Chemical Reactions Chemical Reactions A process in which




- Slides: 12

Chemical Reactions

Chemical Reactions A process in which one substance is changed into another substance

Law of Conservation of Mass During a chemical r e a c t i o n, a t o m s a r e neither created nor destroyed.

A reaction has occured when: z z Color changes Heat changes A gas is produced A precipitate forms

Parts of a Reaction z Reactants y. S u b s t a n c e s t h a t i n t e r a c t z. P r o d u c t s y. N e w s u b s t a n c e s f o r m e d

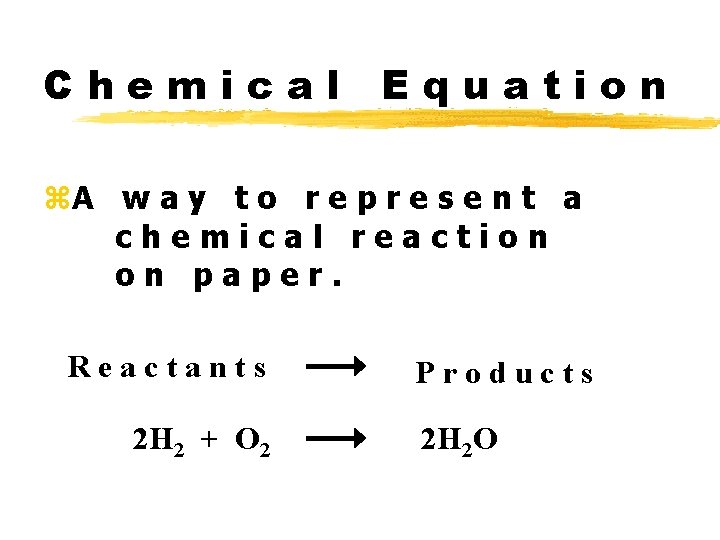
Chemical Equation z. A w a y t o r e p r e s e n t a chemical reaction on paper. Reactants 2 H 2 + O 2 Products 2 H 2 O

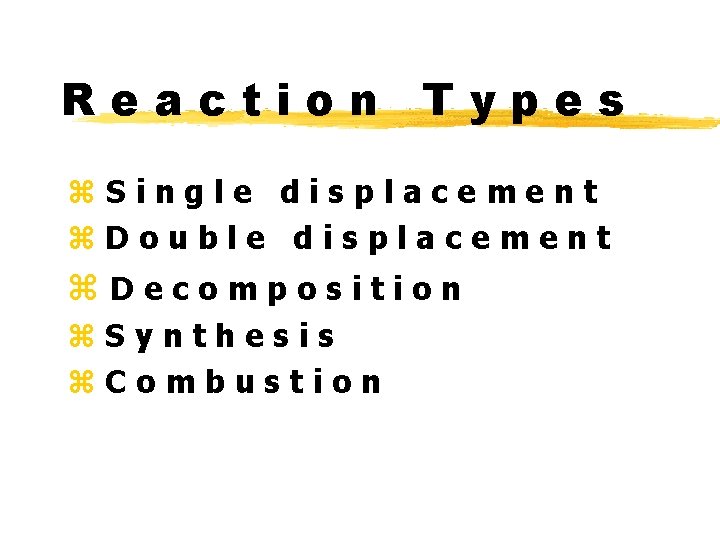
Reaction Types z. Single displacement z. Double displacement z. Decomposition z. Synthesis z. Combustion

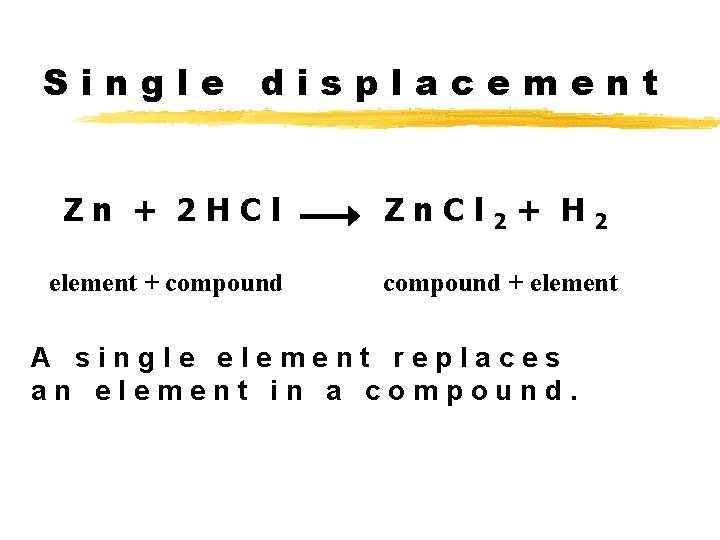
Single displacement Zn + 2 HCl element + compound Zn. Cl 2 + H 2 compound + element A single element replaces an element in a compound.

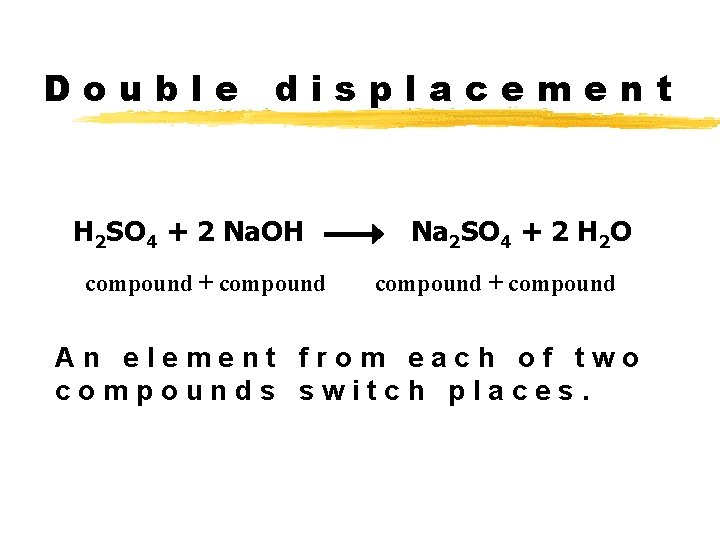
Double displacement H 2 SO 4 + 2 Na. OH compound + compound Na 2 SO 4 + 2 H 2 O compound + compound An element from each of two compounds switch places.

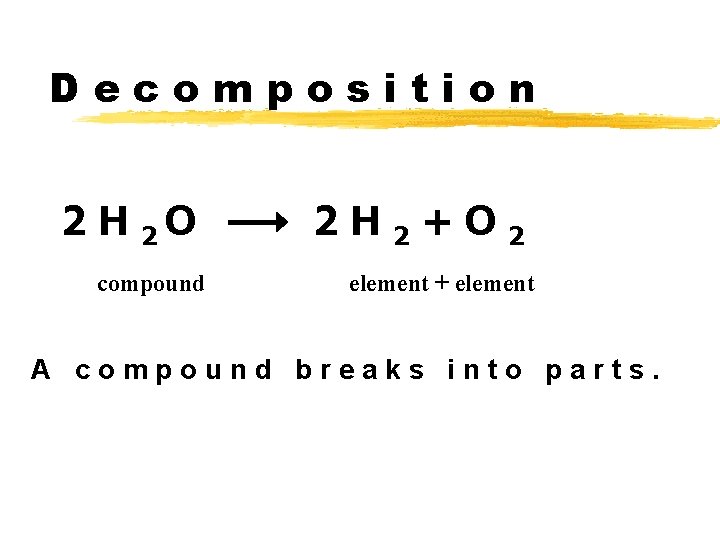
Decomposition 2 H 2 O compound 2 H 2+O 2 element + element A compound breaks into parts.

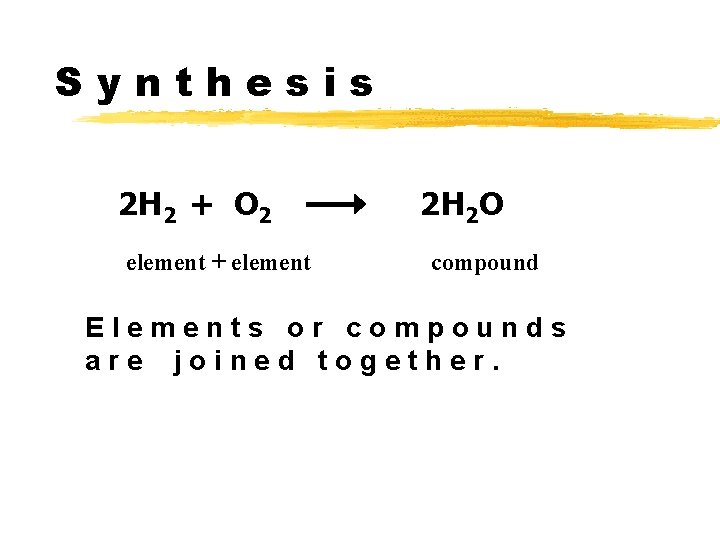
Synthesis 2 H 2 + O 2 element + element 2 H 2 O compound Elements or compounds are joined together.

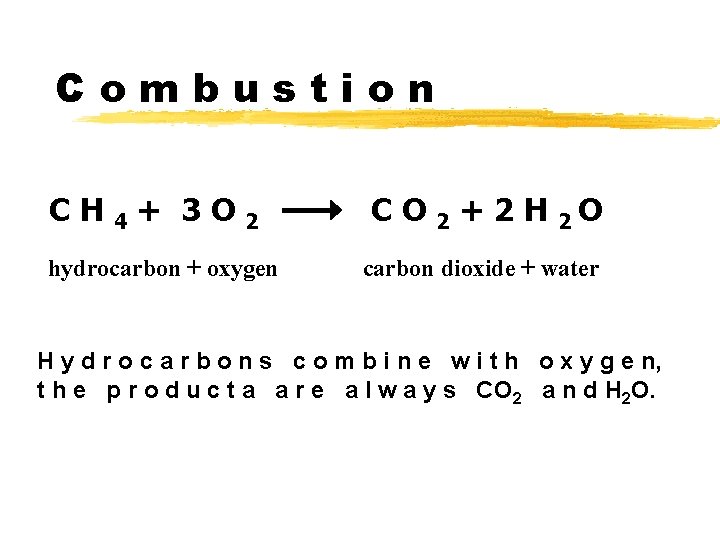
Combustion CH 4+ 3 O 2 hydrocarbon + oxygen C O 2 + 2 H 2 O carbon dioxide + water H y d r o c a r b o n s c o m b i n e w i t h o x y g e n, t h e p r o d u c t a a r e a l w a y s CO 2 a n d H 2 O.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations What is this
What is this Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Market forms of chicken
Market forms of chicken Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Balancing chemical equations definition
Balancing chemical equations definition Balancing redox reactions
Balancing redox reactions Identify types of reactions
Identify types of reactions























