Secondary Cell Nickel Cadmium Ni Cd Cells and




- Slides: 17

Secondary Cell • Nickel Cadmium (Ni. Cd) Cells and Batteries – This type of cell delivers high current. – It can be recharged many times. – It can be stored for long periods of time. – Applications include • Portable power tools. • Alarm systems. • Portable radio and TV equipment.

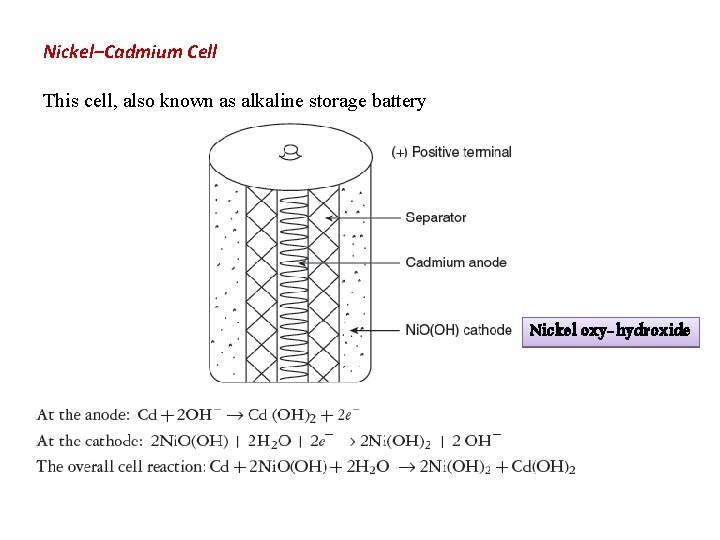
Nickel–Cadmium Cell This cell, also known as alkaline storage battery Nickel oxy-hydroxide

• Nickel Cadmium (Ni. Cd) Cells and Batteries The electrolyte is potassium hydroxide (KOH) its function is to act as a conductor for the transfer of the hydroxyl (OH) ions. – It gives 1. 4 V potential. The products of the reaction are solid hydroxides and they are sticky, cling to the innards of the battery, and remain in place. If current is applied, the reaction can be driven backwards!

Batteries: The Nickel-Cadmium Battery In a nickel-cadmium battery, we can recharge the battery by applying an electrical current from another source Cd(s) + 2 Ni. O(OH)(s) + 2 H 2 O(l) Cd(OH)2(s) + 2 Ni(OH)2(s)

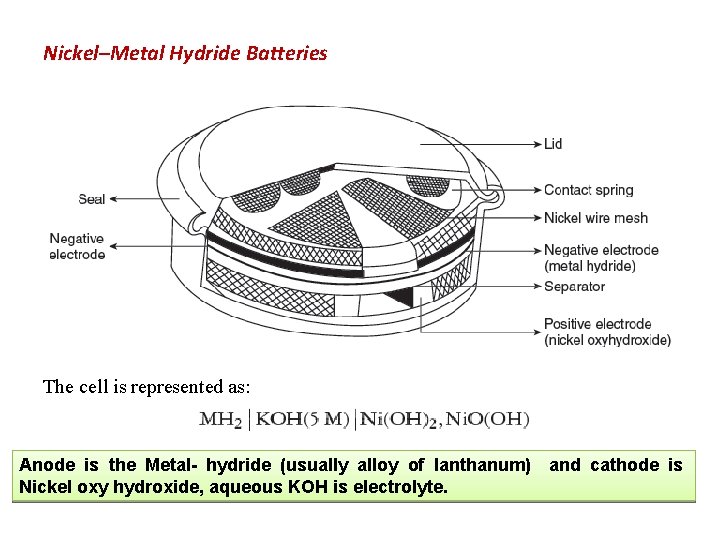
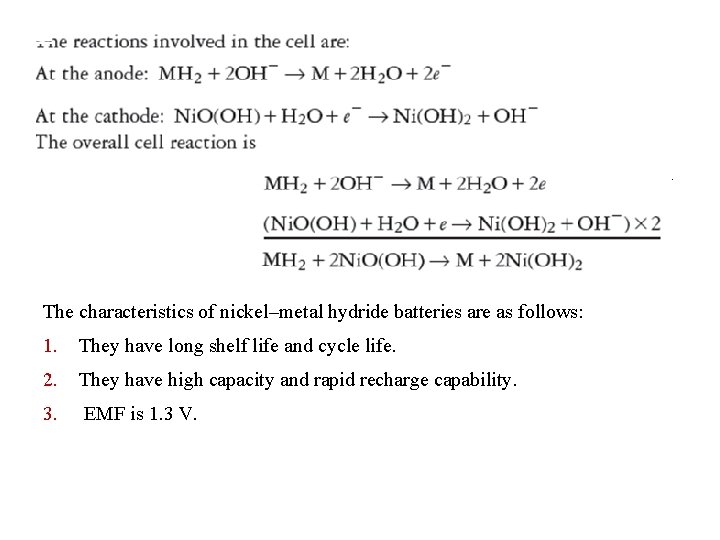
Nickel–Metal Hydride Batteries The cell is represented as: Anode is the Metal- hydride (usually alloy of lanthanum) Nickel oxy hydroxide, aqueous KOH is electrolyte. and cathode is

The characteristics of nickel–metal hydride batteries are as follows: 1. They have long shelf life and cycle life. 2. They have high capacity and rapid recharge capability. 3. EMF is 1. 3 V.

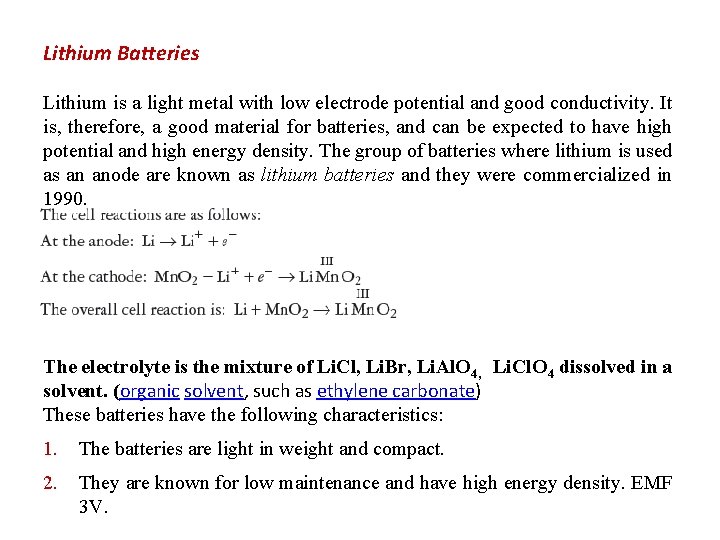
Lithium Batteries Lithium is a light metal with low electrode potential and good conductivity. It is, therefore, a good material for batteries, and can be expected to have high potential and high energy density. The group of batteries where lithium is used as an anode are known as lithium batteries and they were commercialized in 1990. The electrolyte is the mixture of Li. Cl, Li. Br, Li. Al. O 4, Li. Cl. O 4 dissolved in a solvent. (organic solvent, such as ethylene carbonate) These batteries have the following characteristics: 1. The batteries are light in weight and compact. 2. They are known for low maintenance and have high energy density. EMF 3 V.

There are two types of lithium-based batteries available. 1. Lithium batteries 2. Lithium-ion batteries • used as anode. These types of batteries are not rechargeable. • Inlithium-ion batteries, lithium compounds are used as anode. • These batteries are known asre-chargeable batteries. Therefore, Lithium ion batteries are considered as best than pure Lithium based batteries. PH 0101 Unit-5 Lecture-7 8

4. Lithium-ion battery (Li-ion Battery) Li-ion batteries are secondary batteries. • (Graphite) • compounds, typically the three electro-active oxide materials. • Lithium Cobalt-oxide (Li. Co. O 2 ) • Lithium Manganese-oxide (Li. Mn 2 O 4 ) • Lithium Nickel-oxide (Li. Ni. O 2) • Electrolyte: solid lithium-salt electrolytes (Li. PF 6, Li. BF 4, or Li. Cl. O 4) and organic solvents (ether) 9

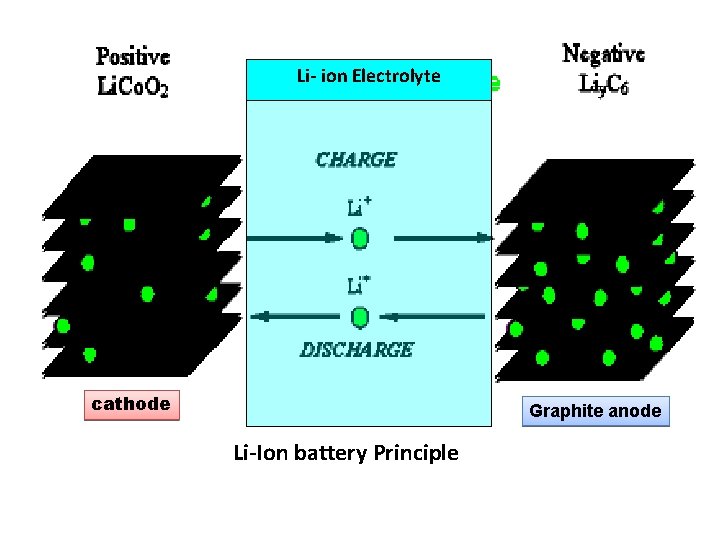
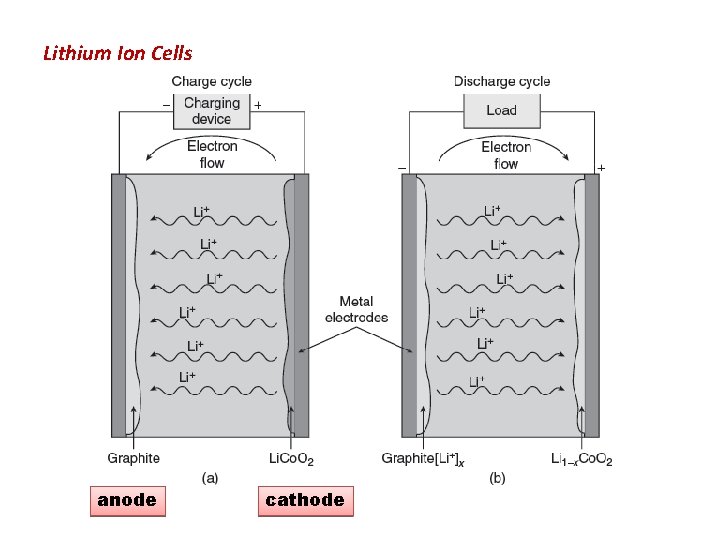
Principle • oxidation reduction reaction, rather lithium ions are transported from one electrode to other through electrolyte (Li salt in organic solvent) • (Li. Co. O 2 ) through Electrolyte during discharging as a result the electron flows through the external circuit to balance the positive charge of Li+. • During charging Li-ion moves to opposite direction so the electron also moves to opposite direction. 10

Li- ion Electrolyte cathode Graphite anode Li-Ion battery Principle

Lithium Ion Cells anode cathode

Applications • • • The Li-ion batteries are used in cameras, calculators implantable device instruments, portable radios and TVs, pagers • They are used to operate laptop computers and phones and aerospace application mobile 13

Fuel Cells The conversion of fuel into electrical energy involves a number of steps and there is loss of energy at every step. The efficiency of the process is around 40%. There is also a viable way of converting the chemical energy of fuel directly into electrical energy through catalytically activated redox reactions. Such devices are called fuel cells. Fuel cell is a galvanic cell in which the electrical energy is directly obtained from redox reaction of the fuel. Comparison with Conventional Galvanic Cells 1. They consist of two catalytic electrodes. 2. The reagents used are fuel and oxidant. 3. The fuel and oxidant are not stored in the cell. 4. No pollutants and hence fuel cells are environmentally friendly. 5. No toxic species are formed in a fuel cell. 6. They do not need charging.

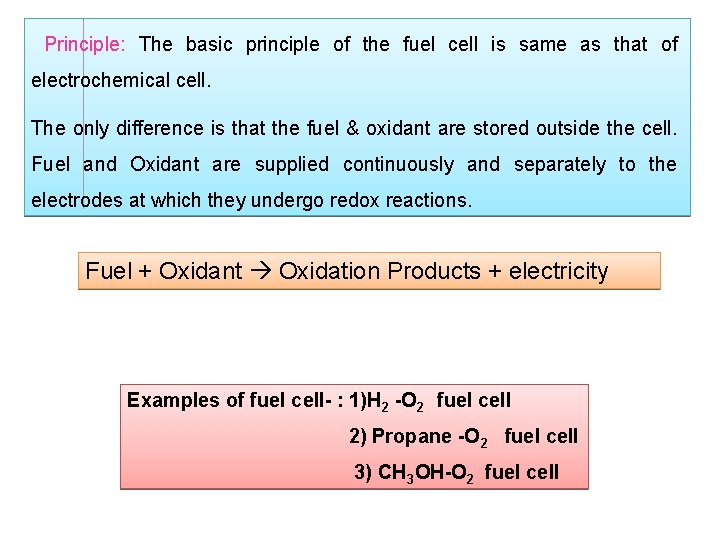
Principle: The basic principle of the fuel cell is same as that of electrochemical cell. The only difference is that the fuel & oxidant are stored outside the cell. Fuel and Oxidant are supplied continuously and separately to the electrodes at which they undergo redox reactions. Fuel + Oxidant Oxidation Products + electricity Examples of fuel cell- : 1)H 2 -O 2 fuel cell 2) Propane -O 2 fuel cell 3) CH 3 OH-O 2 fuel cell

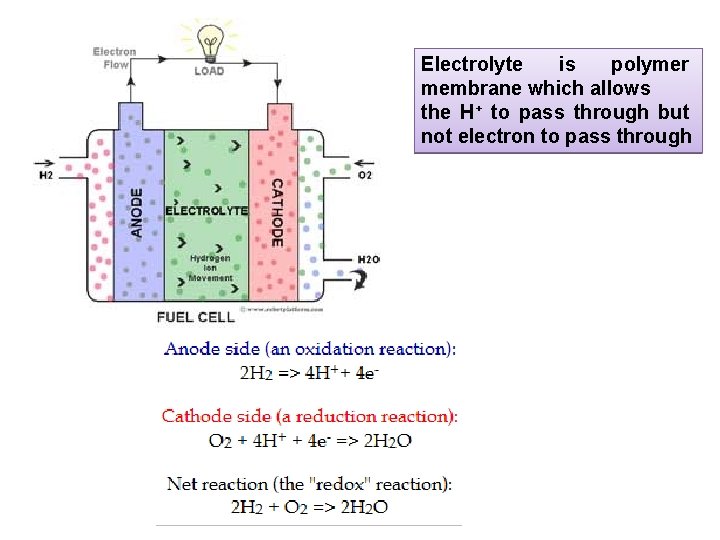
Electrolyte is polymer membrane which allows the H+ to pass through but not electron to pass through

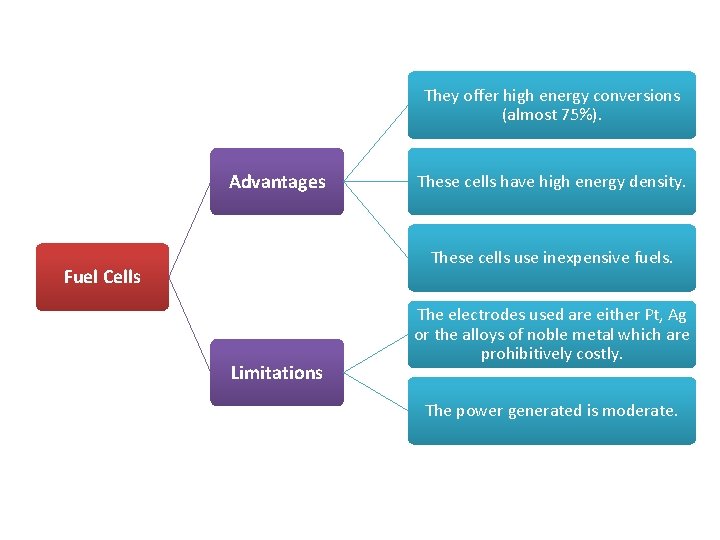
They offer high energy conversions (almost 75%). Advantages These cells have high energy density. These cells use inexpensive fuels. Fuel Cells Limitations The electrodes used are either Pt, Ag or the alloys of noble metal which are prohibitively costly. The power generated is moderate.