Chemical Reactions Neutralization Reactions Environment Neutralization Reactions n







- Slides: 9

Chemical Reactions Neutralization Reactions & Environment

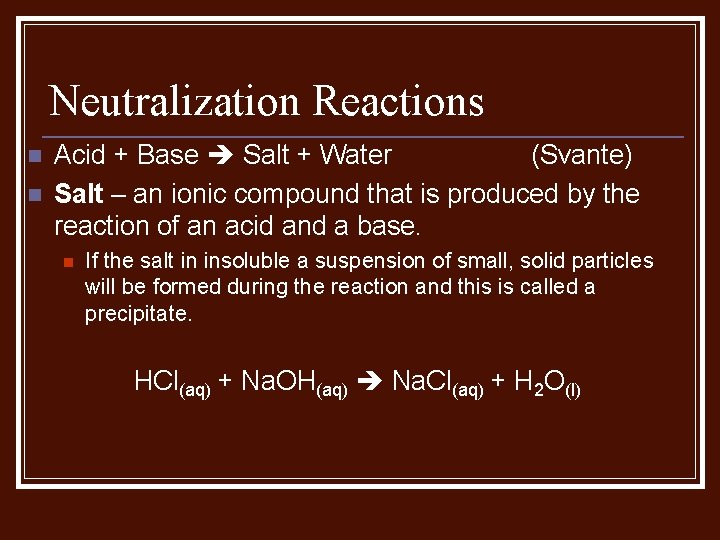
Neutralization Reactions n n Acid + Base Salt + Water (Svante) Salt – an ionic compound that is produced by the reaction of an acid and a base. n If the salt in insoluble a suspension of small, solid particles will be formed during the reaction and this is called a precipitate. HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l)

Neutralization and Environmental Challenges n Acid Precipitation n Sulfuric Acid – H 2 SO 4(aq) n S(s) + O 2(g) SO 2(g) n 2 SO 2(g) + O 2(g) 2 SO 3(g) n SO 3(g) + H 2 O(l) H 2 SO 4(aq)

Neutralization and Environmental Challenges n Acid Precipitation • Different oxides of sulfur (SO 2 and SO 3) and nitrogen (NO and NO 2) are referred to as SOx and NOx. • When they are burned in air they react with water to form acid precipitation.

Neutralization and Environmental Challenges • Normal rain is usually slightly acidic (5. 5 – 6. 2) due to carbon dioxide in the atmosphere: CO 2(g) + H 2 O(l) H 2 CO 3(aq). • n Adding the other acids to the atmosphere increases the acidity of the precipitation and it is normally now between 4 and 5 on the p. H scale. Acid precipitation is soluble in water (it joins water easily) and this poses a threat to lakes and ponds. n As the p. H of a lake approaches 6, insects and other aquatic animals begin to die. As the p. H approaches 5, plants and micro-organisms begin to die. Below 5 and all life in a lake or pond is gone and the water appears crystal clear.

Neutralization and Environmental Challenges n n Heavy Metals - Acid precipitation dissolves metals (aluminum, mercury and copper), which may leach into ground water that feeds rivers and lakes. In high concentrations these metals are harmful, even to us. Ways to combat Acid Precipitation n Calcium carbonate (limestone) reacts with acids in a neutralization reaction. There are three factors that determine how limestone should be added to a lake: n n n 1. Volume of water in the lake 2. Chemical composition and reactivity of its rocks and soil 3. Turnover time

Neutralization and Environmental Challenges n Turnover time refers to the time needed for all the water in the lake to be replaced by natural resources. If turnover time is short liming needs to be done more frequently. n It is important to understand that liming is not a solution. It like treating the symptoms instead of the problem itself. Liming cannot alter streams that are acidic or ice that has a low p. H. Limestone also may kill plants and insects that are sensitive to calcium.

Neutralization and Environmental Challenges n n n Using Chemistry to Control Harmful Emissions Scrubbers – anti-pollution device for removing polluting gases such as sulfur dioxide from industrial smokestack emissions. They can remove up to 95% of SO 2 from smokestack emissions. All sources of coal contain sulfur as a contaminant. When coal burns the sulfur is converted to sulfur dioxide. Calcium carbonate is added with the coal and air as it enters the furnace.

Neutralization and Environmental Challenges n The coal burns and CO 2 and SO 2 are produced. The heat breaks the Ca. CO 3 into CO 2 and Ca. O. In the furnace some of the SO 2 reacts with Ca. O to form Ca. SO 3 (calcium sulfite). Some SO 2 remains unreacted and leave the furnace as exhaust. n This enters the scrubber where Ca. O in water is sprayed on the exhaust gases. Most of the remaining SO 2 reacts with the dissolved Ca. O to again form Ca. SO 3. A wet slurry of Ca. SO 3 is formed and discarded.
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Example of neutralization reaction
Example of neutralization reaction Writing neutralization reactions
Writing neutralization reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Financial environment of business
Financial environment of business Unit 5 chemical reactions answers
Unit 5 chemical reactions answers 10 examples of redox reaction
10 examples of redox reaction