Chemical Reactions Chemical Reactions A process in which













- Slides: 16

Chemical Reactions

Chemical Reactions A process in which one or more substances, are converted into one or more new substances.

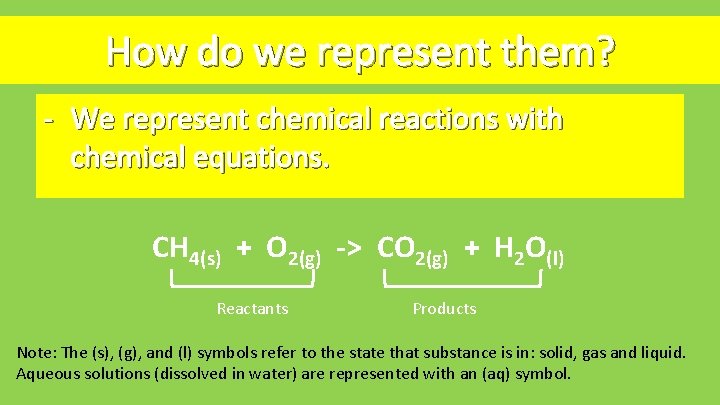
How do we represent them? - We represent chemical reactions with chemical equations. CH 4(s) + O 2(g) -> CO 2(g) + H 2 O(l) Reactants Products Note: The (s), (g), and (l) symbols refer to the state that substance is in: solid, gas and liquid. Aqueous solutions (dissolved in water) are represented with an (aq) symbol.

Can we classify chemical reactions? - There are 5 major types of chemical reactions that occur. - Each can be recognized by analyzing the chemical equation.

Synthesis Rxn A chemical reaction where two or more substances form one new substance. These are also commonly referred to as combination reactions.

2 Al(s) + 3 I 2(s) -> Al 2 I 6(s) Notice, two reactants are being synthesized into one product.

Decomposition Rxn A chemical reaction where one reactant compound breaks apart into two or more products.

H 2 CO 3(aq) H 2 O(l) + CO 2(g) Notice, one reactant is decomposing into two products.

Single Replacement Rxn A chemical change in which one element will replace one element in another compound.

Cu. SO 4(s) + Fe(s) -> Cu(s) + Fe. SO 4(s) Notice, the Iron (Fe) is replacing the Copper (Cu). Only one element is being replaced.

Double Replacement Rxns A chemical reaction where positive ions of one compound displaces the positive ion of another compound.

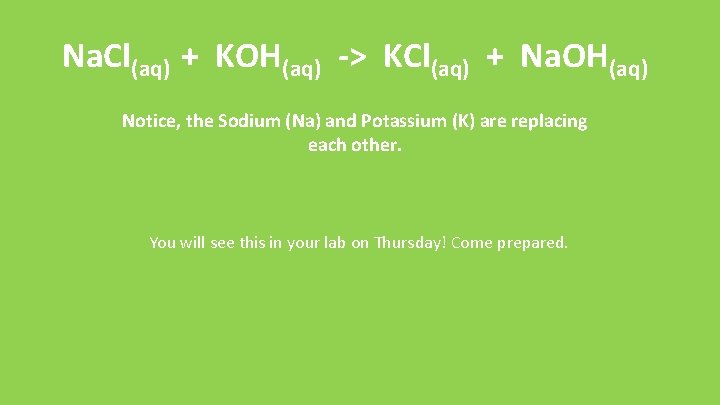
Na. Cl(aq) + KOH(aq) -> KCl(aq) + Na. OH(aq) Notice, the Sodium (Na) and Potassium (K) are replacing each other. You will see this in your lab on Thursday! Come prepared.

Combustion Rxns A process in which an element or compound (usually carbon based) reacts with oxygen, producing carbon dioxide, water, heat and light.

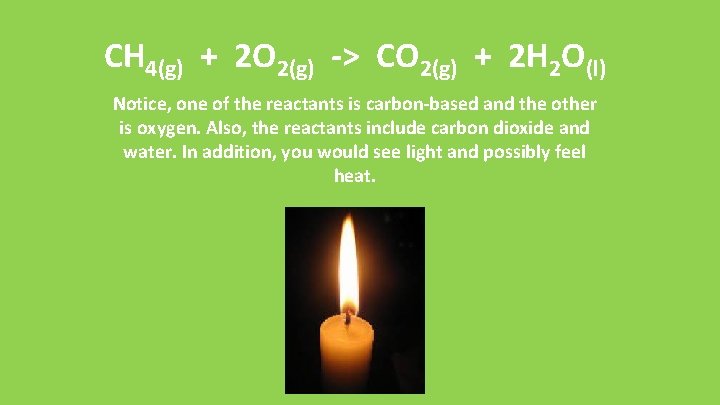
CH 4(g) + 2 O 2(g) -> CO 2(g) + 2 H 2 O(l) Notice, one of the reactants is carbon-based and the other is oxygen. Also, the reactants include carbon dioxide and water. In addition, you would see light and possibly feel heat.

How do we know chemical reactions are taking place? - Production of a gas (often bubbles can be seen) Precipitate forms Color change Temperature change Light Change in volume Change in conductivity And more…

Classify the following reactions… - H 2 O(l) - > H 2(g) + O 2(g) 2 C 2 H 6(g) + 7 O 2(g) -> 4 CO 2(g) + 6 H 2 O(l) 6 Li(s) + N 2(g) -> 2 Li 3 N(s) Mg(s) + Cu. SO 4(aq) -> Cu(s) + Mg. SO 4(s)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal What is this
What is this Redox examples
Redox examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers 10 market forms of poultry
10 market forms of poultry Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Examples of chemical change
Examples of chemical change Redox rules
Redox rules How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions