22 6 Elementary reactions Elementary reactions reactions which

- Slides: 23

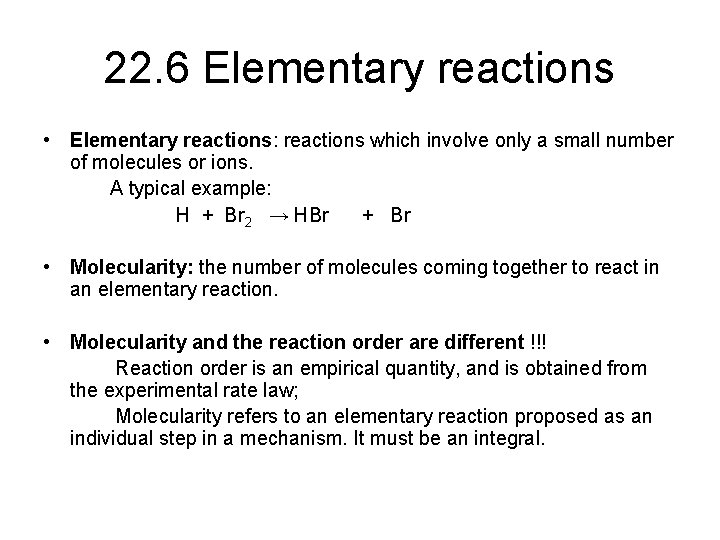
22. 6 Elementary reactions • Elementary reactions: reactions which involve only a small number of molecules or ions. A typical example: H + Br 2 → HBr + Br • Molecularity: the number of molecules coming together to react in an elementary reaction. • Molecularity and the reaction order are different !!! Reaction order is an empirical quantity, and is obtained from the experimental rate law; Molecularity refers to an elementary reaction proposed as an individual step in a mechanism. It must be an integral.

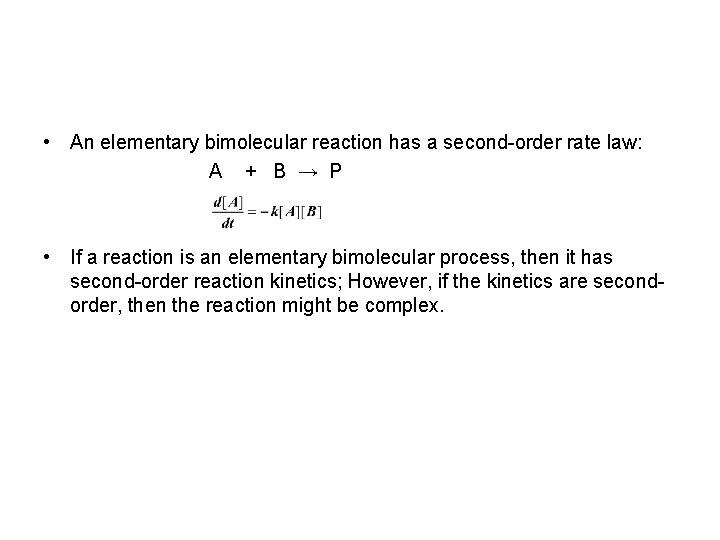
• An elementary bimolecular reaction has a second-order rate law: A + B → P • If a reaction is an elementary bimolecular process, then it has second-order reaction kinetics; However, if the kinetics are secondorder, then the reaction might be complex.

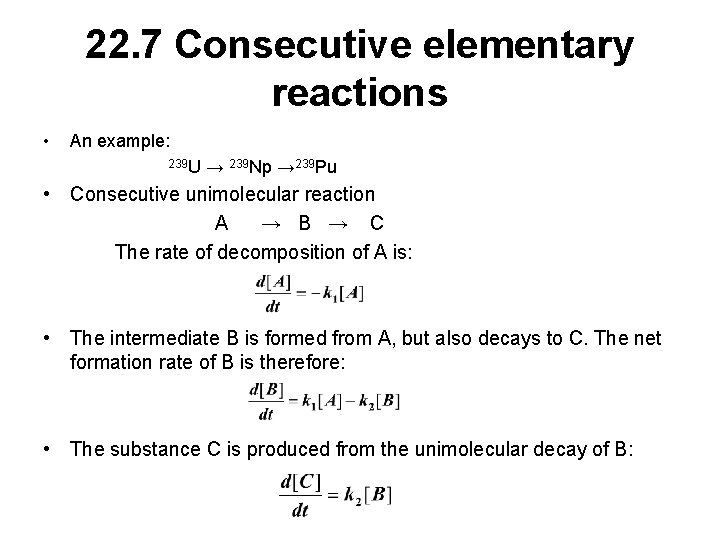
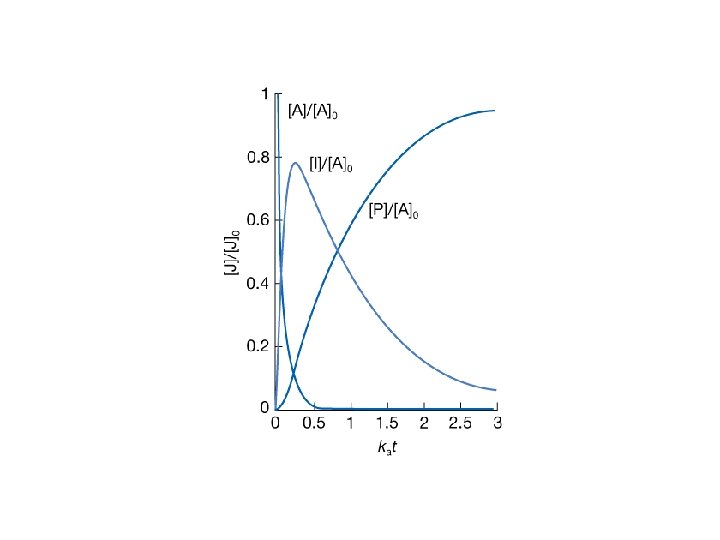
22. 7 Consecutive elementary reactions • An example: 239 U → 239 Np → 239 Pu • Consecutive unimolecular reaction A → B → C The rate of decomposition of A is: • The intermediate B is formed from A, but also decays to C. The net formation rate of B is therefore: • The substance C is produced from the unimolecular decay of B:

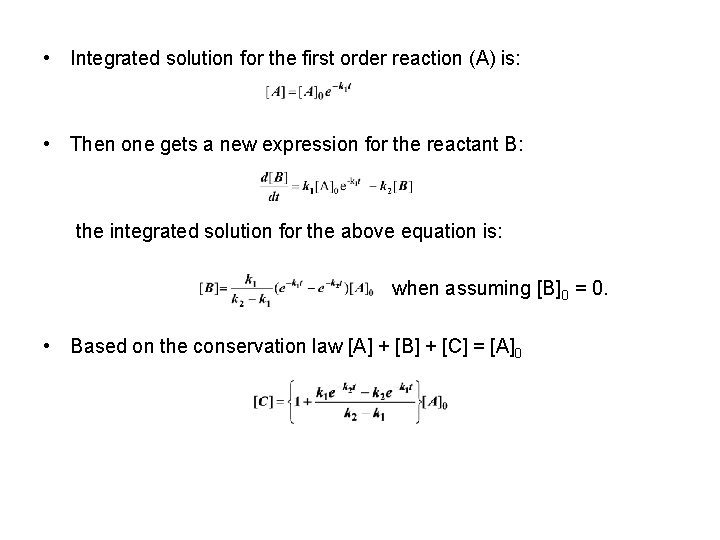
• Integrated solution for the first order reaction (A) is: • Then one gets a new expression for the reactant B: the integrated solution for the above equation is: when assuming [B] 0 = 0. • Based on the conservation law [A] + [B] + [C] = [A]0


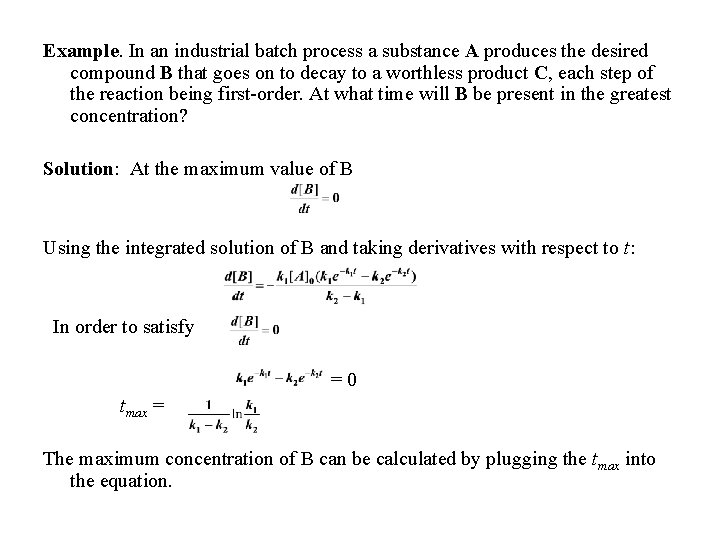
Example. In an industrial batch process a substance A produces the desired compound B that goes on to decay to a worthless product C, each step of the reaction being first-order. At what time will B be present in the greatest concentration? Solution: At the maximum value of B Using the integrated solution of B and taking derivatives with respect to t: In order to satisfy =0 tmax = The maximum concentration of B can be calculated by plugging the tmax into the equation.

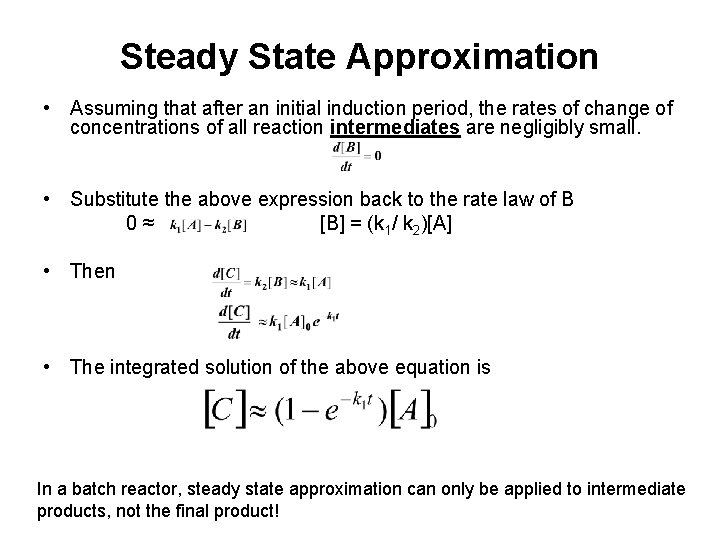
Steady State Approximation • Assuming that after an initial induction period, the rates of change of concentrations of all reaction intermediates are negligibly small. • Substitute the above expression back to the rate law of B 0≈ [B] = (k 1/ k 2)[A] • Then • The integrated solution of the above equation is In a batch reactor, steady state approximation can only be applied to intermediate products, not the final product!

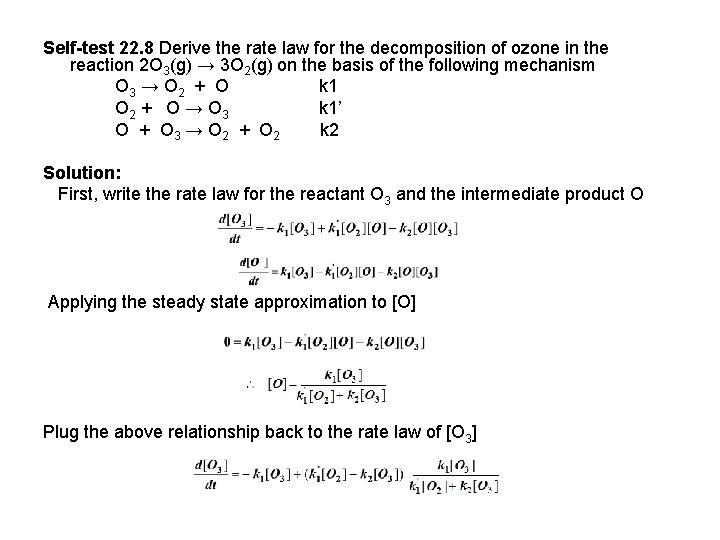
Self-test 22. 8 Derive the rate law for the decomposition of ozone in the reaction 2 O 3(g) → 3 O 2(g) on the basis of the following mechanism O 3 → O 2 + O k 1 O 2 + O → O 3 k 1’ O + O 3 → O 2 + O 2 k 2 Solution: First, write the rate law for the reactant O 3 and the intermediate product O Applying the steady state approximation to [O] Plug the above relationship back to the rate law of [O 3]

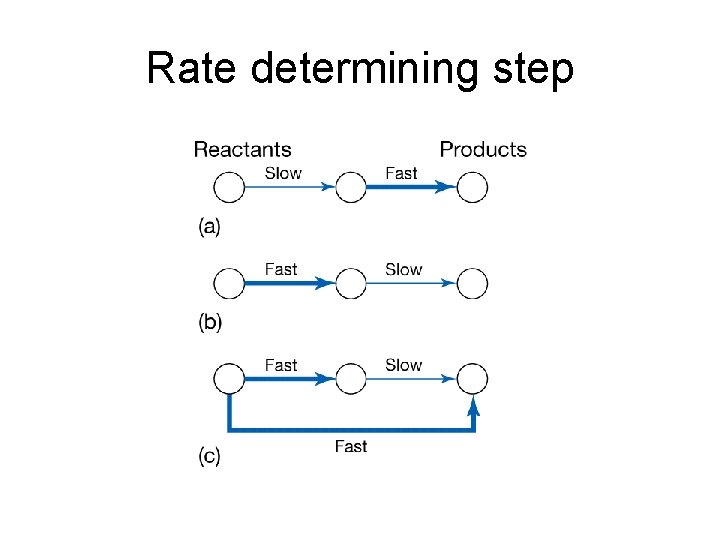
Rate determining step

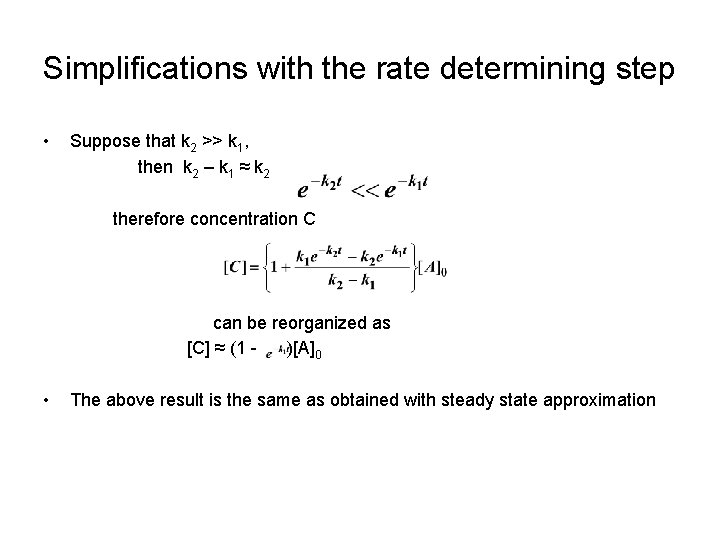
Simplifications with the rate determining step • Suppose that k 2 >> k 1, then k 2 – k 1 ≈ k 2 therefore concentration C can be reorganized as [C] ≈ (1 )[A]0 • The above result is the same as obtained with steady state approximation

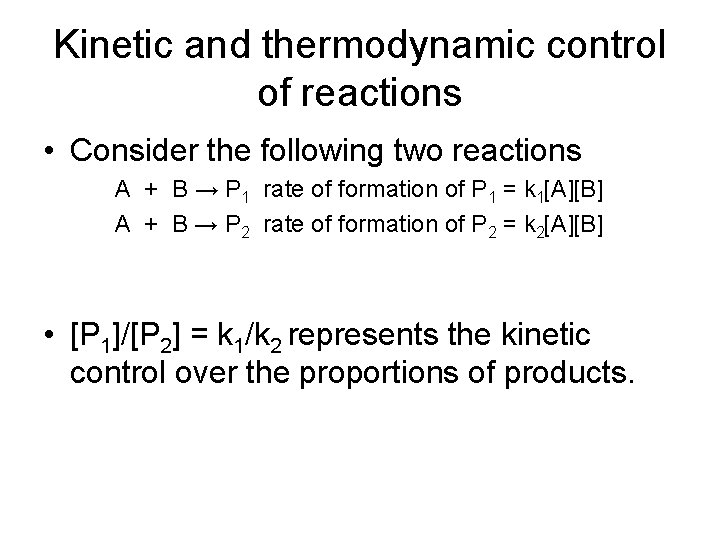
Kinetic and thermodynamic control of reactions • Consider the following two reactions A + B → P 1 rate of formation of P 1 = k 1[A][B] A + B → P 2 rate of formation of P 2 = k 2[A][B] • [P 1]/[P 2] = k 1/k 2 represents the kinetic control over the proportions of products.

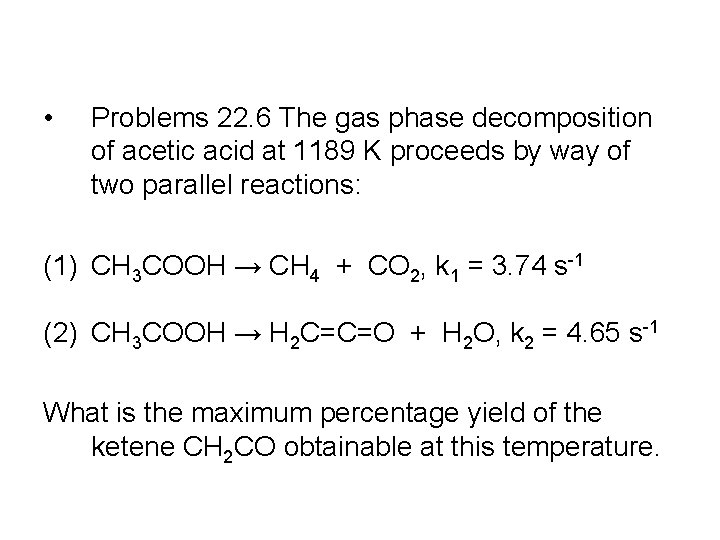
• Problems 22. 6 The gas phase decomposition of acetic acid at 1189 K proceeds by way of two parallel reactions: (1) CH 3 COOH → CH 4 + CO 2, k 1 = 3. 74 s-1 (2) CH 3 COOH → H 2 C=C=O + H 2 O, k 2 = 4. 65 s-1 What is the maximum percentage yield of the ketene CH 2 CO obtainable at this temperature.

Pre-equilibrium • Consider the reaction: A + B ↔ I → P when k 1’ >> k 2, the intermediate product, I, could reach an equilibrium with the reactants A and B. • Knowing that A, B, and I are in equilibrium, one gets: and • When expressing the rate of formation of the product P in terms of the reactants, we get

Self-test 22. 9: Show that the pre-equilibrium mechanism in which 2 A ↔ I followed by I + B → P results in an overall third-order reaction. Solution: A+ A↔I I+B→P k 1, k 1’ k 2 write the rate law for the product P Because I, and A are in pre-equilibrium so [I] = K [A]2 Therefore, the overall reaction order is 3.

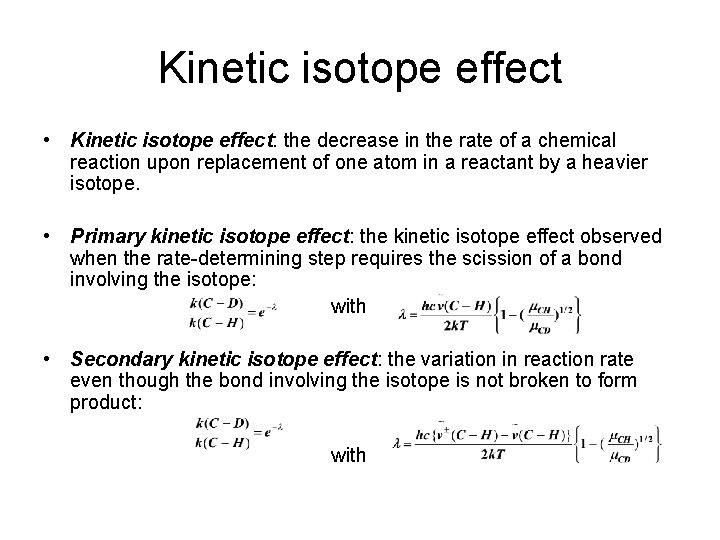
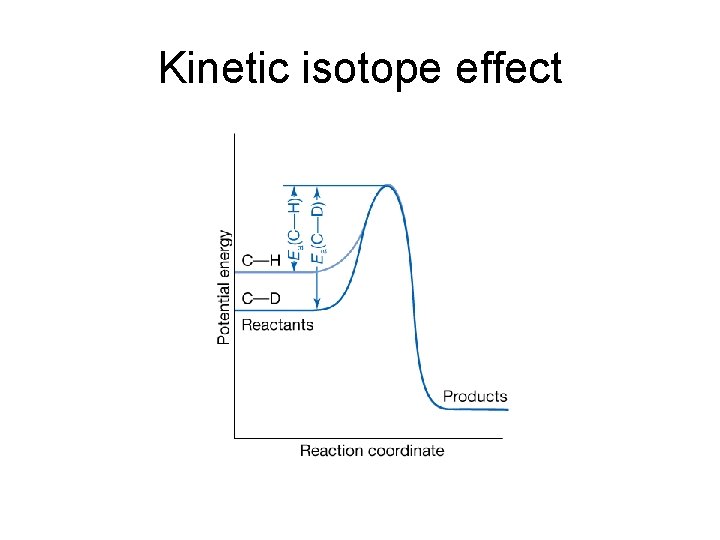
Kinetic isotope effect • Kinetic isotope effect: the decrease in the rate of a chemical reaction upon replacement of one atom in a reactant by a heavier isotope. • Primary kinetic isotope effect: the kinetic isotope effect observed when the rate-determining step requires the scission of a bond involving the isotope: with • Secondary kinetic isotope effect: the variation in reaction rate even though the bond involving the isotope is not broken to form product: with

Kinetic isotope effect

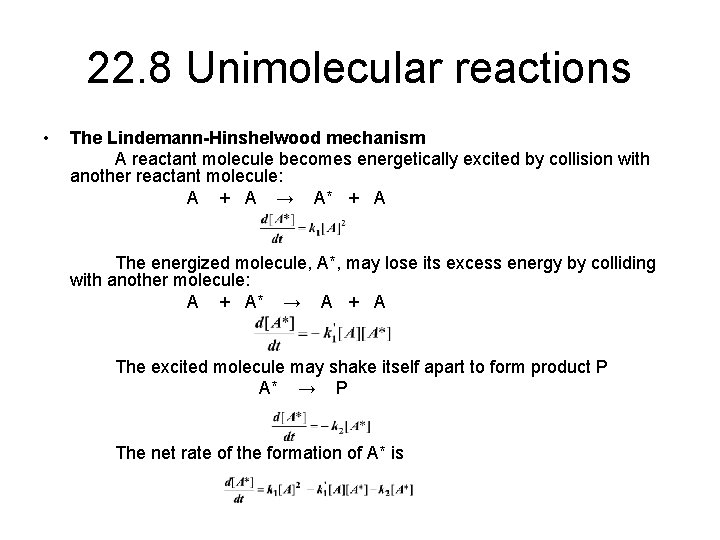
22. 8 Unimolecular reactions • The Lindemann-Hinshelwood mechanism A reactant molecule becomes energetically excited by collision with another reactant molecule: A + A → A* + A The energized molecule, A*, may lose its excess energy by colliding with another molecule: A + A* → A + A The excited molecule may shake itself apart to form product P A* → P The net rate of the formation of A* is

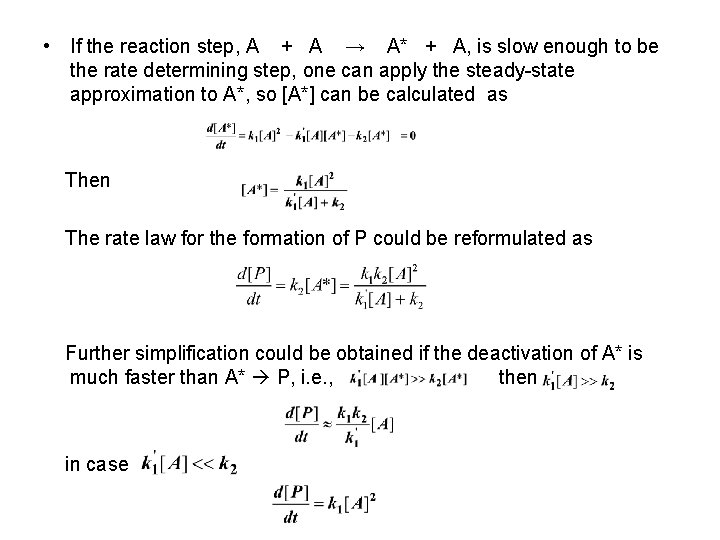
• If the reaction step, A + A → A* + A, is slow enough to be the rate determining step, one can apply the steady-state approximation to A*, so [A*] can be calculated as Then The rate law for the formation of P could be reformulated as Further simplification could be obtained if the deactivation of A* is much faster than A* P, i. e. , then in case

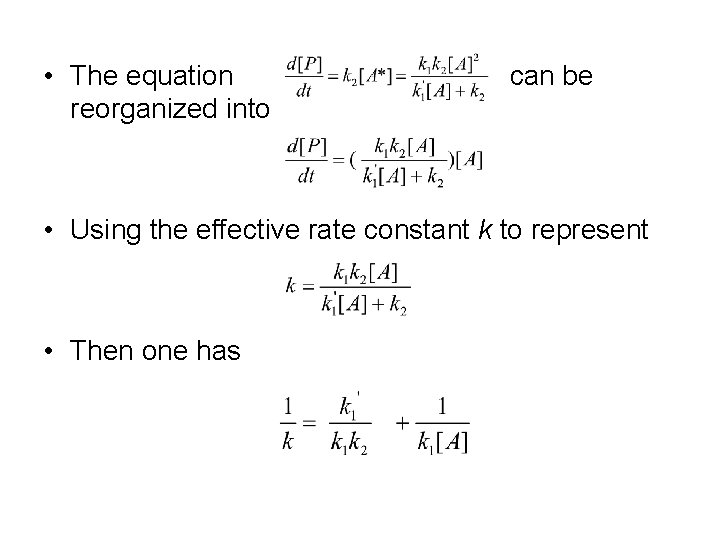
• The equation reorganized into can be • Using the effective rate constant k to represent • Then one has

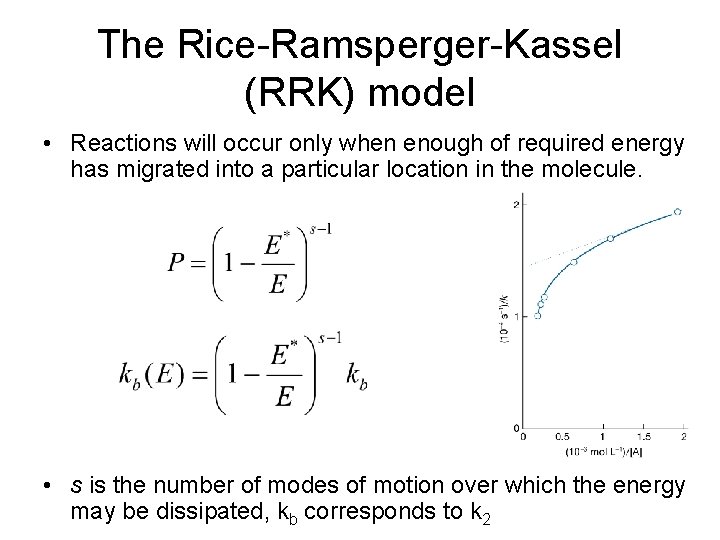
The Rice-Ramsperger-Kassel (RRK) model • Reactions will occur only when enough of required energy has migrated into a particular location in the molecule. • s is the number of modes of motion over which the energy may be dissipated, kb corresponds to k 2

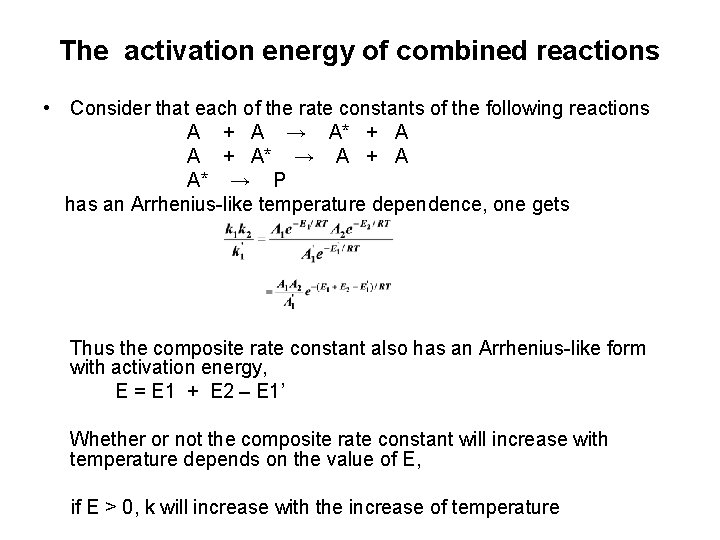
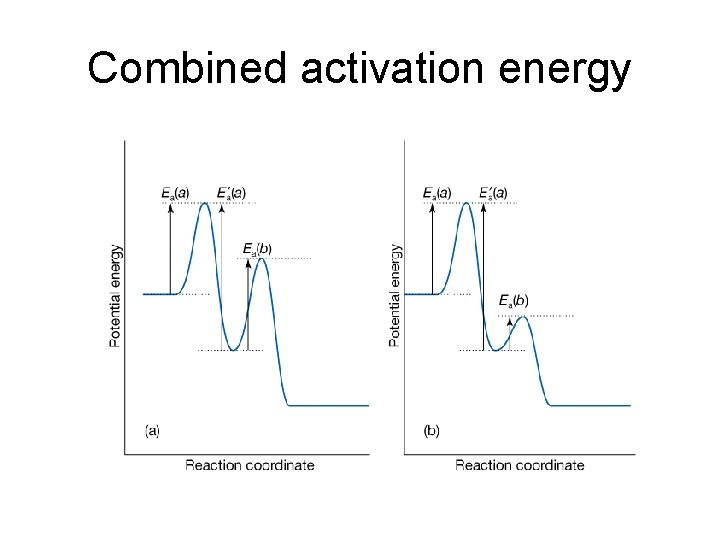
The activation energy of combined reactions • Consider that each of the rate constants of the following reactions A + A → A* + A A + A* → A + A A* → P has an Arrhenius-like temperature dependence, one gets Thus the composite rate constant also has an Arrhenius-like form with activation energy, E = E 1 + E 2 – E 1’ Whether or not the composite rate constant will increase with temperature depends on the value of E, if E > 0, k will increase with the increase of temperature

Combined activation energy

• Theoretical problem 22. 20 The reaction mechanism A 2 ↔ A + A (fast) A + B → P (slow) Involves an intermediate A. Deduce the rate law for the reaction. • Solution: