Types of Reactions There are 5 types of







- Slides: 20

Types of Reactions • There are 5 types of reactions – Combination – Decomposition – Single Displacement – Double Displacement – Combustion

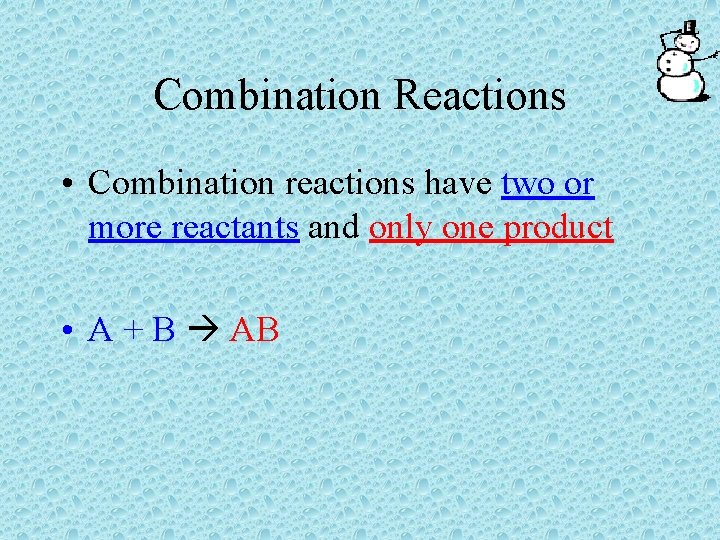
Combination Reactions • Combination reactions have two or more reactants and only one product • A + B AB

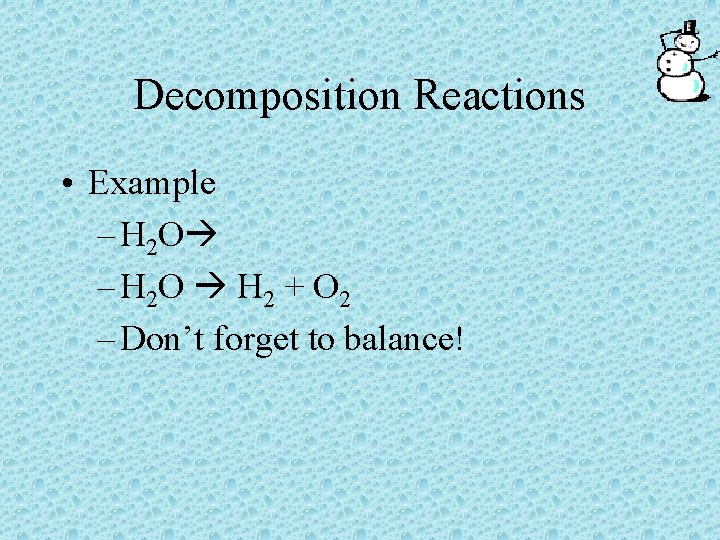
Decomposition Reactions • Decomposition reactions have only one reactant which breaks down into two or more products • AB A + B

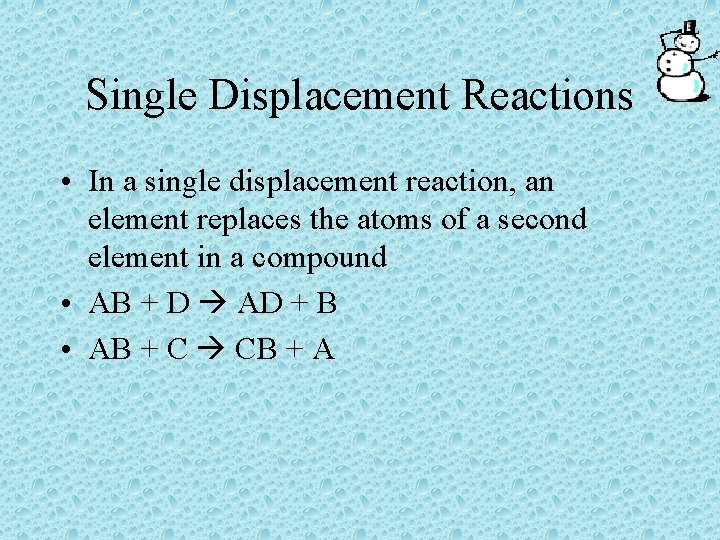
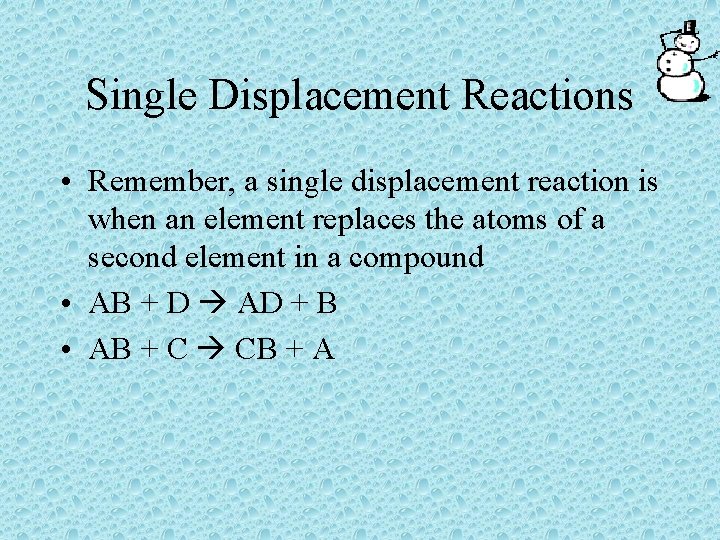
Single Displacement Reactions • In a single displacement reaction, an element replaces the atoms of a second element in a compound • AB + D AD + B • AB + C CB + A

Double Displacement Reactions • Remember, these involve the exchange of positive ions (or negative ions) between two compounds • AB + CD AD + CB

Combustion • When an organic compounds reacts with oxygen to produce water and either CO or CO 2 • Complete combustion always ends with CO 2 and H 2 O • Incomplete combustion always ends with CO and H 2 O

Combination Reactions • When two nonmetals react, or a transition metal reacts with a nonmetal, more than one product is often possible • With two nonmetals it is hard to predict the product • With the transition metal reacts it depends on the charge being used

Combination Reactions • Example – Fe(s) + S(s) Fe. S(s) iron (II) sulfide – Fe(s) + S(s) Fe 2 S 3(s) iron (III) sulfide – Make sure to balance when finished

Combination Reactions • When a metal from one of the first two groups reacts with a nonmetal there is only one possible product • Use the oxidation number (charge) to determine the final compound

Combination Reactions • • • Example K(s) + Cl 2(g) K+ and Cl-1 so KCl K(s) + Cl 2(g) KCl(s) Make sure to balance

Decomposition Reactions • Remember, a decomposition reaction has only one reactant which breaks down into two or more products • AB A + B

Decomposition Reactions • The difficulty with decomposition reactions is the products can be any combination of elements or compounds found in the reactant • It is usually very difficult to predict decomposition products

Decomposition Reactions • Example – H 2 O H 2 + O 2 – Don’t forget to balance!

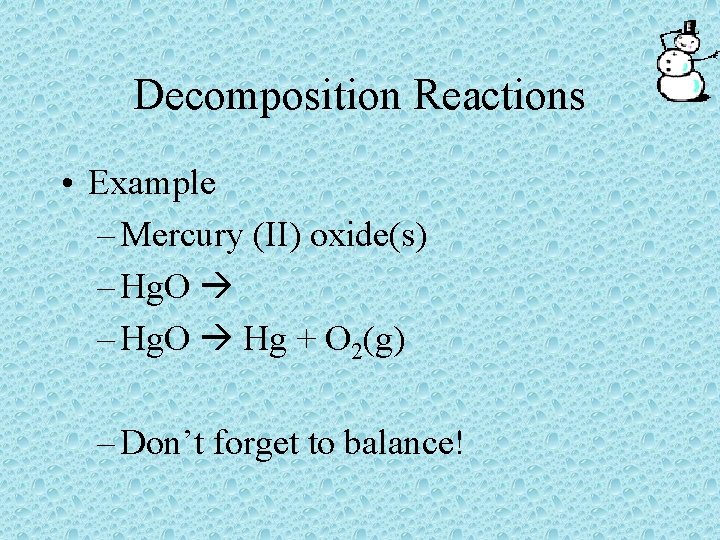
Decomposition Reactions • Example – Mercury (II) oxide(s) – Hg. O Hg + O 2(g) – Don’t forget to balance!

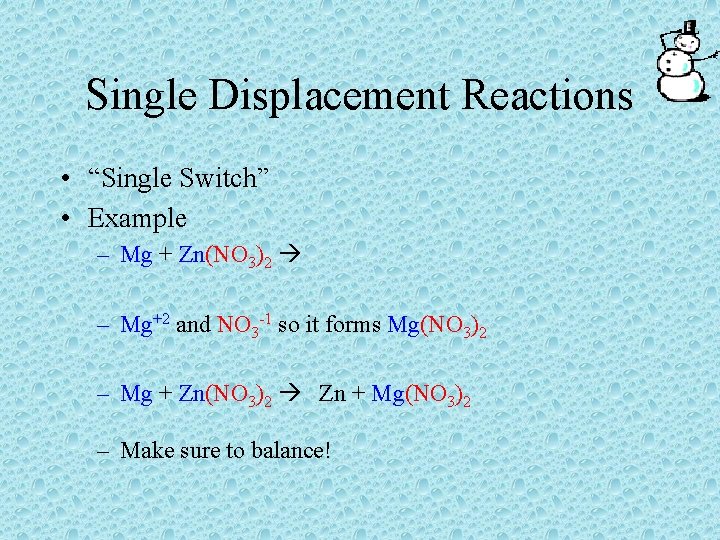
Single Displacement Reactions • Remember, a single displacement reaction is when an element replaces the atoms of a second element in a compound • AB + D AD + B • AB + C CB + A

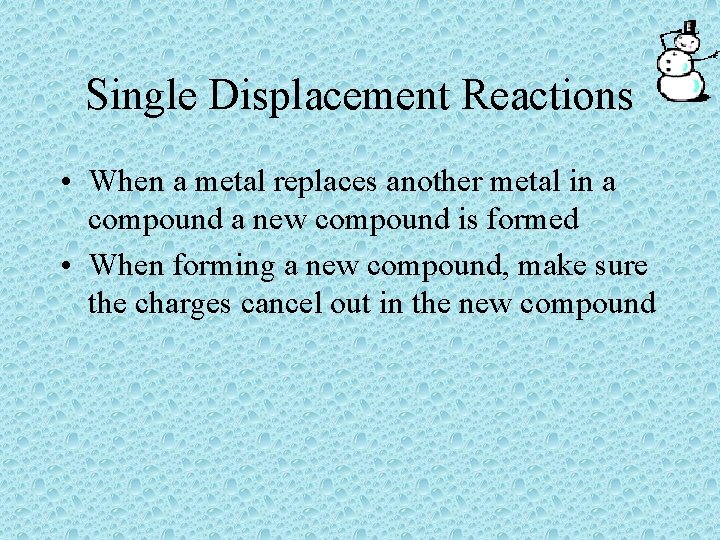
Single Displacement Reactions • When a metal replaces another metal in a compound a new compound is formed • When forming a new compound, make sure the charges cancel out in the new compound

Single Displacement Reactions • “Single Switch” • Example – Mg + Zn(NO 3)2 – Mg+2 and NO 3 -1 so it forms Mg(NO 3)2 – Mg + Zn(NO 3)2 Zn + Mg(NO 3)2 – Make sure to balance!

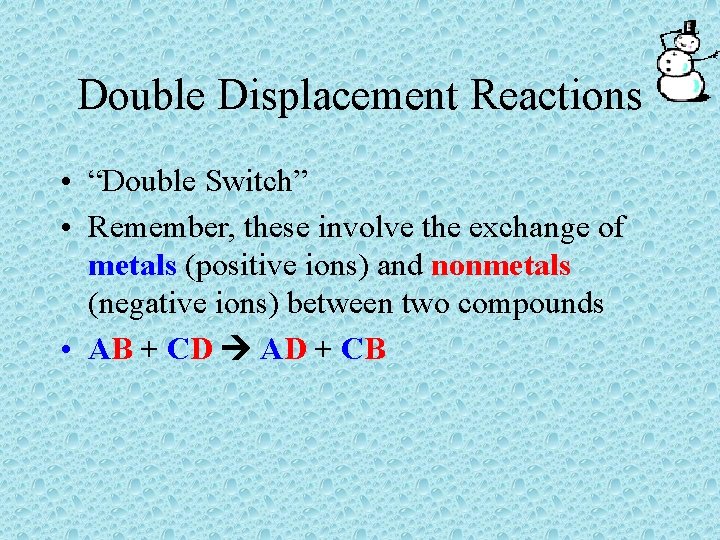
Double Displacement Reactions • “Double Switch” • Remember, these involve the exchange of metals (positive ions) and nonmetals (negative ions) between two compounds • AB + CD AD + CB

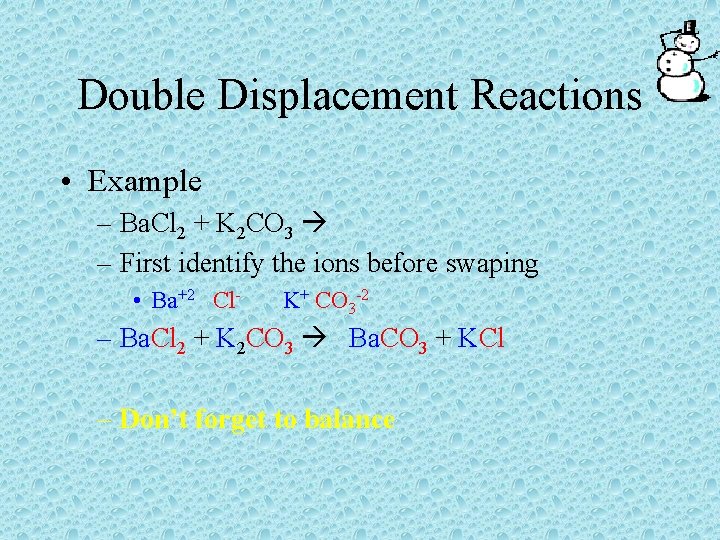
Double Displacement Reactions • Example – Ba. Cl 2 + K 2 CO 3 – First identify the ions before swaping • Ba+2 Cl- K+ CO 3 -2 – Ba. Cl 2 + K 2 CO 3 Ba. CO 3 + KCl – Don’t forget to balance

Combustion • When an organic compounds reacts with oxygen to produce water and either CO or CO 2 • Complete combustion always ends with CO 2 and H 2 O
 Insidan region jh
Insidan region jh Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers How to write half reactions
How to write half reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Balancing redox reactions
Balancing redox reactions Types of reaction
Types of reaction Five types of reactions
Five types of reactions Different types of reactions
Different types of reactions Types of reactions chemistry
Types of reactions chemistry 5 types of chemical reactions
5 types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions U 235 fission products
U 235 fission products Four types of chemical reactions
Four types of chemical reactions 5 types of reactions chemistry
5 types of reactions chemistry Five chemical change
Five chemical change 5 general types of chemical reactions
5 general types of chemical reactions Decomposition reactions
Decomposition reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions