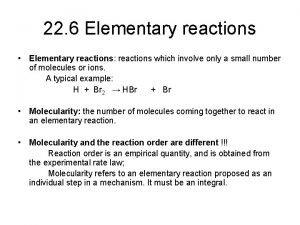
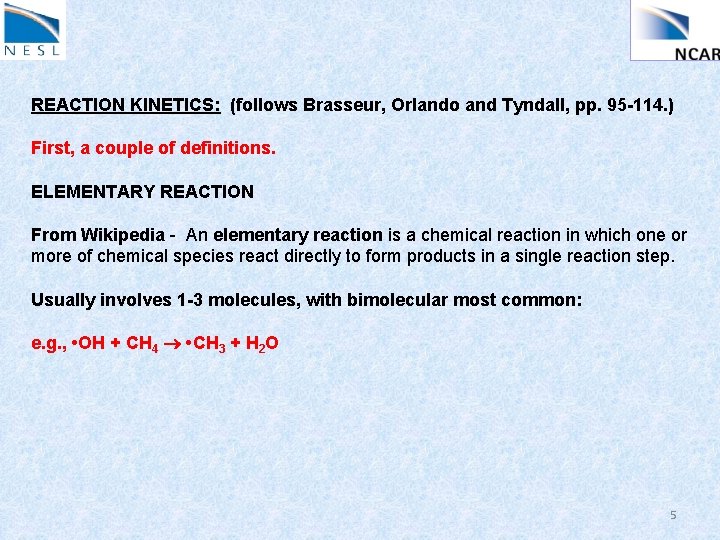
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) First, a couple of definitions. ELEMENTARY REACTION From Wikipedia - An elementary reaction is a chemical reaction in which one or more of chemical species react directly to form products in a single reaction step. Usually involves 1 -3 molecules, with bimolecular most common: e. g. , • OH + CH 4 • CH 3 + H 2 O 5

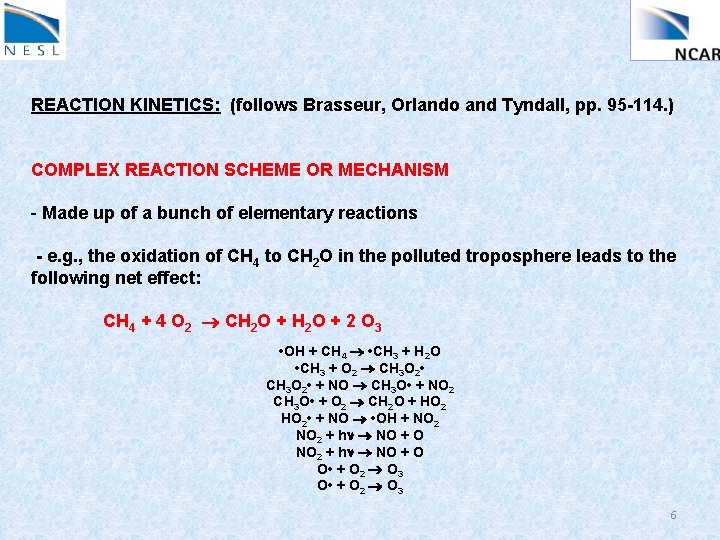
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) COMPLEX REACTION SCHEME OR MECHANISM - Made up of a bunch of elementary reactions - e. g. , the oxidation of CH 4 to CH 2 O in the polluted troposphere leads to the following net effect: CH 4 + 4 O 2 CH 2 O + 2 O 3 • OH + CH 4 • CH 3 + H 2 O • CH 3 + O 2 CH 3 O 2 • + NO CH 3 O • + NO 2 CH 3 O • + O 2 CH 2 O + HO 2 • + NO • OH + NO 2 + hn NO + O O • + O 2 O 3 6

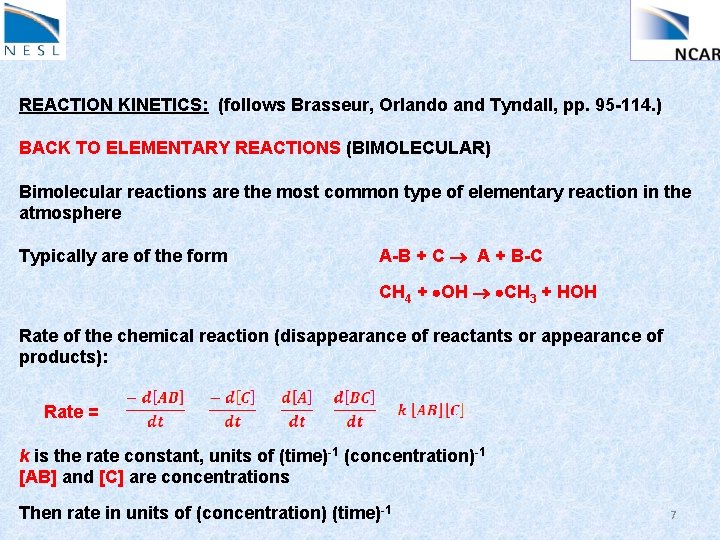
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) BACK TO ELEMENTARY REACTIONS (BIMOLECULAR) Bimolecular reactions are the most common type of elementary reaction in the atmosphere Typically are of the form A-B + C A + B-C CH 4 + OH CH 3 + HOH Rate of the chemical reaction (disappearance of reactants or appearance of products): Rate = k is the rate constant, units of (time)-1 (concentration)-1 [AB] and [C] are concentrations Then rate in units of (concentration) (time)-1 7

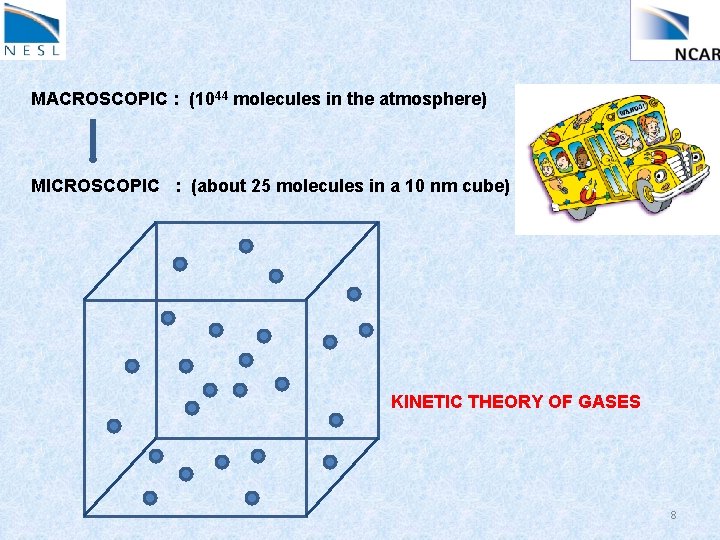
MACROSCOPIC : (1044 molecules in the atmosphere) MICROSCOPIC : (about 25 molecules in a 10 nm cube) KINETIC THEORY OF GASES 8

KINETIC THEORY OF GASES: (most any Physical Chemistry text, including Adamson, 1979; Atkins, 1986). 1) Molecules Move !! (they have kinetic energy): Average Velocity: For N 2, can show that c is about 4 x 104 cm/sec at 298 K (225 miles / hr). They traverse our 10 nm cube in 25 picosec! 9

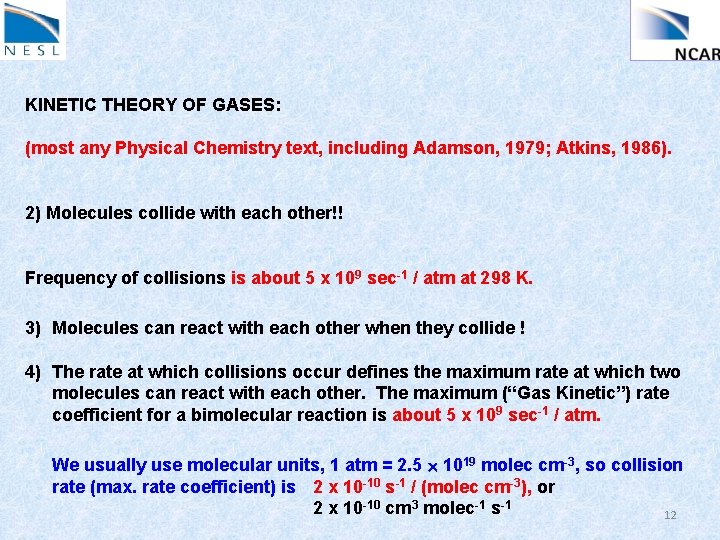
KINETIC THEORY OF GASES: (most any Physical Chemistry text, including Adamson, 1979; Atkins, 1986). 2) Molecules collide with each other!! Frequency of collisions is about 5 x 109 sec-1 / atm at 298 K. 10

KINETIC THEORY OF GASES: (most any Physical Chemistry text, including Adamson, 1979; Atkins, 1986). 2) Molecules collide with each other!! Frequency of collisions is about 5 x 109 sec-1 / atm at 298 K. 3) Molecules can react with each other when they collide ! 11

KINETIC THEORY OF GASES: (most any Physical Chemistry text, including Adamson, 1979; Atkins, 1986). 2) Molecules collide with each other!! Frequency of collisions is about 5 x 109 sec-1 / atm at 298 K. 3) Molecules can react with each other when they collide ! 4) The rate at which collisions occur defines the maximum rate at which two molecules can react with each other. The maximum (“Gas Kinetic”) rate coefficient for a bimolecular reaction is about 5 x 109 sec-1 / atm. We usually use molecular units, 1 atm = 2. 5 1019 molec cm-3, so collision rate (max. rate coefficient) is 2 x 10 -10 s-1 / (molec cm-3), or 2 x 10 -10 cm 3 molec-1 s-1 12

REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) What determines the value of the rate constant? ? ? Recall: Frequency of collisions is about 2 x 10 -10 cm 3 molec-1 s-1 at 298 K. 13

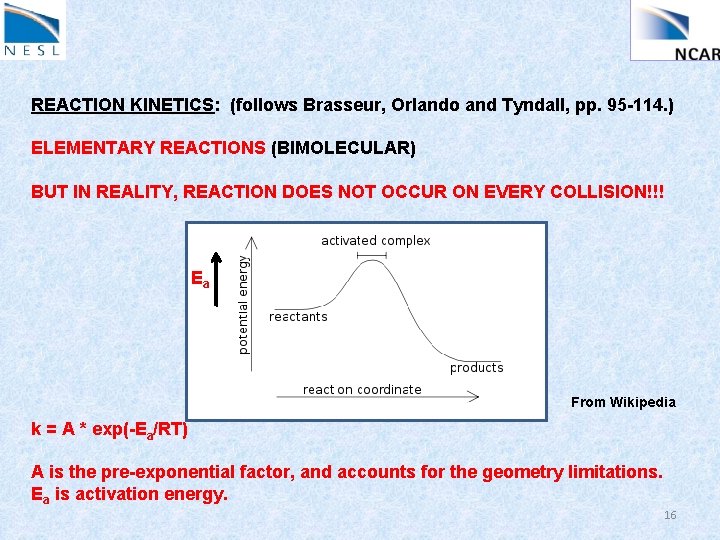
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) BUT IN REALITY, REACTION DOES NOT OCCUR ON EVERY COLLISION!!! CH 4 + OH CH 3 + HOH Why NOT? – any ideas? 14

REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) BUT IN REALITY, REACTION DOES NOT OCCUR ON EVERY COLLISION!!! Two main reasons: 1) There are energetic limitations. Colliding molecules must possess sufficient energy to overcome an ‘activation energy’ that typically exists. 2) Also, there may be ‘geometrical limitations’. Molecules must approach each other in such a way that the appropriate bonds can break / form. k = A * exp(-Ea/RT) 15

REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) BUT IN REALITY, REACTION DOES NOT OCCUR ON EVERY COLLISION!!! Ea From Wikipedia k = A * exp(-Ea/RT) A is the pre-exponential factor, and accounts for the geometry limitations. Ea is activation energy. 16

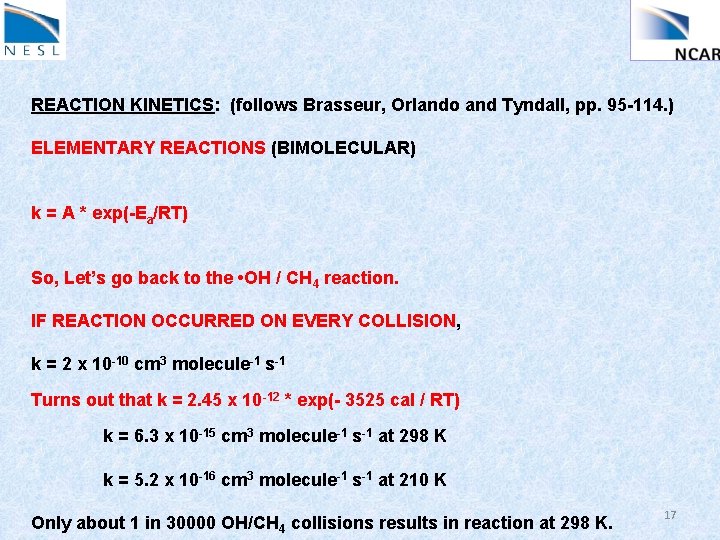
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) k = A * exp(-Ea/RT) So, Let’s go back to the • OH / CH 4 reaction. IF REACTION OCCURRED ON EVERY COLLISION, k = 2 x 10 -10 cm 3 molecule-1 s-1 Turns out that k = 2. 45 x 10 -12 * exp(- 3525 cal / RT) k = 6. 3 x 10 -15 cm 3 molecule-1 s-1 at 298 K k = 5. 2 x 10 -16 cm 3 molecule-1 s-1 at 210 K Only about 1 in 30000 OH/CH 4 collisions results in reaction at 298 K. 17

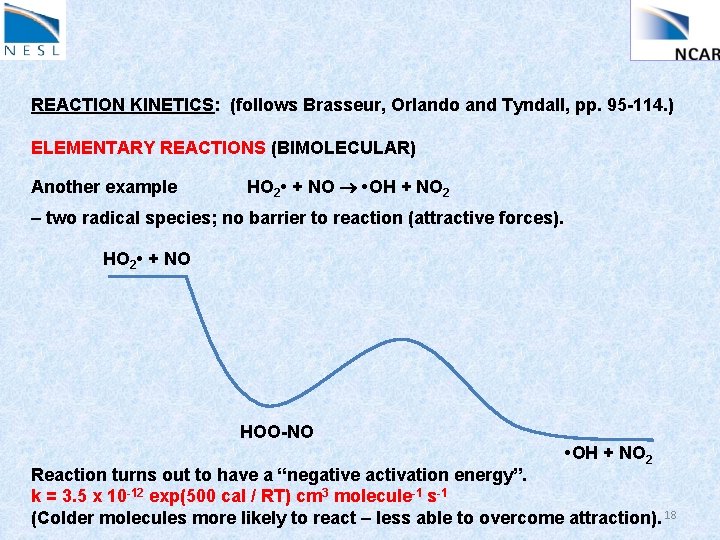
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) Another example HO 2 • + NO • OH + NO 2 – two radical species; no barrier to reaction (attractive forces). HO 2 • + NO HOO-NO • OH + NO 2 Reaction turns out to have a “negative activation energy”. k = 3. 5 x 10 -12 exp(500 cal / RT) cm 3 molecule-1 s-1 (Colder molecules more likely to react – less able to overcome attraction). 18

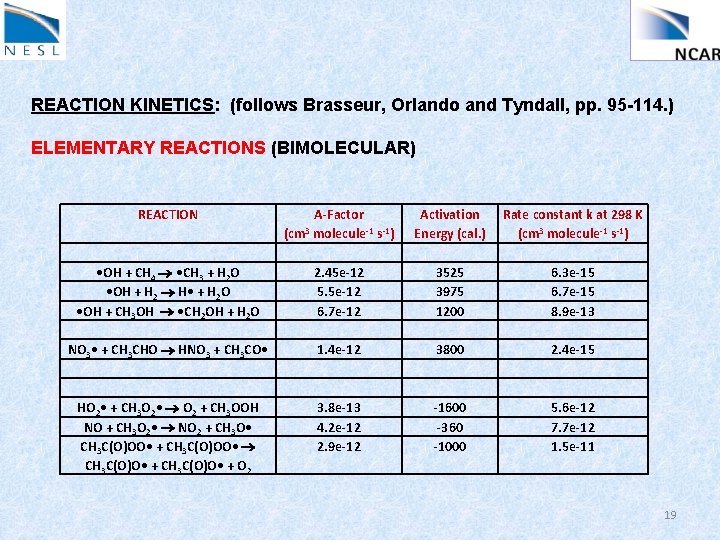
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) ELEMENTARY REACTIONS (BIMOLECULAR) REACTION A-Factor (cm 3 molecule-1 s-1) Activation Energy (cal. ) Rate constant k at 298 K (cm 3 molecule-1 s-1) • OH + CH 4 • CH 3 + H 2 O • OH + H 2 H • + H 2 O • OH + CH 3 OH • CH 2 OH + H 2 O 2. 45 e-12 5. 5 e-12 6. 7 e-12 3525 3975 1200 6. 3 e-15 6. 7 e-15 8. 9 e-13 NO 3 • + CH 3 CHO HNO 3 + CH 3 CO • 1. 4 e-12 3800 2. 4 e-15 HO 2 • + CH 3 O 2 • O 2 + CH 3 OOH NO + CH 3 O 2 • NO 2 + CH 3 O • CH 3 C(O)OO • + CH 3 C(O)OO • CH 3 C(O)O • + O 2 3. 8 e-13 4. 2 e-12 2. 9 e-12 -1600 -360 -1000 5. 6 e-12 7. 7 e-12 1. 5 e-11 19

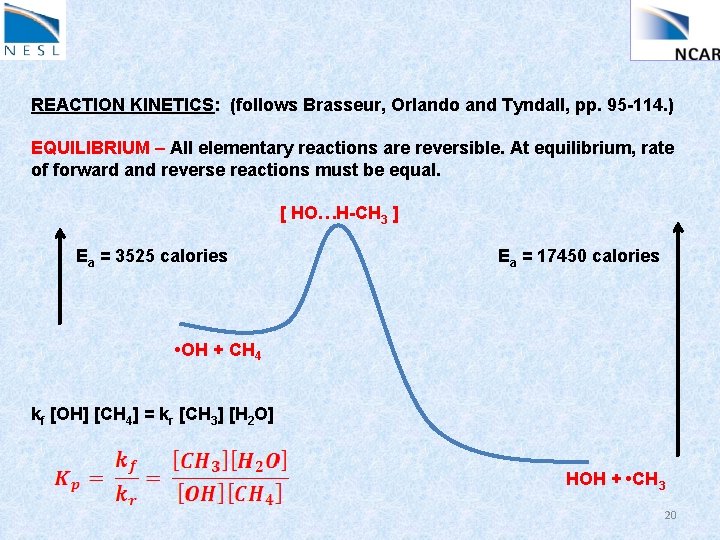
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) EQUILIBRIUM – All elementary reactions are reversible. At equilibrium, rate of forward and reverse reactions must be equal. [ HO…H-CH 3 ] Ea = 3525 calories Ea = 17450 calories • OH + CH 4 kf [OH] [CH 4] = kr [CH 3] [H 2 O] HOH + • CH 3 20

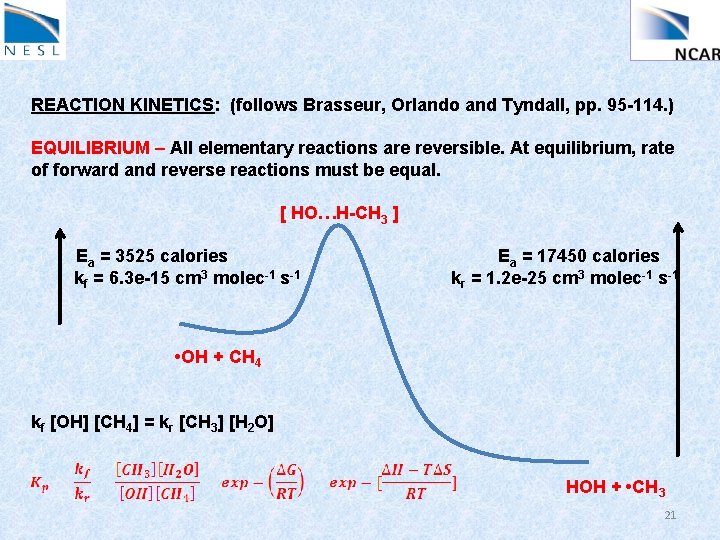
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) EQUILIBRIUM – All elementary reactions are reversible. At equilibrium, rate of forward and reverse reactions must be equal. [ HO…H-CH 3 ] Ea = 3525 calories kf = 6. 3 e-15 cm 3 molec-1 s-1 Ea = 17450 calories kr = 1. 2 e-25 cm 3 molec-1 s-1 • OH + CH 4 kf [OH] [CH 4] = kr [CH 3] [H 2 O] HOH + • CH 3 21

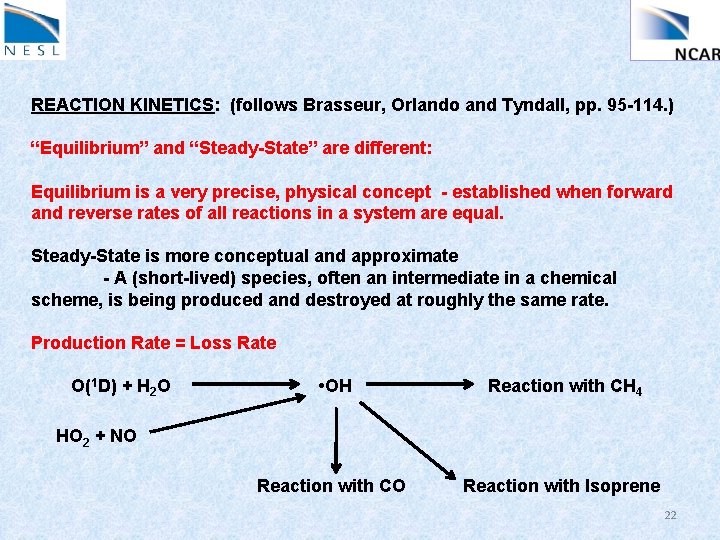
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) “Equilibrium” and “Steady-State” are different: Equilibrium is a very precise, physical concept - established when forward and reverse rates of all reactions in a system are equal. Steady-State is more conceptual and approximate - A (short-lived) species, often an intermediate in a chemical scheme, is being produced and destroyed at roughly the same rate. Production Rate = Loss Rate O(1 D) + H 2 O • OH Reaction with CH 4 Reaction with CO Reaction with Isoprene HO 2 + NO 22

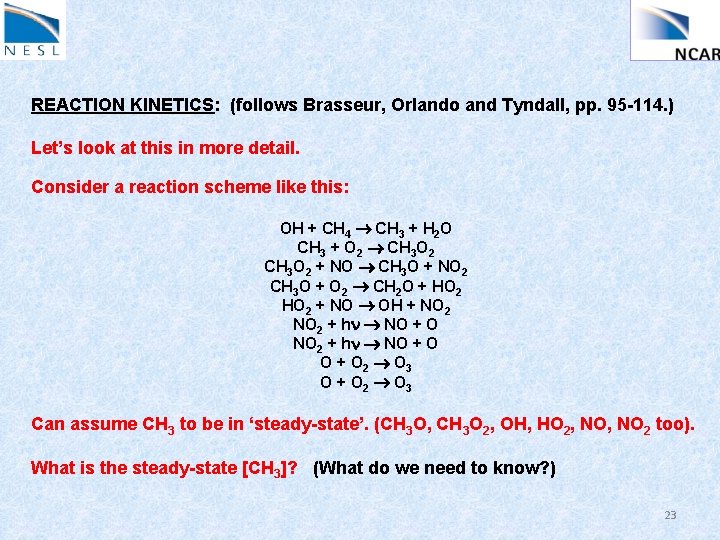
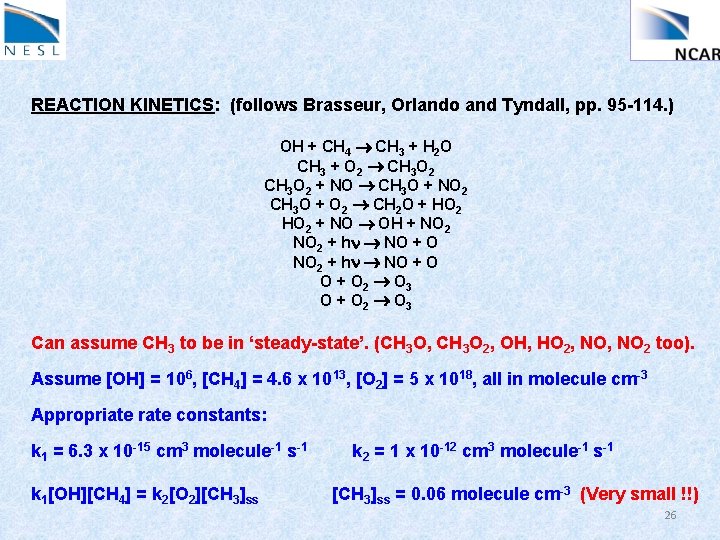
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) Let’s look at this in more detail. Consider a reaction scheme like this: OH + CH 4 CH 3 + H 2 O CH 3 + O 2 CH 3 O 2 + NO CH 3 O + NO 2 CH 3 O + O 2 CH 2 O + HO 2 + NO OH + NO 2 + hn NO + O 2 O 3 O + O 2 O 3 Can assume CH 3 to be in ‘steady-state’. (CH 3 O, CH 3 O 2, OH, HO 2, NO 2 too). What is the steady-state [CH 3]? (What do we need to know? ) 23

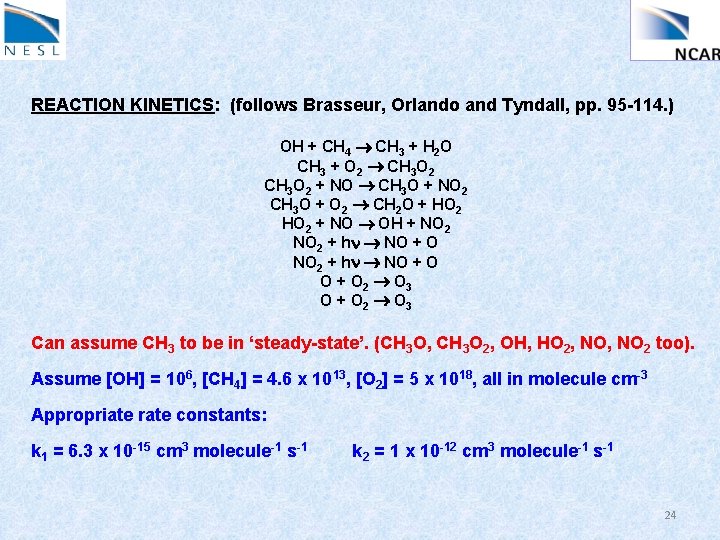
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) OH + CH 4 CH 3 + H 2 O CH 3 + O 2 CH 3 O 2 + NO CH 3 O + NO 2 CH 3 O + O 2 CH 2 O + HO 2 + NO OH + NO 2 + hn NO + O 2 O 3 O + O 2 O 3 Can assume CH 3 to be in ‘steady-state’. (CH 3 O, CH 3 O 2, OH, HO 2, NO 2 too). Assume [OH] = 106, [CH 4] = 4. 6 x 1013, [O 2] = 5 x 1018, all in molecule cm-3 Appropriate rate constants: k 1 = 6. 3 x 10 -15 cm 3 molecule-1 s-1 k 2 = 1 x 10 -12 cm 3 molecule-1 s-1 24

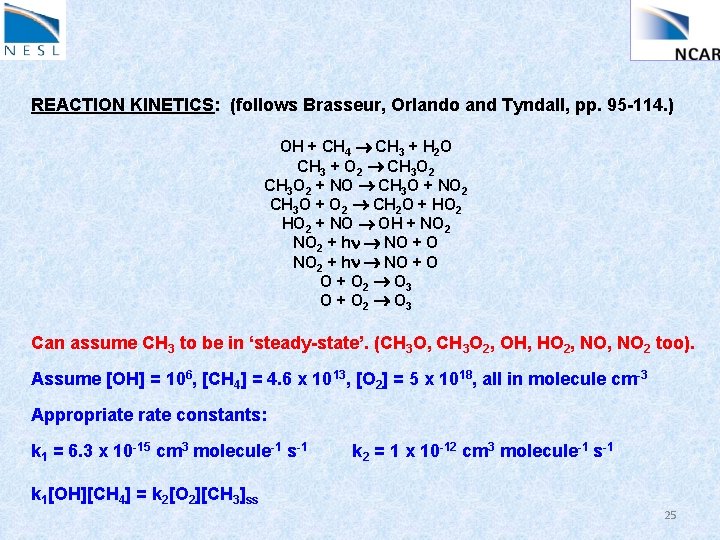
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) OH + CH 4 CH 3 + H 2 O CH 3 + O 2 CH 3 O 2 + NO CH 3 O + NO 2 CH 3 O + O 2 CH 2 O + HO 2 + NO OH + NO 2 + hn NO + O 2 O 3 O + O 2 O 3 Can assume CH 3 to be in ‘steady-state’. (CH 3 O, CH 3 O 2, OH, HO 2, NO 2 too). Assume [OH] = 106, [CH 4] = 4. 6 x 1013, [O 2] = 5 x 1018, all in molecule cm-3 Appropriate rate constants: k 1 = 6. 3 x 10 -15 cm 3 molecule-1 s-1 k 2 = 1 x 10 -12 cm 3 molecule-1 s-1 k 1[OH][CH 4] = k 2[O 2][CH 3]ss 25

REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) OH + CH 4 CH 3 + H 2 O CH 3 + O 2 CH 3 O 2 + NO CH 3 O + NO 2 CH 3 O + O 2 CH 2 O + HO 2 + NO OH + NO 2 + hn NO + O 2 O 3 O + O 2 O 3 Can assume CH 3 to be in ‘steady-state’. (CH 3 O, CH 3 O 2, OH, HO 2, NO 2 too). Assume [OH] = 106, [CH 4] = 4. 6 x 1013, [O 2] = 5 x 1018, all in molecule cm-3 Appropriate rate constants: k 1 = 6. 3 x 10 -15 cm 3 molecule-1 s-1 k 1[OH][CH 4] = k 2[O 2][CH 3]ss k 2 = 1 x 10 -12 cm 3 molecule-1 s-1 [CH 3]ss = 0. 06 molecule cm-3 (Very small !!) 26

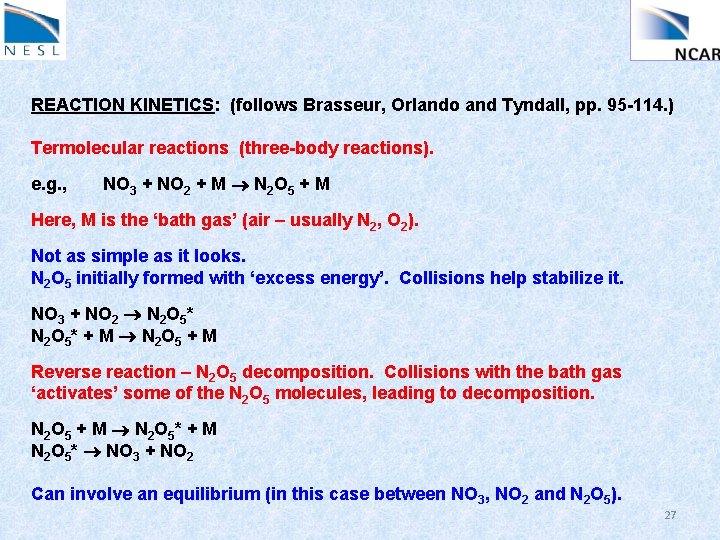
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) Termolecular reactions (three-body reactions). e. g. , NO 3 + NO 2 + M N 2 O 5 + M Here, M is the ‘bath gas’ (air – usually N 2, O 2). Not as simple as it looks. N 2 O 5 initially formed with ‘excess energy’. Collisions help stabilize it. NO 3 + NO 2 N 2 O 5* N 2 O 5 * + M N 2 O 5 + M Reverse reaction – N 2 O 5 decomposition. Collisions with the bath gas ‘activates’ some of the N 2 O 5 molecules, leading to decomposition. N 2 O 5 + M N 2 O 5 * + M N 2 O 5* NO 3 + NO 2 Can involve an equilibrium (in this case between NO 3, NO 2 and N 2 O 5). 27

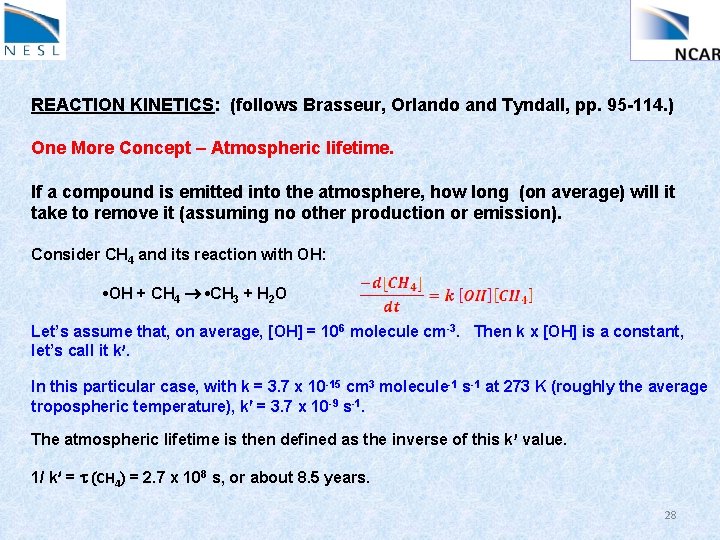
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) One More Concept – Atmospheric lifetime. If a compound is emitted into the atmosphere, how long (on average) will it take to remove it (assuming no other production or emission). Consider CH 4 and its reaction with OH: • OH + CH 4 • CH 3 + H 2 O Let’s assume that, on average, [OH] = 106 molecule cm-3. Then k x [OH] is a constant, let’s call it k. In this particular case, with k = 3. 7 x 10 -15 cm 3 molecule-1 s-1 at 273 K (roughly the average tropospheric temperature), k = 3. 7 x 10 -9 s-1. The atmospheric lifetime is then defined as the inverse of this k value. 1/ k = t (CH 4) = 2. 7 x 108 s, or about 8. 5 years. 28

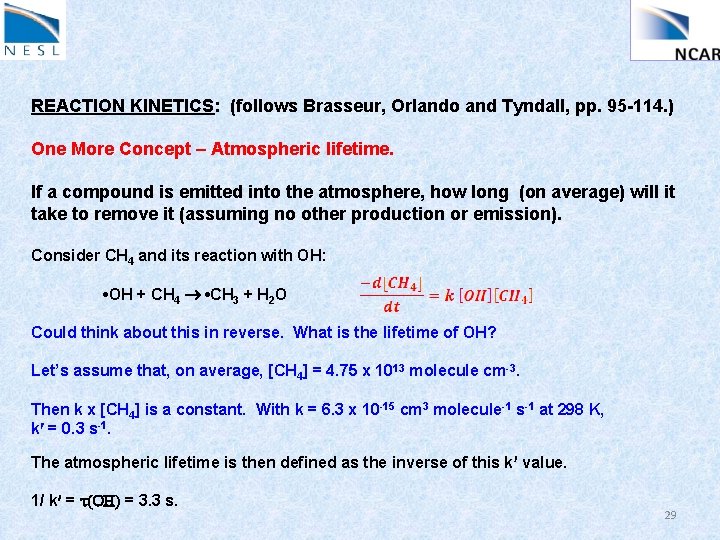
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) One More Concept – Atmospheric lifetime. If a compound is emitted into the atmosphere, how long (on average) will it take to remove it (assuming no other production or emission). Consider CH 4 and its reaction with OH: • OH + CH 4 • CH 3 + H 2 O Could think about this in reverse. What is the lifetime of OH? Let’s assume that, on average, [CH 4] = 4. 75 x 1013 molecule cm-3. Then k x [CH 4] is a constant. With k = 6. 3 x 10 -15 cm 3 molecule-1 s-1 at 298 K, k = 0. 3 s-1. The atmospheric lifetime is then defined as the inverse of this k value. 1/ k = t(OH) = 3. 3 s. 29

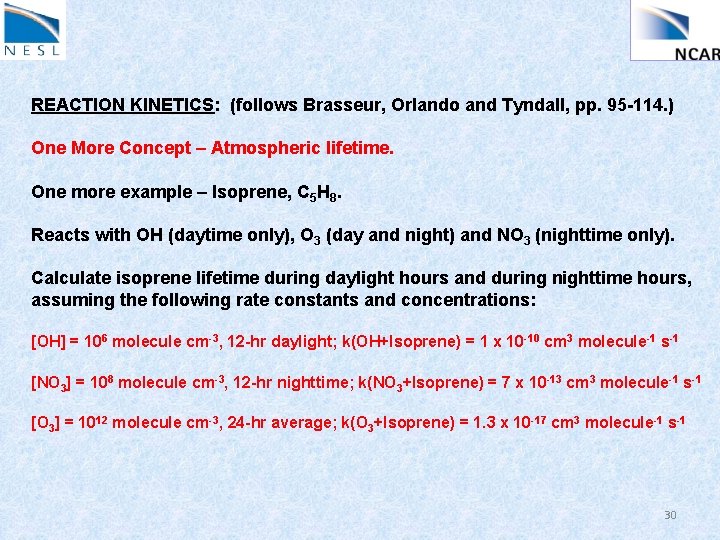
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) One More Concept – Atmospheric lifetime. One more example – Isoprene, C 5 H 8. Reacts with OH (daytime only), O 3 (day and night) and NO 3 (nighttime only). Calculate isoprene lifetime during daylight hours and during nighttime hours, assuming the following rate constants and concentrations: [OH] = 106 molecule cm-3, 12 -hr daylight; k(OH+Isoprene) = 1 x 10 -10 cm 3 molecule-1 s-1 [NO 3] = 108 molecule cm-3, 12 -hr nighttime; k(NO 3+Isoprene) = 7 x 10 -13 cm 3 molecule-1 s-1 [O 3] = 1012 molecule cm-3, 24 -hr average; k(O 3+Isoprene) = 1. 3 x 10 -17 cm 3 molecule-1 s-1 30

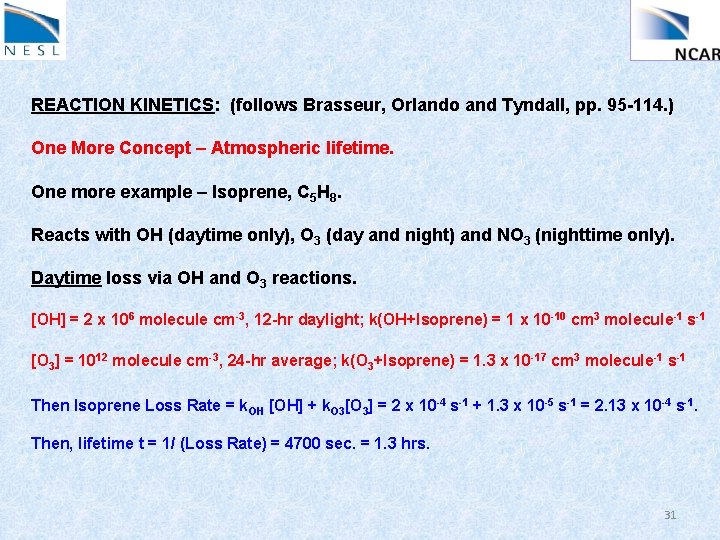
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) One More Concept – Atmospheric lifetime. One more example – Isoprene, C 5 H 8. Reacts with OH (daytime only), O 3 (day and night) and NO 3 (nighttime only). Daytime loss via OH and O 3 reactions. [OH] = 2 x 106 molecule cm-3, 12 -hr daylight; k(OH+Isoprene) = 1 x 10 -10 cm 3 molecule-1 s-1 [O 3] = 1012 molecule cm-3, 24 -hr average; k(O 3+Isoprene) = 1. 3 x 10 -17 cm 3 molecule-1 s-1 Then Isoprene Loss Rate = k. OH [OH] + k. O 3[O 3] = 2 x 10 -4 s-1 + 1. 3 x 10 -5 s-1 = 2. 13 x 10 -4 s-1. Then, lifetime t = 1/ (Loss Rate) = 4700 sec. = 1. 3 hrs. 31

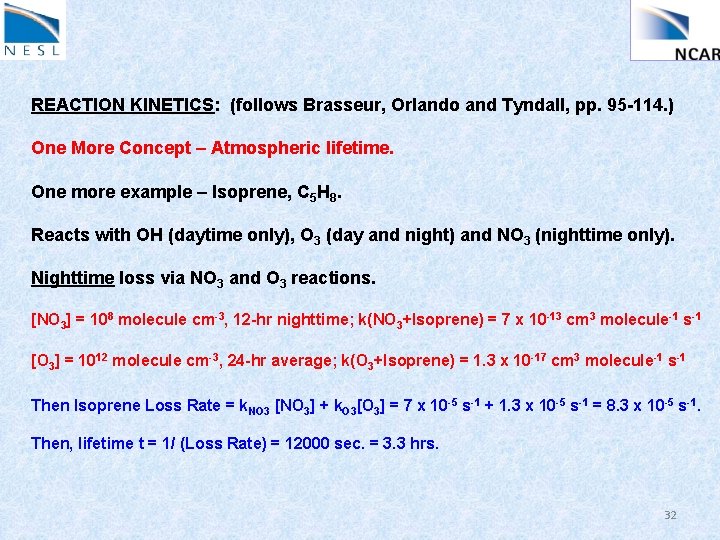
REACTION KINETICS: (follows Brasseur, Orlando and Tyndall, pp. 95 -114. ) One More Concept – Atmospheric lifetime. One more example – Isoprene, C 5 H 8. Reacts with OH (daytime only), O 3 (day and night) and NO 3 (nighttime only). Nighttime loss via NO 3 and O 3 reactions. [NO 3] = 108 molecule cm-3, 12 -hr nighttime; k(NO 3+Isoprene) = 7 x 10 -13 cm 3 molecule-1 s-1 [O 3] = 1012 molecule cm-3, 24 -hr average; k(O 3+Isoprene) = 1. 3 x 10 -17 cm 3 molecule-1 s-1 Then Isoprene Loss Rate = k. NO 3 [NO 3] + k. O 3[O 3] = 7 x 10 -5 s-1 + 1. 3 x 10 -5 s-1 = 8. 3 x 10 -5 s-1. Then, lifetime t = 1/ (Loss Rate) = 12000 sec. = 3. 3 hrs. 32

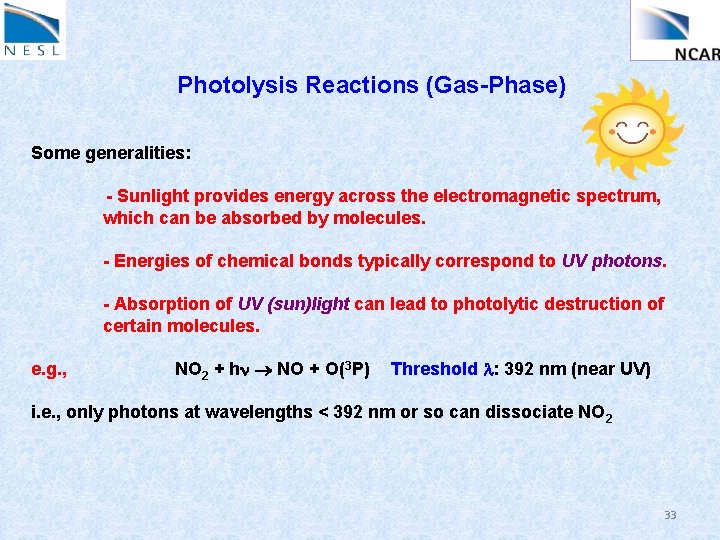
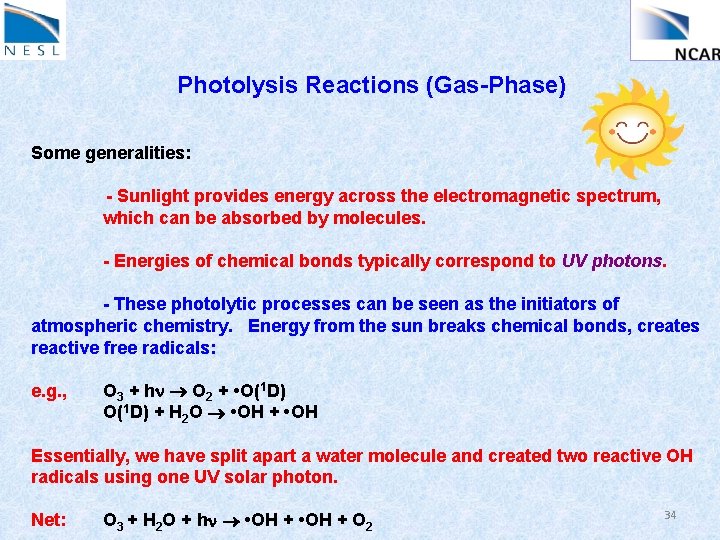
Photolysis Reactions (Gas-Phase) Some generalities: - Sunlight provides energy across the electromagnetic spectrum, which can be absorbed by molecules. - Energies of chemical bonds typically correspond to UV photons. - Absorption of UV (sun)light can lead to photolytic destruction of certain molecules. e. g. , NO 2 + hn NO + O(3 P) Threshold l: 392 nm (near UV) i. e. , only photons at wavelengths < 392 nm or so can dissociate NO 2 33

Photolysis Reactions (Gas-Phase) Some generalities: - Sunlight provides energy across the electromagnetic spectrum, which can be absorbed by molecules. - Energies of chemical bonds typically correspond to UV photons. - These photolytic processes can be seen as the initiators of atmospheric chemistry. Energy from the sun breaks chemical bonds, creates reactive free radicals: e. g. , O 3 + hn O 2 + • O(1 D) + H 2 O • OH + • OH Essentially, we have split apart a water molecule and created two reactive OH radicals using one UV solar photon. Net: O 3 + H 2 O + hn • OH + O 2 34

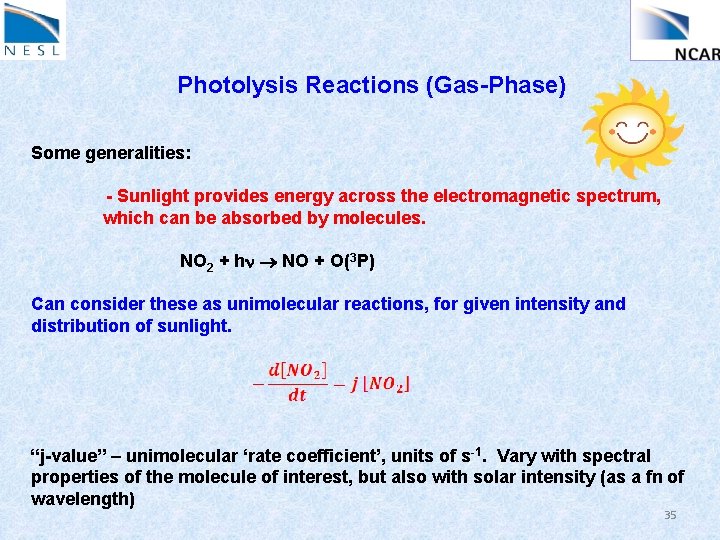
Photolysis Reactions (Gas-Phase) Some generalities: - Sunlight provides energy across the electromagnetic spectrum, which can be absorbed by molecules. NO 2 + hn NO + O(3 P) Can consider these as unimolecular reactions, for given intensity and distribution of sunlight. “j-value” – unimolecular ‘rate coefficient’, units of s-1. Vary with spectral properties of the molecule of interest, but also with solar intensity (as a fn of wavelength) 35

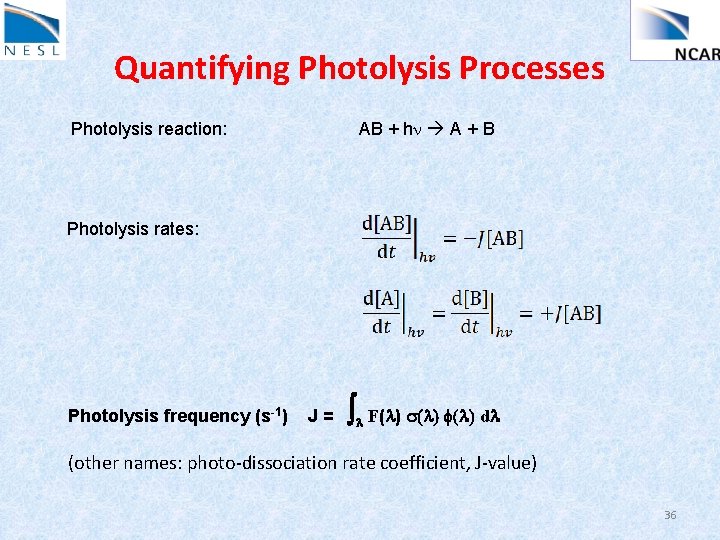
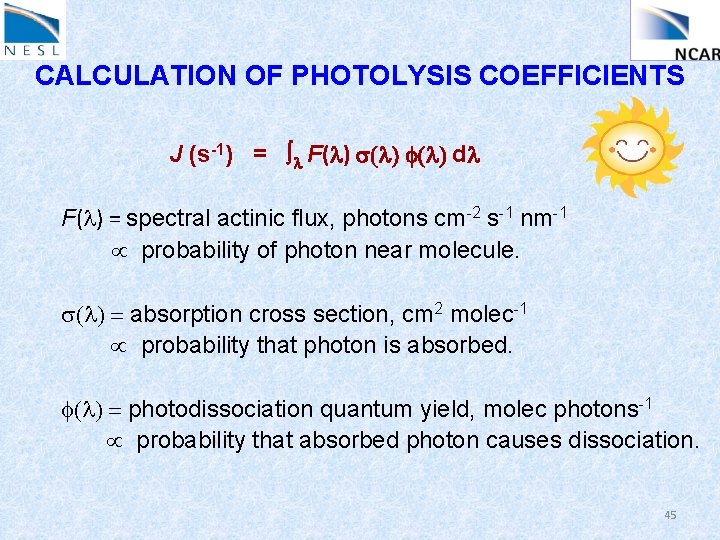
Quantifying Photolysis Processes Photolysis reaction: AB + hn A + B Photolysis rates: Photolysis frequency (s-1) J= l F(l) s(l) f(l) dl (other names: photo-dissociation rate coefficient, J-value) 36

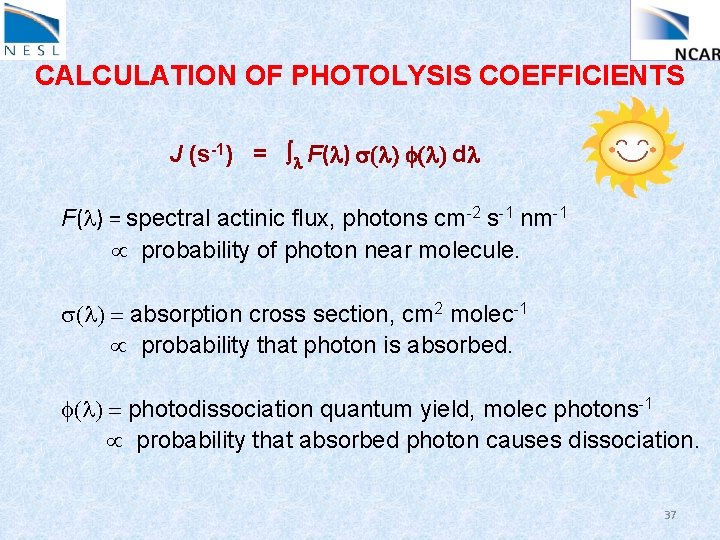
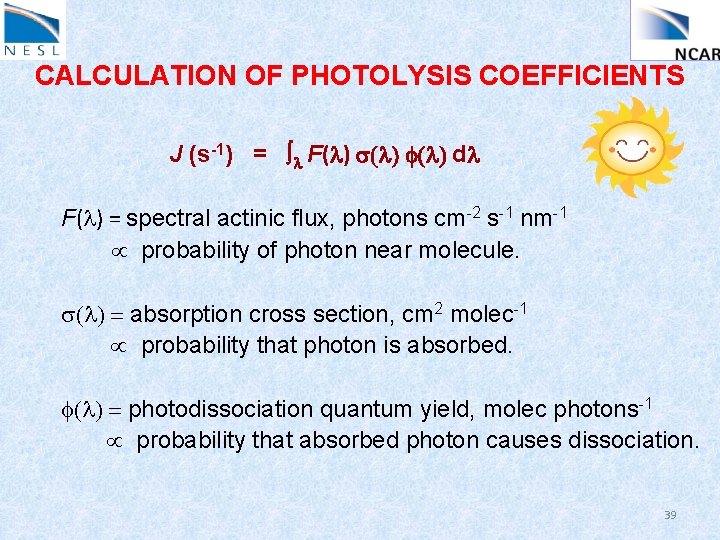
CALCULATION OF PHOTOLYSIS COEFFICIENTS J (s-1) = l F(l) s(l) f(l) dl F(l) = spectral actinic flux, photons cm-2 s-1 nm-1 probability of photon near molecule. s(l) = absorption cross section, cm 2 molec-1 probability that photon is absorbed. f(l) = photodissociation quantum yield, molec photons-1 probability that absorbed photon causes dissociation. 37

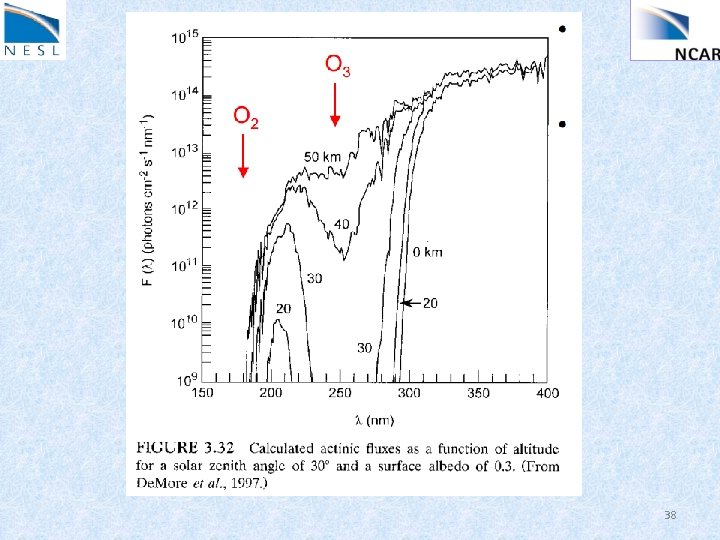
38

CALCULATION OF PHOTOLYSIS COEFFICIENTS J (s-1) = l F(l) s(l) f(l) dl F(l) = spectral actinic flux, photons cm-2 s-1 nm-1 probability of photon near molecule. s(l) = absorption cross section, cm 2 molec-1 probability that photon is absorbed. f(l) = photodissociation quantum yield, molec photons-1 probability that absorbed photon causes dissociation. 39

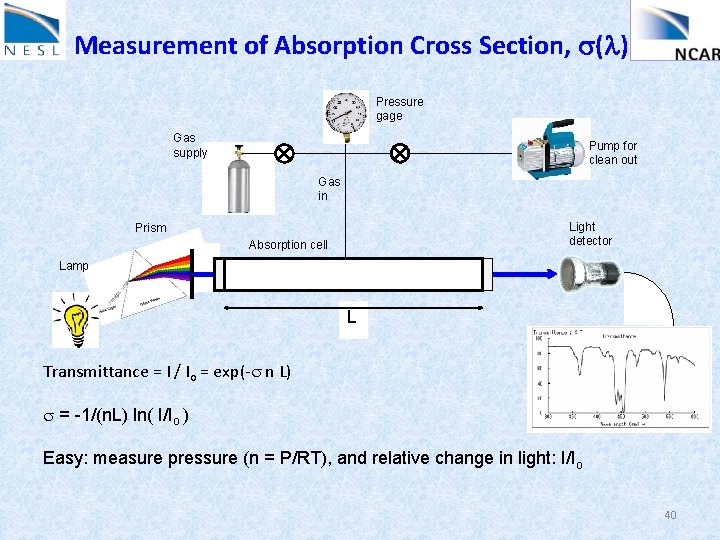
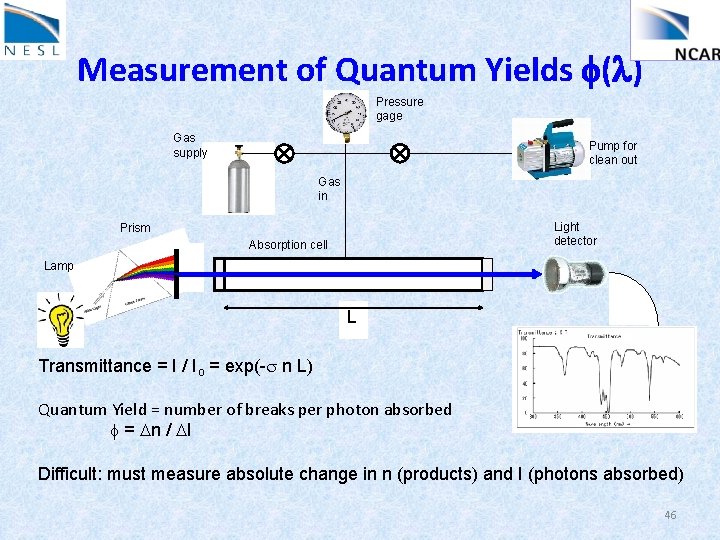
Measurement of Absorption Cross Section, s(l) Pressure gage Gas supply Pump for clean out Gas in Light detector Prism Absorption cell Lamp L Transmittance = I / Io = exp(-s n L) s = -1/(n. L) ln( I/Io ) Easy: measure pressure (n = P/RT), and relative change in light: I/Io 40

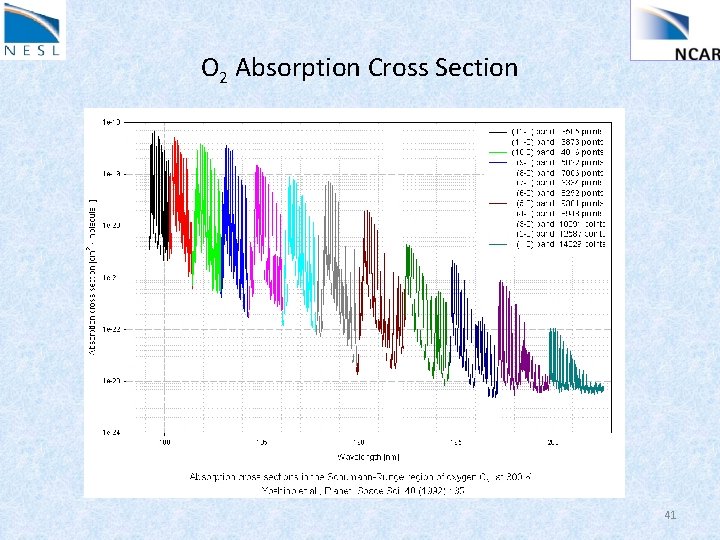
O 2 Absorption Cross Section 41

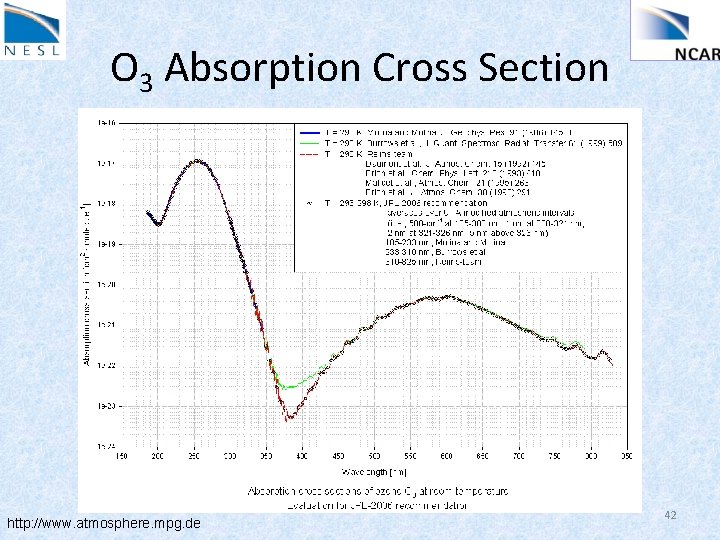
O 3 Absorption Cross Section http: //www. atmosphere. mpg. de 42

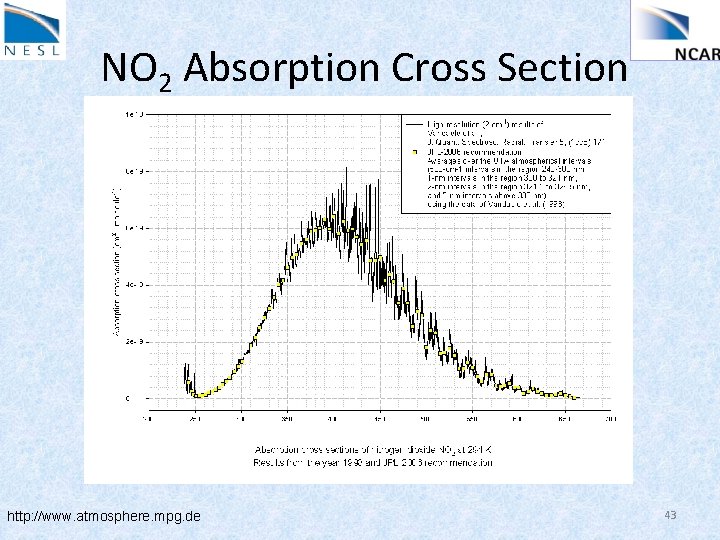
NO 2 Absorption Cross Section http: //www. atmosphere. mpg. de 43

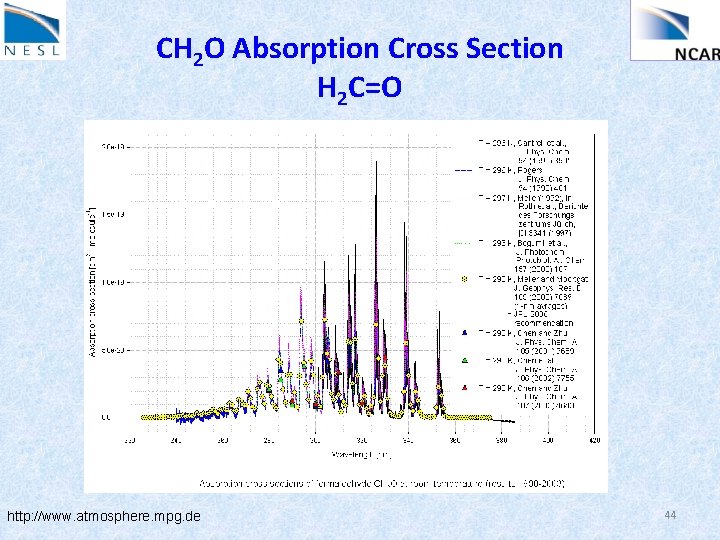
CH 2 O Absorption Cross Section H 2 C=O http: //www. atmosphere. mpg. de 44

CALCULATION OF PHOTOLYSIS COEFFICIENTS J (s-1) = l F(l) s(l) f(l) dl F(l) = spectral actinic flux, photons cm-2 s-1 nm-1 probability of photon near molecule. s(l) = absorption cross section, cm 2 molec-1 probability that photon is absorbed. f(l) = photodissociation quantum yield, molec photons-1 probability that absorbed photon causes dissociation. 45

Measurement of Quantum Yields f(l) Pressure gage Gas supply Pump for clean out Gas in Light detector Prism Absorption cell Lamp L Transmittance = I / Io = exp(-s n L) Quantum Yield = number of breaks per photon absorbed f = Dn / DI Difficult: must measure absolute change in n (products) and I (photons absorbed) 46

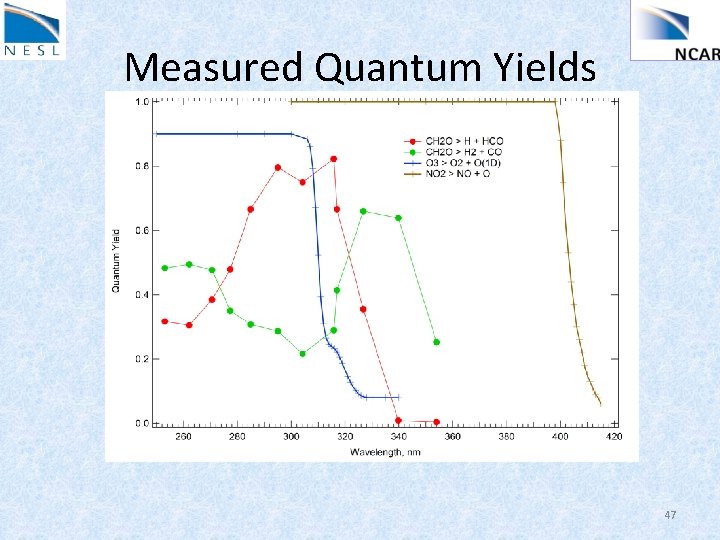
Measured Quantum Yields 47

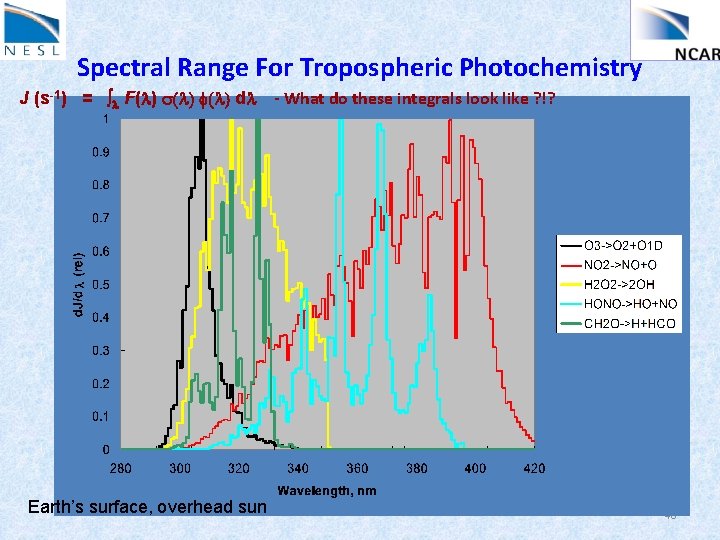
Spectral Range For Tropospheric Photochemistry J (s-1) = l F(l) s(l) f(l) dl Earth’s surface, overhead sun - What do these integrals look like ? !? 48

Aerosol Fundamentals Aerosol - A gaseous suspension of fine solid or liquid particles. http: //www. thefreedictionary. com/aerosol “We have in this fine dust [aerosols] a most beautiful illustration of how the little things in the world work great effects by virtue of their numbers. ” John Aitken (1839 -1919) -John Aitken, 1880 49

Why should we care about aerosol? Air quality and Human health Global Climate Radiation, Chemistry, Rainfall Reactive surfaces/volume where chemistry can happen Aerosols are important from the molecular to the global scale 50

Aerosols are the principle component of what we perceive as “smog” Submicron aerosols are primarily responsible for visibility reduction. Pasadena, CA, on a clear day (hills are 7 km away) Environmental Protection Agency (EPA) PM 2. 5 15 g /m 3 (annual average) PM 10 150 g /m 3 (24 hour) regulations. See national map of compliance at: http: //en. wikipedia. org/wiki/File: Pm 25 -24 asuper. gif Pasadena, CA, on a bad smog day 51

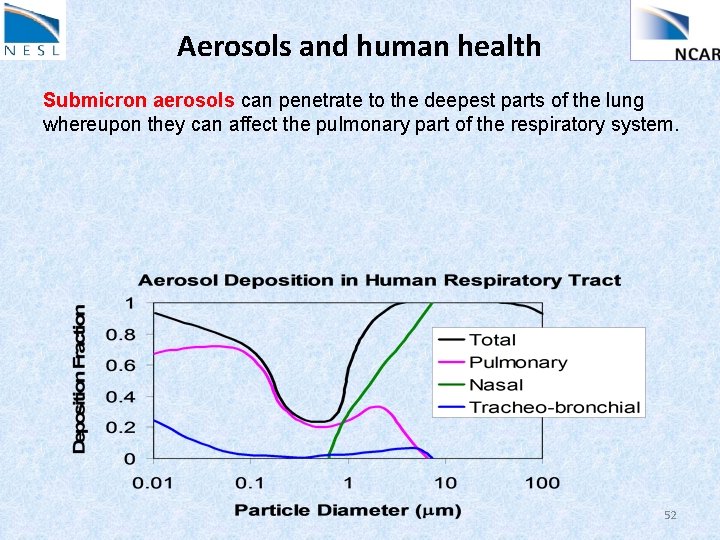
Aerosols and human health Submicron aerosols can penetrate to the deepest parts of the lung whereupon they can affect the pulmonary part of the respiratory system. 52

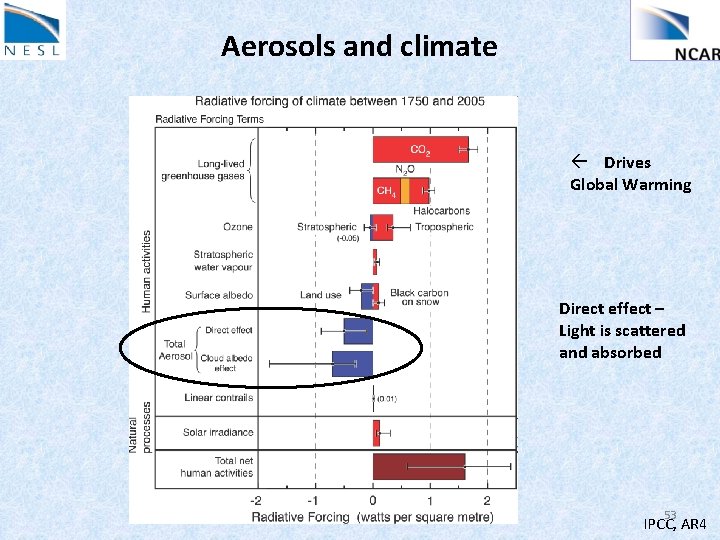
Aerosols and climate ß Drives Global Warming Direct effect – Light is scattered and absorbed 53 IPCC, AR 4

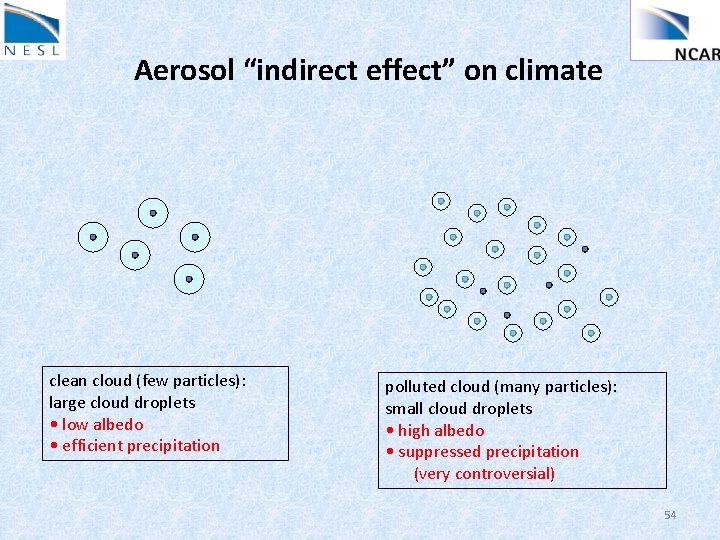
Aerosol “indirect effect” on climate clean cloud (few particles): large cloud droplets • low albedo • efficient precipitation polluted cloud (many particles): small cloud droplets • high albedo • suppressed precipitation (very controversial) 54

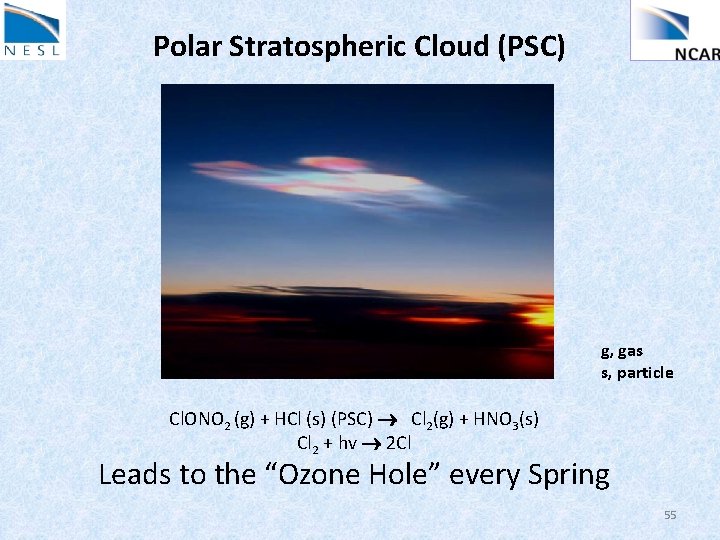
Polar Stratospheric Cloud (PSC) g, gas s, particle Cl. ONO 2 (g) + HCl (s) (PSC) Cl 2(g) + HNO 3(s) Cl 2 + hv 2 Cl Leads to the “Ozone Hole” every Spring 55

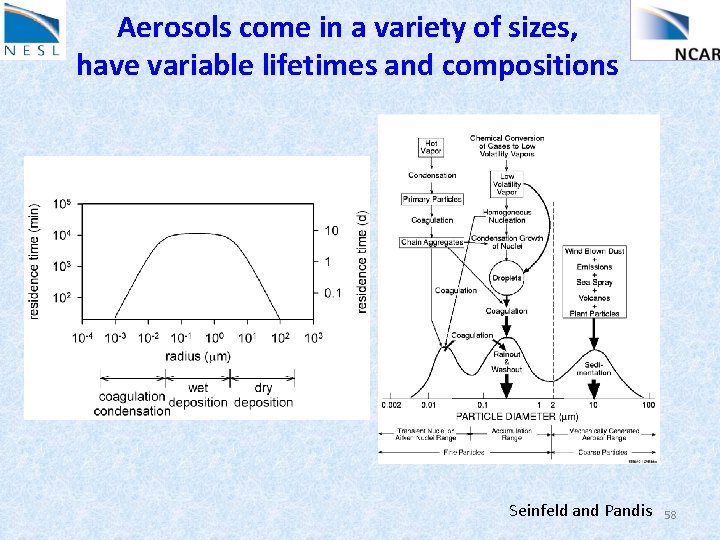
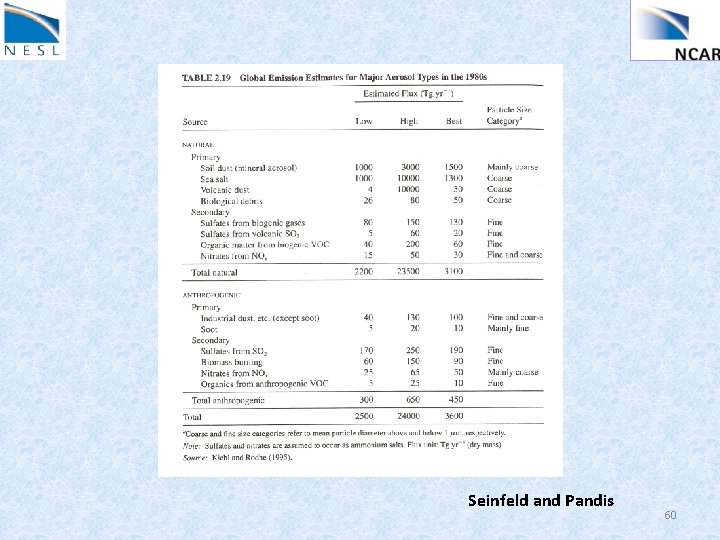
Aerosols : solid and liquid particles suspended in the air Size: nm to 100 microns (range of 105) Lifetime: Troposphere (hours to days) Stratosphere (year) Primary aerosol: emitted directly into the air Secondary aerosol: gas to particle conversion • Composition: sulfate, nitrate, ammonium, sodium, chloride, trace metals, crustal, water, carbonaceous: elemental: emitted directly into the air (e. g. diesel soot) organic: a) directly by sources (e. g combustion, plant leaf) b) condensation of low volatile organic gases 56

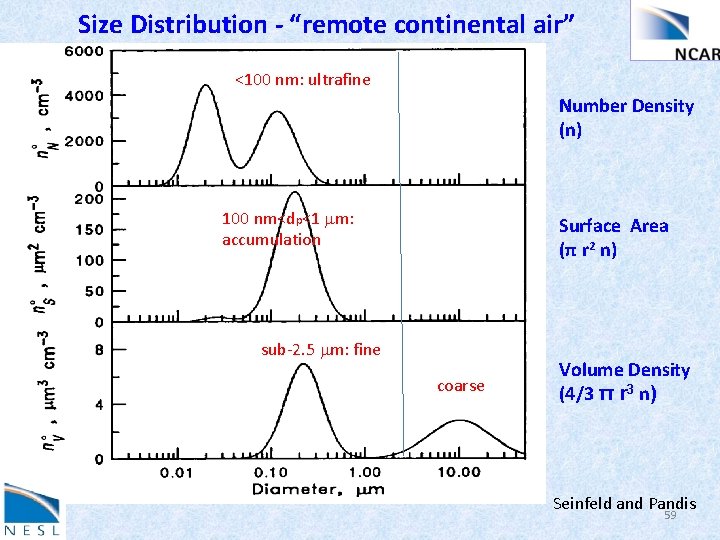
“fine” diameters D < 2. 5 microns sulfate, ammonium, organic carbon, elemental carbon Nucleation (Aitken) mode - 0. 005 to 0. 01 microns condensation of vapors Accumulation mode 0. 1 to 2. 5 microns coagulation “coarse” diameters D > 2. 5 microns natural dust (e. g. desert) mechanical processes crustal materials biogenic (pollen, plant fragments) 57

Aerosols come in a variety of sizes, have variable lifetimes and compositions Seinfeld and Pandis 58

Size Distribution - “remote continental air” <100 nm: ultrafine Number Density (n) 100 nm<dp<1 mm: accumulation Surface Area (π r 2 n) sub-2. 5 mm: fine coarse Volume Density (4/3 π r 3 n) Seinfeld and Pandis 59

Seinfeld and Pandis 60

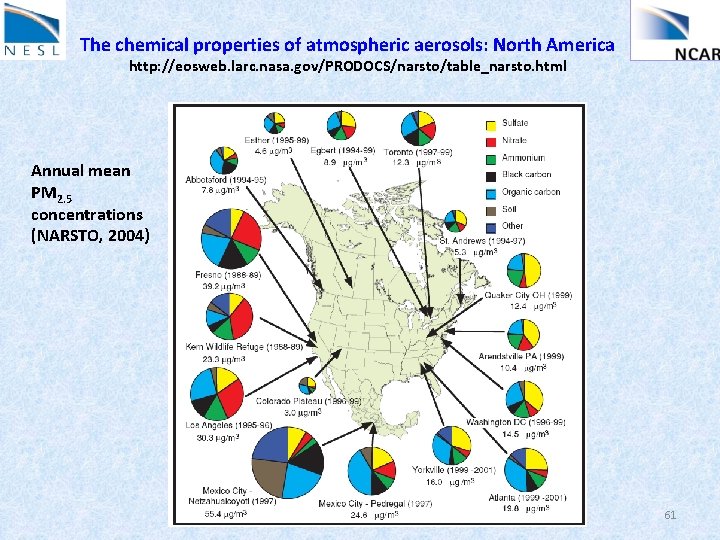
The chemical properties of atmospheric aerosols: North America http: //eosweb. larc. nasa. gov/PRODOCS/narsto/table_narsto. html Annual mean PM 2. 5 concentrations (NARSTO, 2004) 61

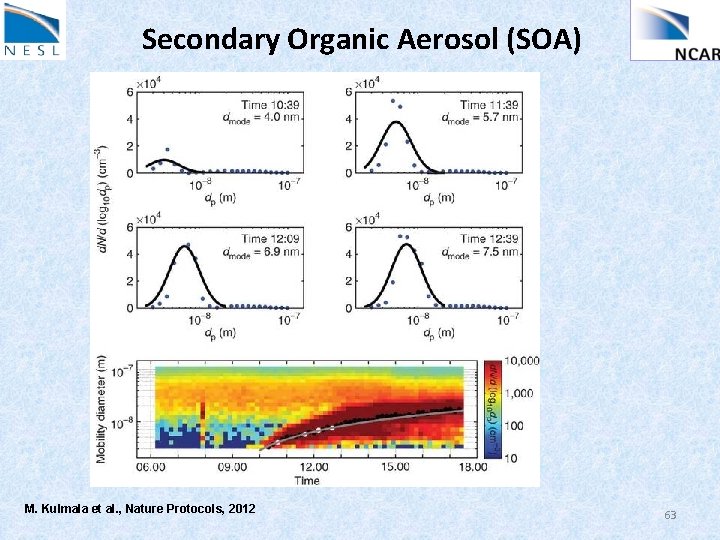
Secondary Organic Aerosol (SOA) SOA accounts for large fraction of submicron particulate mass Arises from nucleation / condensation of low volatility products of oxidation… We’ll talk more about organic chemistry later, but basic idea is that more oxygen on a molecule usually means lower vapor pressure E. g. , Biogenic terpenes, C 10 H 16, can be oxidized to make things like C 10 H 16 O 2, then C 10 H 16 O 4. Some of these products nucleate new particles, or add on to existing ones. Jimenez, Science, 326, Dec 2009 Odum, Env Sci Tech, 30, 2580, 1996 Ervens, JGR, 109, 2004 62

Secondary Organic Aerosol (SOA) M. Kulmala et al. , Nature Protocols, 2012 63

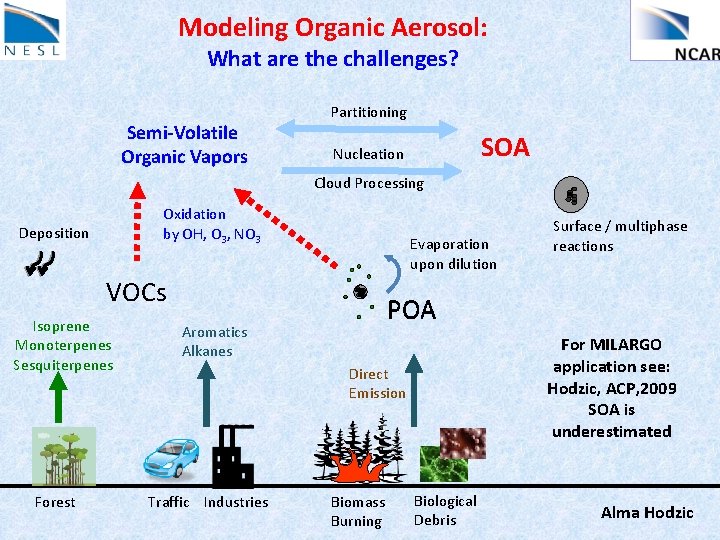
Modeling Organic Aerosol: What are the challenges? Semi-Volatile Organic Vapors Partitioning SOA Nucleation Cloud Processing Oxidation by OH, O 3, NO 3 Deposition Evaporation upon dilution VOCs Isoprene Monoterpenes Sesquiterpenes Forest POA Aromatics Alkanes For MILARGO application see: Hodzic, ACP, 2009 SOA is underestimated Direct Emission Traffic Industries Surface / multiphase reactions Biomass Burning Biological Debris Alma Hodzic

Cloud Chemistry (more generally, Aqueous Phase Chemistry) Chemistry occurring in or on liquid particles (cloud drops, rain drops, fog droplets, aerosols) Cloud droplets Will focus on two aspects of this in terms of atmospheric composition: 1) Scavenging of oxidized gases (nitric acid, sulfuric acid) 2) Oxidative Chemistry (sulfur species, carbon species) 65

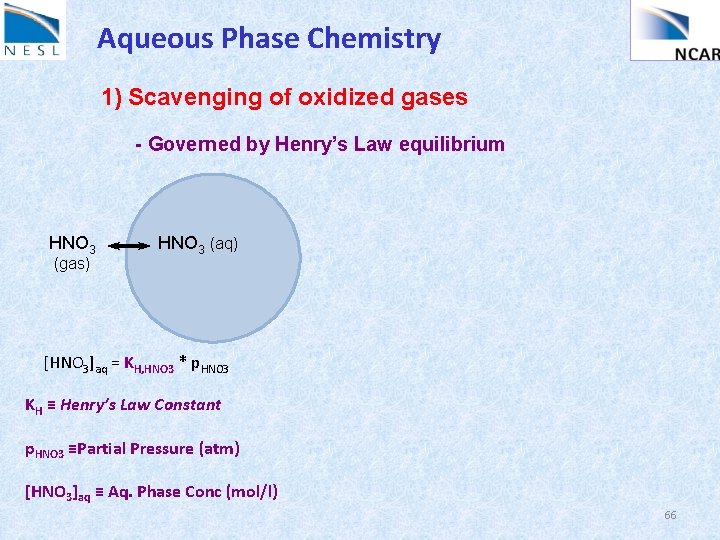
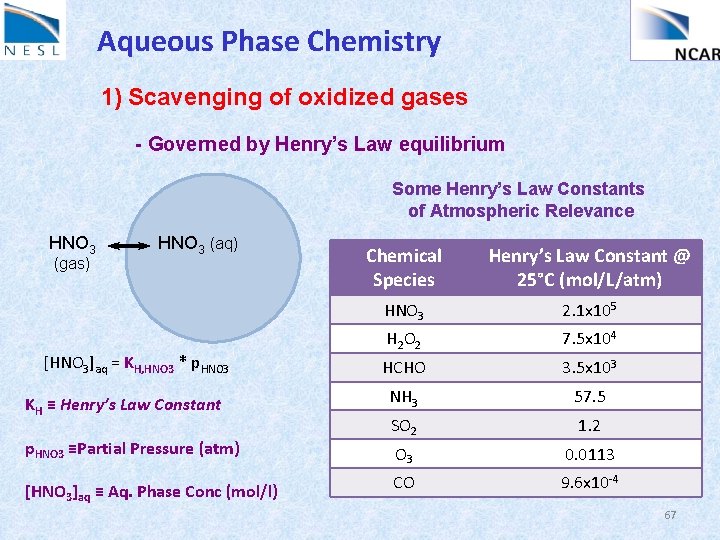
Aqueous Phase Chemistry 1) Scavenging of oxidized gases - Governed by Henry’s Law equilibrium HNO 3 (gas) HNO 3 (aq) [HNO 3]aq = KH, HNO 3 * p. HNO 3 KH ≡ Henry’s Law Constant p. HNO 3 ≡Partial Pressure (atm) [HNO 3]aq ≡ Aq. Phase Conc (mol/l) 66

Aqueous Phase Chemistry 1) Scavenging of oxidized gases - Governed by Henry’s Law equilibrium Some Henry’s Law Constants of Atmospheric Relevance HNO 3 (gas) HNO 3 (aq) [HNO 3]aq = KH, HNO 3 * p. HNO 3 KH ≡ Henry’s Law Constant p. HNO 3 ≡Partial Pressure (atm) [HNO 3]aq ≡ Aq. Phase Conc (mol/l) Chemical Species Henry’s Law Constant @ 25°C (mol/L/atm) HNO 3 2. 1 x 105 H 2 O 2 7. 5 x 104 HCHO 3. 5 x 103 NH 3 57. 5 SO 2 1. 2 O 3 0. 0113 CO 9. 6 x 10 -4 67

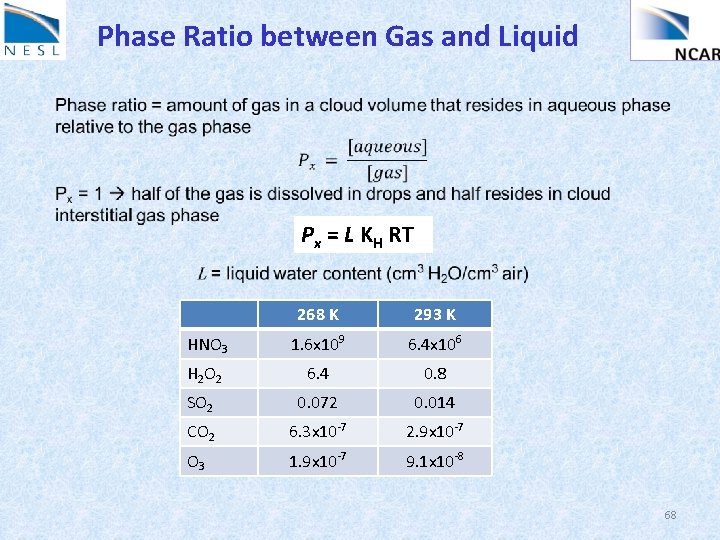
Phase Ratio between Gas and Liquid Px = L KH RT 268 K 293 K HNO 3 1. 6 x 109 6. 4 x 106 H 2 O 2 6. 4 0. 8 SO 2 0. 072 0. 014 CO 2 6. 3 x 10 -7 2. 9 x 10 -7 O 3 1. 9 x 10 -7 9. 1 x 10 -8 68

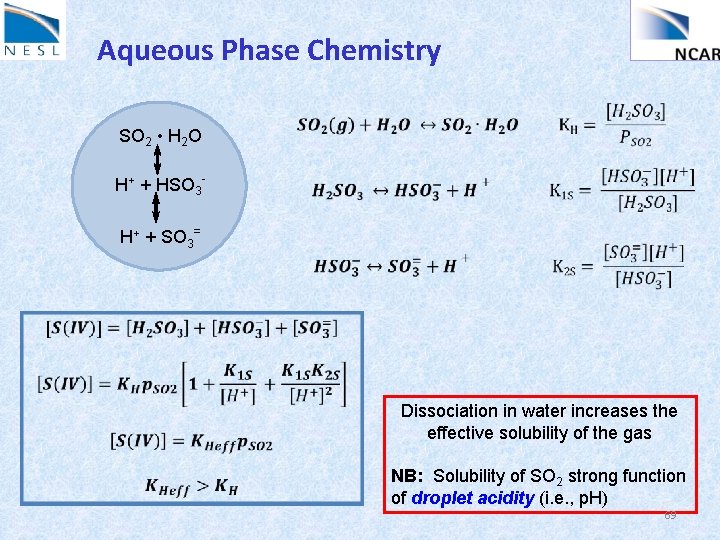
Aqueous Phase Chemistry SO 2 • H 2 O H+ + HSO 3 H+ + SO 3= Dissociation in water increases the effective solubility of the gas NB: Solubility of SO 2 strong function of droplet acidity (i. e. , p. H) 69

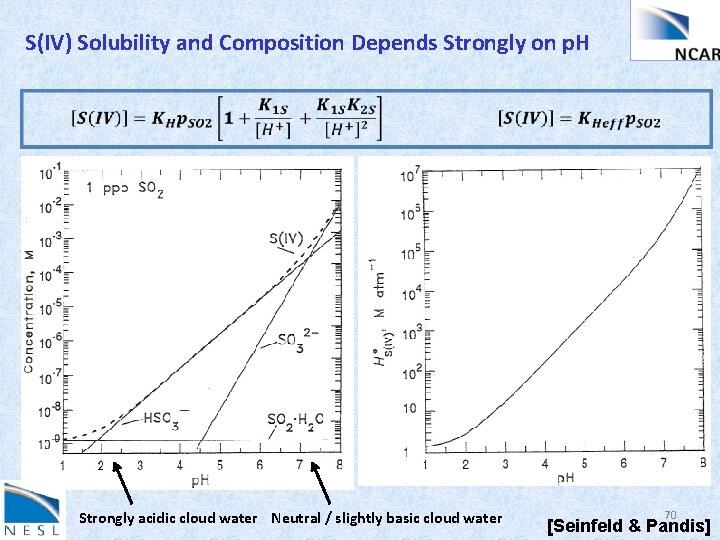
S(IV) Solubility and Composition Depends Strongly on p. H Strongly acidic cloud water Neutral / slightly basic cloud water 70 [Seinfeld & Pandis]

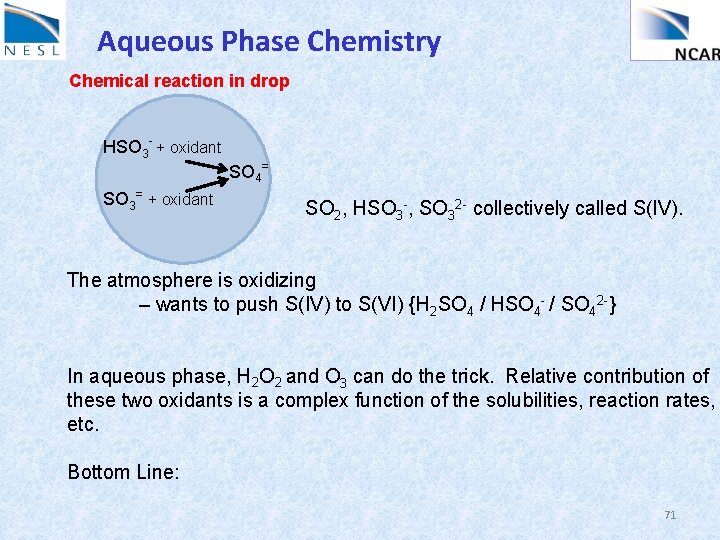
Aqueous Phase Chemistry Chemical reaction in drop HSO 3 - + oxidant SO 3= + oxidant SO 4= SO 2, HSO 3 -, SO 32 - collectively called S(IV). The atmosphere is oxidizing – wants to push S(IV) to S(VI) {H 2 SO 4 / HSO 4 - / SO 42 -} In aqueous phase, H 2 O 2 and O 3 can do the trick. Relative contribution of these two oxidants is a complex function of the solubilities, reaction rates, etc. Bottom Line: 71

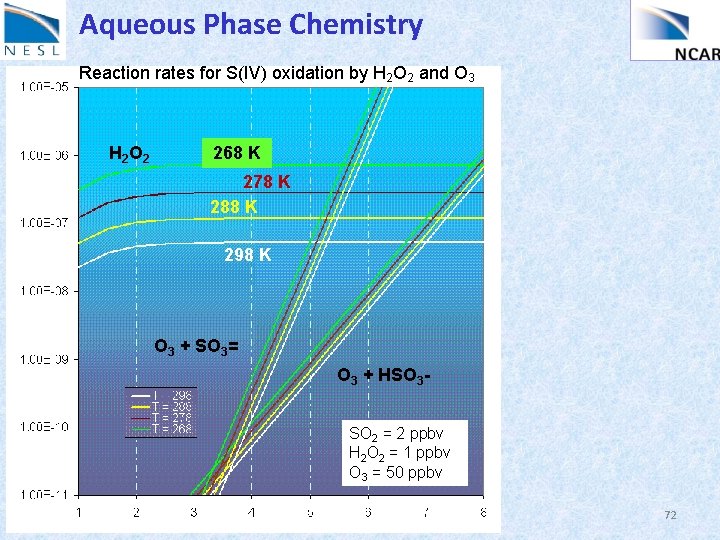
Aqueous Phase Chemistry Reaction rates for S(IV) oxidation by H 2 O 2 and O 3 H 2 O 2 268 K 278 K 288 K 298 K O 3 + SO 3= O 3 + HSO 3 SO 2 = 2 ppbv H 2 O 2 = 1 ppbv O 3 = 50 ppbv 72

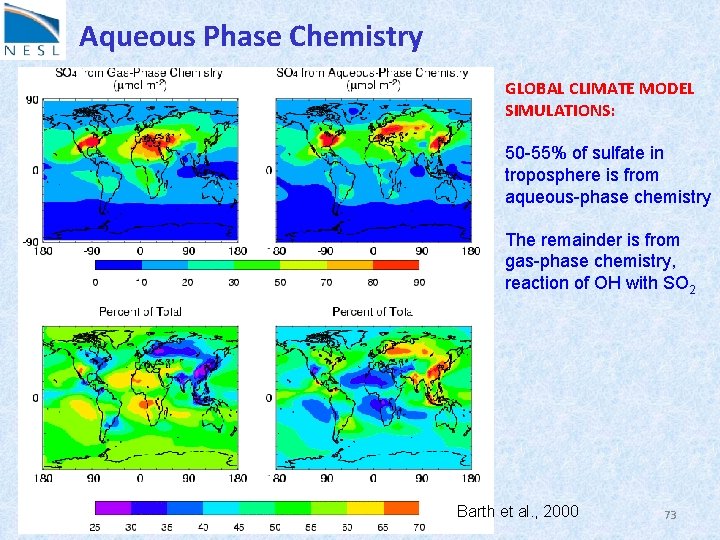
Aqueous Phase Chemistry GLOBAL CLIMATE MODEL SIMULATIONS: 50 -55% of sulfate in troposphere is from aqueous-phase chemistry The remainder is from gas-phase chemistry, reaction of OH with SO 2 Barth et al. , 2000 73

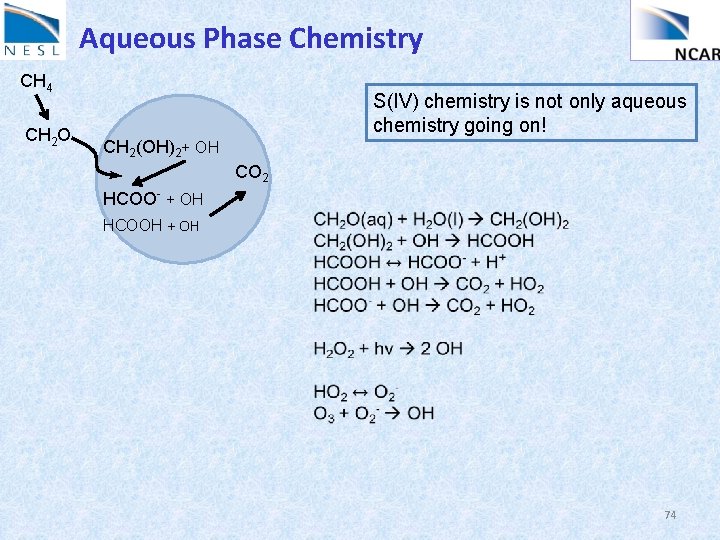
Aqueous Phase Chemistry CH 4 CH 2 O S(IV) chemistry is not only aqueous chemistry going on! CH 2(OH)2+ OH CO 2 HCOO- + OH HCOOH + OH 74

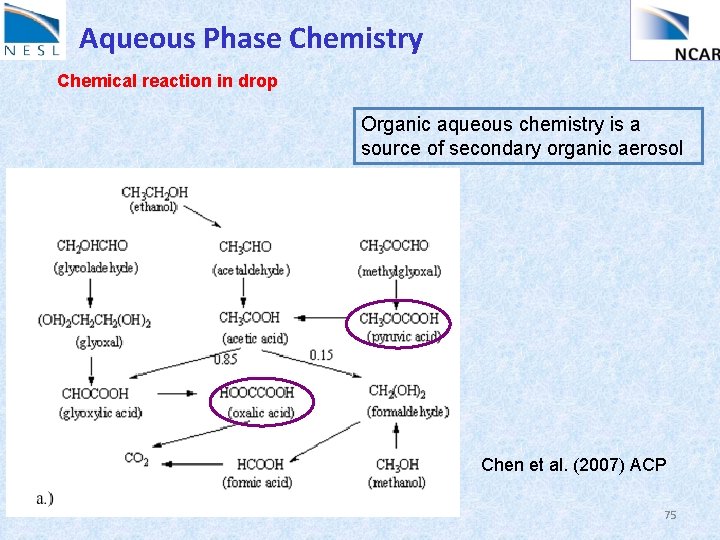
Aqueous Phase Chemistry Chemical reaction in drop Organic aqueous chemistry is a source of secondary organic aerosol Chen et al. (2007) ACP 75
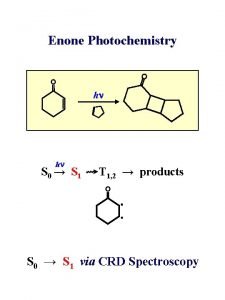
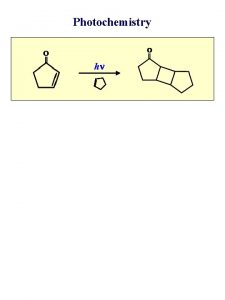
 Laws of photochemistry
Laws of photochemistry Grothus draper law slideshare
Grothus draper law slideshare Photochemistry
Photochemistry Photochemistry
Photochemistry Rhodopsin cycle
Rhodopsin cycle Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions 10 examples of redox reaction
10 examples of redox reaction Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Application of heat transfer
Application of heat transfer Nuclear decays and reactions section 2
Nuclear decays and reactions section 2 Reversible and irreversible reactions
Reversible and irreversible reactions How to do redox reactions
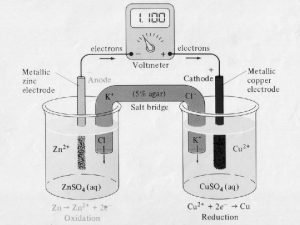
How to do redox reactions Define concentration cells
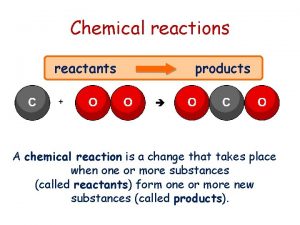
Define concentration cells Chemical reactions reactants and products
Chemical reactions reactants and products Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry 18.3 reversible reactions and equilibrium
18.3 reversible reactions and equilibrium Anode cathode reduction oxidation
Anode cathode reduction oxidation Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Propanal + kcn
Propanal + kcn Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry The age of exploration outcome china and japan's reactions
The age of exploration outcome china and japan's reactions What was the outcome of china's age of exploration?
What was the outcome of china's age of exploration? Chemical reactions of copper and percent yield report sheet
Chemical reactions of copper and percent yield report sheet What is released or absorbed whenever chemical
What is released or absorbed whenever chemical The ni-cd cell delivers a potential difference of
The ni-cd cell delivers a potential difference of Endothermic and exothermic reactions grade 11
Endothermic and exothermic reactions grade 11 Reactions of benzene and its derivatives
Reactions of benzene and its derivatives Aldehyde protecting group
Aldehyde protecting group Diol formation from alkene
Diol formation from alkene Reactivity series
Reactivity series Stereoselective and stereospecific reaction
Stereoselective and stereospecific reaction What is an oxidation reaction
What is an oxidation reaction Structure of chlorophyll
Structure of chlorophyll Alkyne reactions
Alkyne reactions Key terms radioactivity and nuclear reactions
Key terms radioactivity and nuclear reactions Endotermik tepkime
Endotermik tepkime Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Mta security fundamentals exam questions and answers
Mta security fundamentals exam questions and answers Jk flip flop
Jk flip flop Jk flip flop
Jk flip flop Logic and computer design fundamentals
Logic and computer design fundamentals Logic and computer design fundamentals
Logic and computer design fundamentals Activity and exercise fundamentals of nursing
Activity and exercise fundamentals of nursing Chapter 74 ase questions
Chapter 74 ase questions Tire wheel and wheel bearing fundamentals
Tire wheel and wheel bearing fundamentals Fundamentals of data and signals
Fundamentals of data and signals Chapter 1 understanding health and wellness answer key
Chapter 1 understanding health and wellness answer key Nrf customer service and sales
Nrf customer service and sales Stress and adaptation fundamentals of nursing
Stress and adaptation fundamentals of nursing Fundamentals of the nervous system and nervous tissue
Fundamentals of the nervous system and nervous tissue No slip condition
No slip condition Database processing fundamentals design and implementation
Database processing fundamentals design and implementation Productivity = output/input
Productivity = output/input Process instrumentation ppt
Process instrumentation ppt Fundamentals of planning and developing tourism
Fundamentals of planning and developing tourism Fundamentals of data and signals
Fundamentals of data and signals Forensic science fundamentals and investigations chapter 6
Forensic science fundamentals and investigations chapter 6 Fundamentals of futures and options markets
Fundamentals of futures and options markets Cscatt
Cscatt Fundamentals in food service operations
Fundamentals in food service operations Chapter 1 understanding your health and wellness
Chapter 1 understanding your health and wellness Evolution and fundamentals of business
Evolution and fundamentals of business Pain management nursing
Pain management nursing Thermal conduction resistance
Thermal conduction resistance Fundamentals of the nervous system and nervous tissue
Fundamentals of the nervous system and nervous tissue Reclaiming and redefining the fundamentals of care
Reclaiming and redefining the fundamentals of care Capillary force
Capillary force