Neutralization Reactions Neutralization Reactions n This is a











- Slides: 13

+ Neutralization Reactions

+ Neutralization Reactions n. This is a type of double displacement reaction n. An acid and base combine to form water and a salt Acid + Base A salt + Water

+ n. Water is formed when the H+ ions from the acid bond with the OH- ions from the base to form H 2 O(l) n. The salt (a metal bonds with a non-metal) is an ionic compound which is formed from the negative ion of the acid, and the positive ion of the base n The salt is typically aqueous (aq)

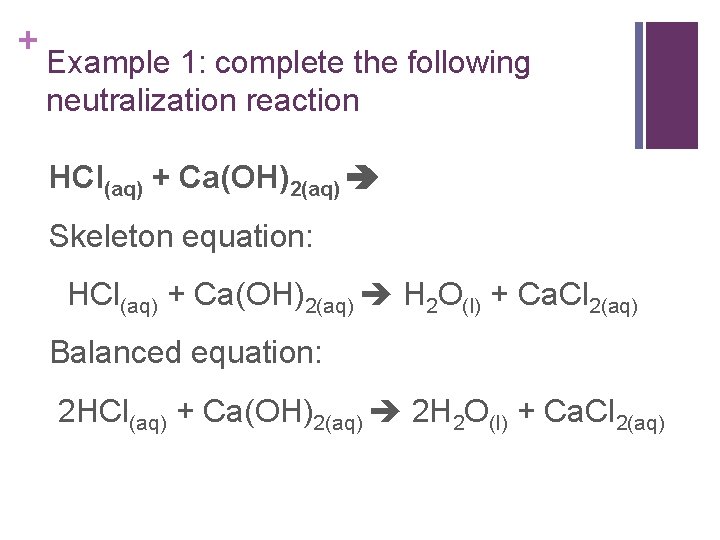
+ Example 1: complete the following neutralization reaction HCl(aq) + Ca(OH)2(aq) Skeleton equation: HCl(aq) + Ca(OH)2(aq) H 2 O(l) + Ca. Cl 2(aq) Balanced equation: 2 HCl(aq) + Ca(OH)2(aq) 2 H 2 O(l) + Ca. Cl 2(aq)

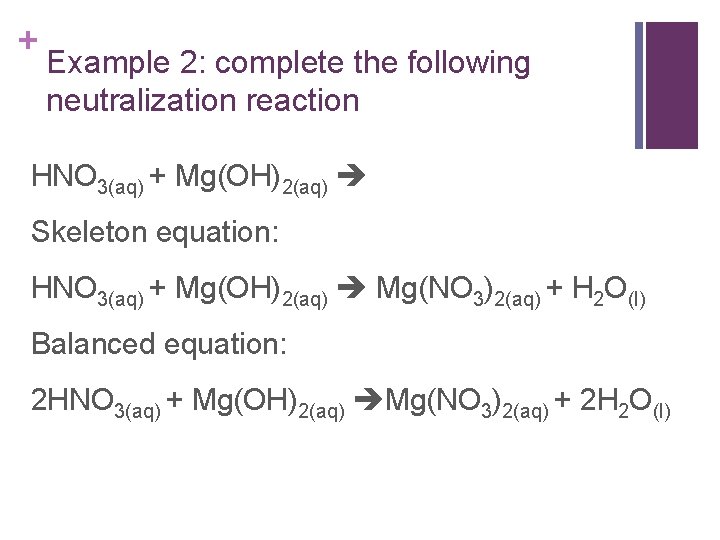
+ Example 2: complete the following neutralization reaction HNO 3(aq) + Mg(OH)2(aq) Skeleton equation: HNO 3(aq) + Mg(OH)2(aq) Mg(NO 3)2(aq) + H 2 O(l) Balanced equation: 2 HNO 3(aq) + Mg(OH)2(aq) Mg(NO 3)2(aq) + 2 H 2 O(l)

Handout “Acidic Oceans” +

+ How the oceans become acidic n. CO 2 from the air dissolves in the water forming carbonic acid (H 2 CO 3) CO 2 + H 2 O H 2 CO 3 n. The carbonic acid is very soluble in water so it dissociates into hydrogen and bicarbonate ions. H 2 CO 3 H+ + HCO 3 -

+ n Carbonate ions (CO 32 -) are naturally found in the oceans. n Many ocean organisms need calcium carbonate to make their shells and skeletons. n When the oceans become acidic, the calcium carbonate in the shells and skeletons become weaker because it dissociates (breaks apart into the positive and negative ions) Ca. CO 3 Ca 2+ + CO 32 -

+ Consequences of More Acidic Oceans First Problem: n Since the oceans are more acidic, the hydrogen ions will combine with the carbonate ions forming bicarbonate ions. n This uses up the carbonate ions, so ocean organisms are not able to use it to make their shells and skeleton. n As a result, the health and survival of these organisms is threatened. n It has been reported that some oysters and clams have holes in their shells.

+ Second Problem: n With the reduction of carbonate ions in the ocean, the ability of ocean organism to make their shells and skeletons is greatly affected. n If the bottom of the food chain (plankton) is decreased because they cannot form a skeleton, then the whole food chain collapses. n This will affect the whole ocean ecosystems as well as the human population that depends on food from the oceans.

+ Third Problem: n Corals use carbonate ions in the building of reefs. n Coral reefs support a great number of organisms that humans depend on. n They also act as a barrier to protect coastlines from damage due to storms. n With the decrease in p. H levels, they are affected just like other ocean organisms. They will not be able to build their structures, and their reefs will be weakened.

+ Analyze and Communicate 1. Brainstorm a list of possible solutions for the problems described in this feature. What changes could people make in their lives that could contribute to solutions? 2. What other local and global problems are caused by the fact that we rely so greatly on fossil fuels?
