Aim How to write neutralization reactions Reactions of


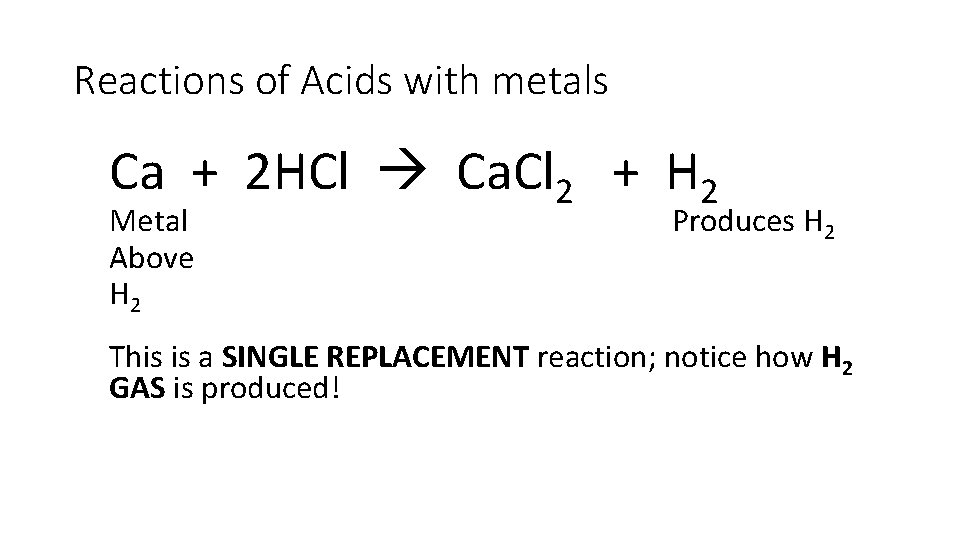
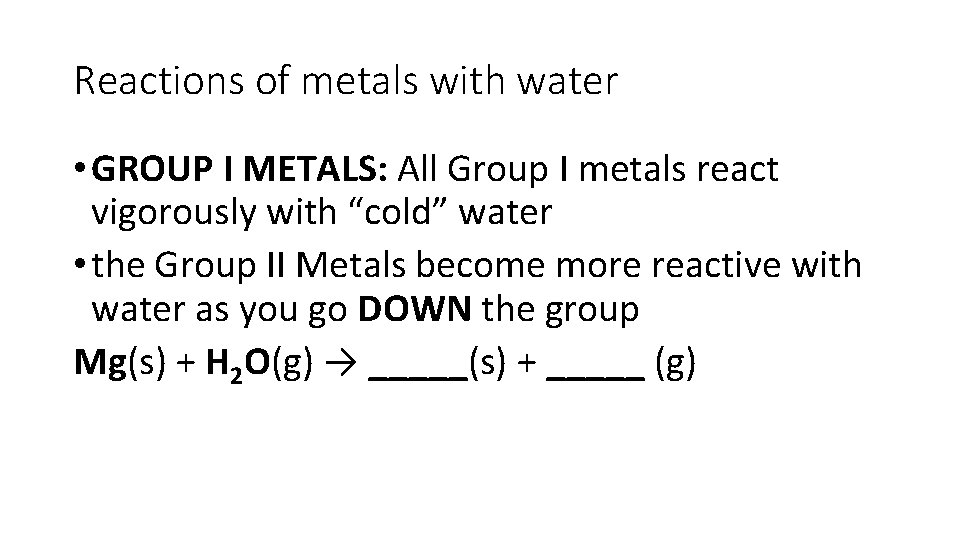

- Slides: 10

Aim: How to write neutralization reactions

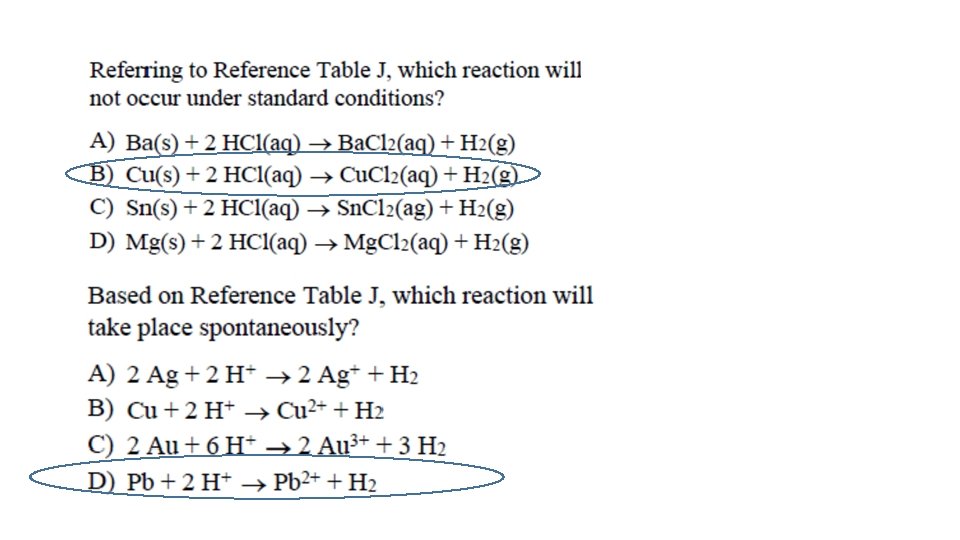
Reactions of Acids with metals • According to Table J, any METAL LOCATED ABOVE H 2 WILL REACT WITH AN ACID to produce H 2 gas and a salt • Example: Which metal, Mg or Cu will react with HCl? ____

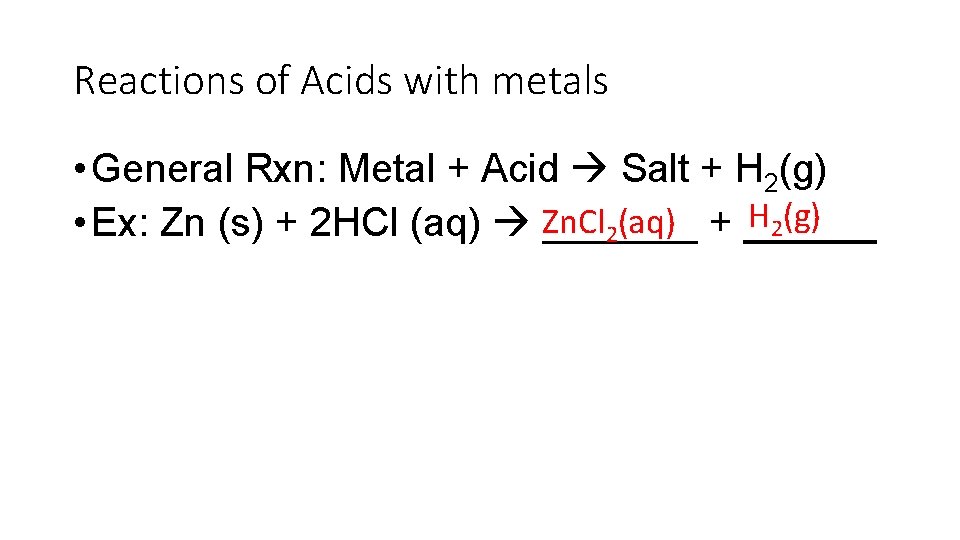
Reactions of Acids with metals • General Rxn: Metal + Acid Salt + H 2(g) H (g) (aq) • Ex: Zn (s) + 2 HCl (aq) Zn. Cl _______ + ______ 2 2
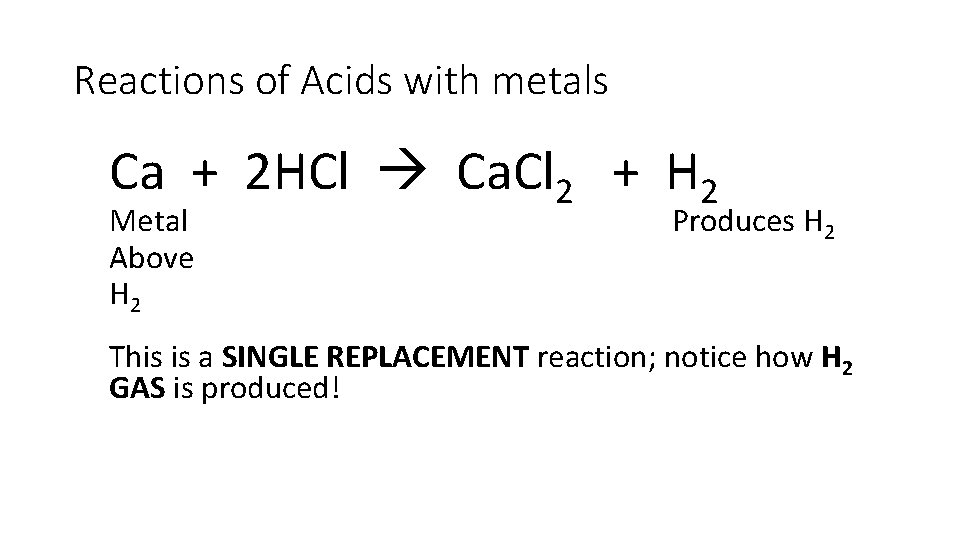
Reactions of Acids with metals Ca + 2 HCl Ca. Cl 2 + H 2 Metal Above H 2 Produces H 2 This is a SINGLE REPLACEMENT reaction; notice how H 2 GAS is produced!
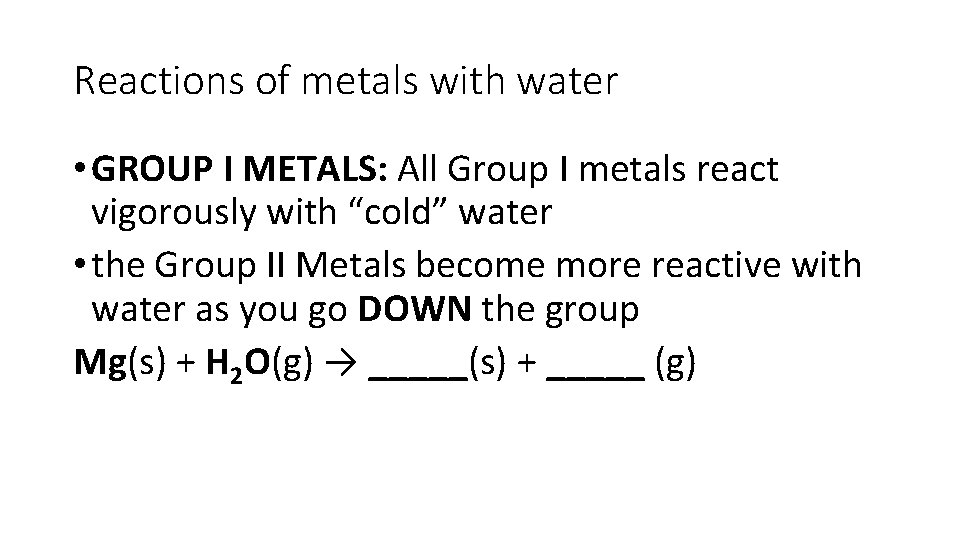
Reactions of metals with water • GROUP I METALS: All Group I metals react vigorously with “cold” water • the Group II Metals become more reactive with water as you go DOWN the group Mg(s) + H 2 O(g) → _____(s) + _____ (g)


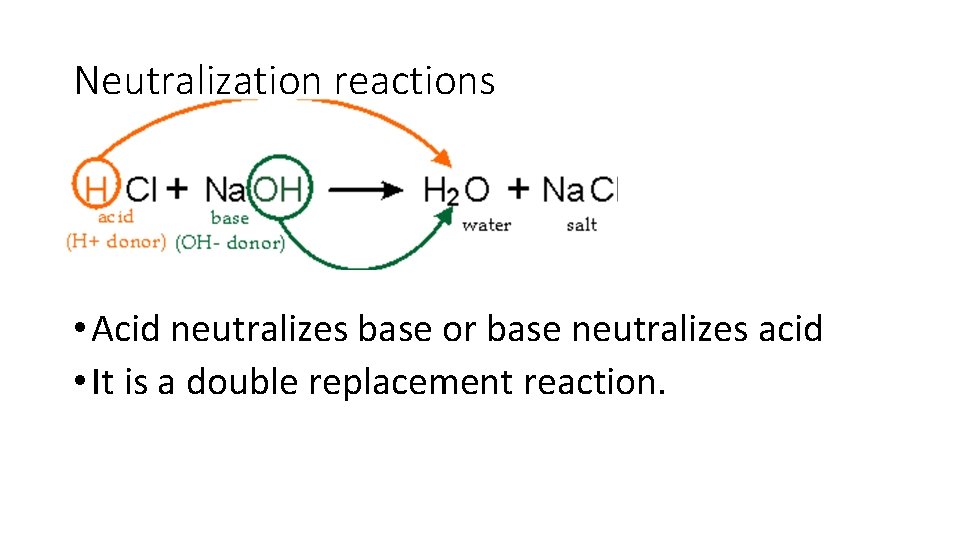
Neutralization reactions • Neutralization occurs: • # of H+ ions = # of OH- ions (equivalent or equal amounts) • when an Arrhenius acid an Arrhenius base react to form WATER and a SALT • Example: Antacid for upset stomach neutralizes the acid in stomach and makes a neutral salt to provide relief

Neutralization reactions HCl + Na. OH Na. Cl + H 2 O Acid Base Salt water • Acid neutralizes base or base neutralizes acid • It is a double replacement reaction.

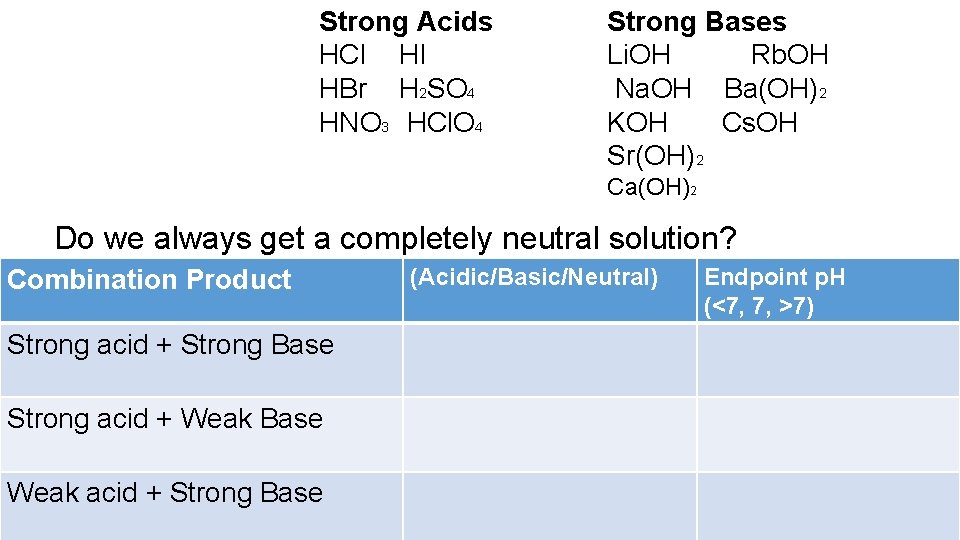
Strong Acids HCl HI HBr H 2 SO 4 HNO 3 HCl. O 4 Strong Bases Li. OH Rb. OH Na. OH Ba(OH)2 KOH Cs. OH Sr(OH)2 Ca(OH)2 Do we always get a completely neutral solution? Combination Product Strong acid + Strong Base Strong acid + Weak Base Weak acid + Strong Base (Acidic/Basic/Neutral) Endpoint p. H (<7, 7, >7)

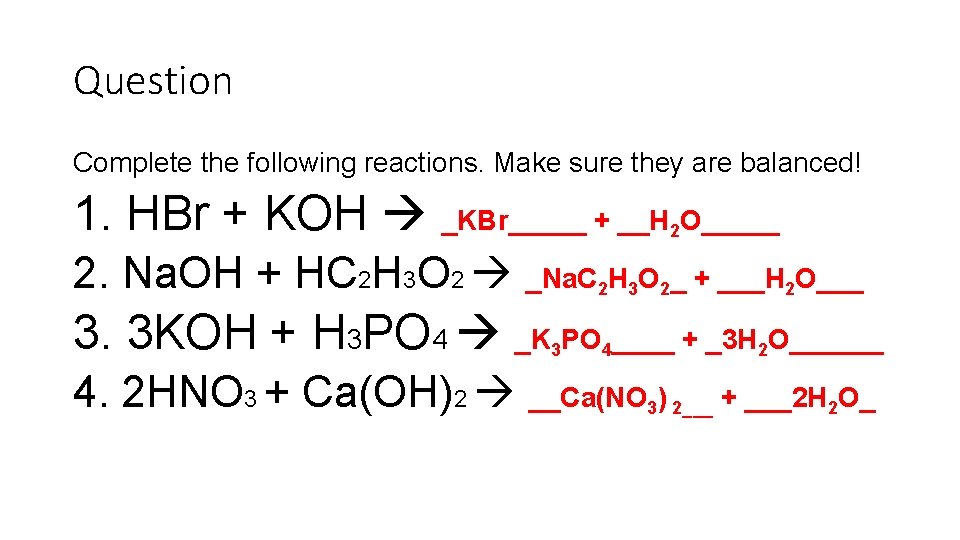
Question Complete the following reactions. Make sure they are balanced! 1. HBr + KOH _KBr_____ + __H O_____ 2 2. Na. OH + HC 2 H 3 O 2 _Na. C 2 H 3 O 2_ + ___H 2 O___ 3. 3 KOH + H 3 PO 4 _K PO ____ + _3 H O______ 3 4 2 4. 2 HNO 3 + Ca(OH)2 __Ca(NO 3) 2___ + ___2 H 2 O_
 Example of neutralization reaction
Example of neutralization reaction Writing neutralization reactions
Writing neutralization reactions How to write half reactions
How to write half reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Titration equation
Titration equation Whats a neutralization reaction
Whats a neutralization reaction Positional neutralization
Positional neutralization Base catalyzed hydrolysis
Base catalyzed hydrolysis