11 E coli molecule 1 water H 2




- Slides: 50

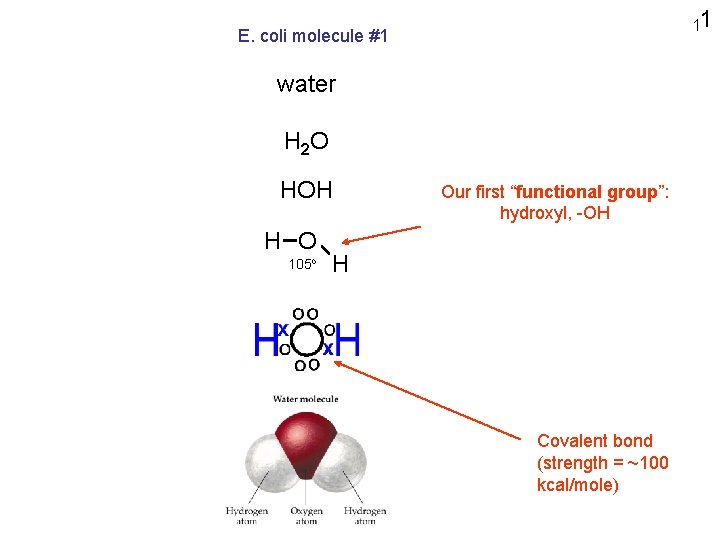
11 E. coli molecule #1 water H 2 O HOH H O 105 o Our first “functional group”: hydroxyl, -OH H Covalent bond (strength = ~100 kcal/mole)

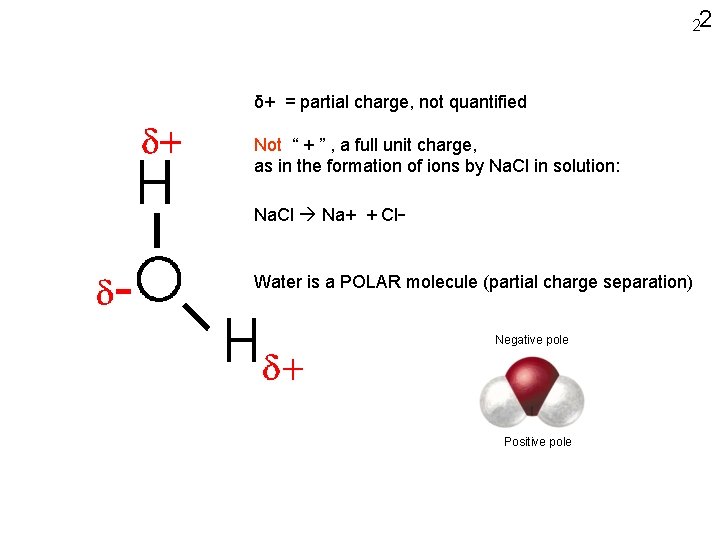
22 δ+ = partial charge, not quantified Not “ + ” , a full unit charge, as in the formation of ions by Na. Cl in solution: Na. Cl Na+ + Cl- Water is a POLAR molecule (partial charge separation) Negative pole Positive pole

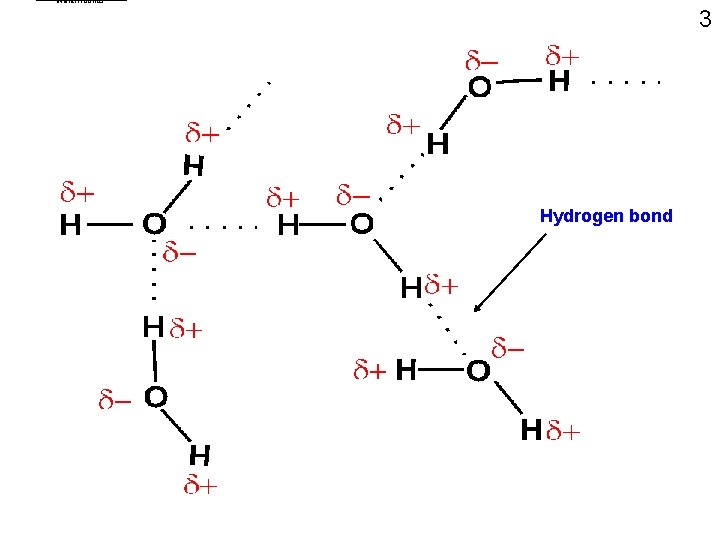
water. Hbonds 3 Hydrogen bond

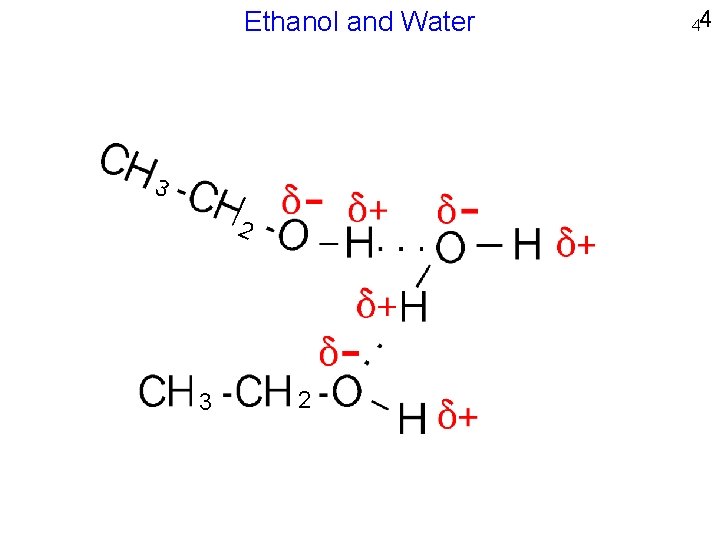
Ethanol and Water 3 2 44

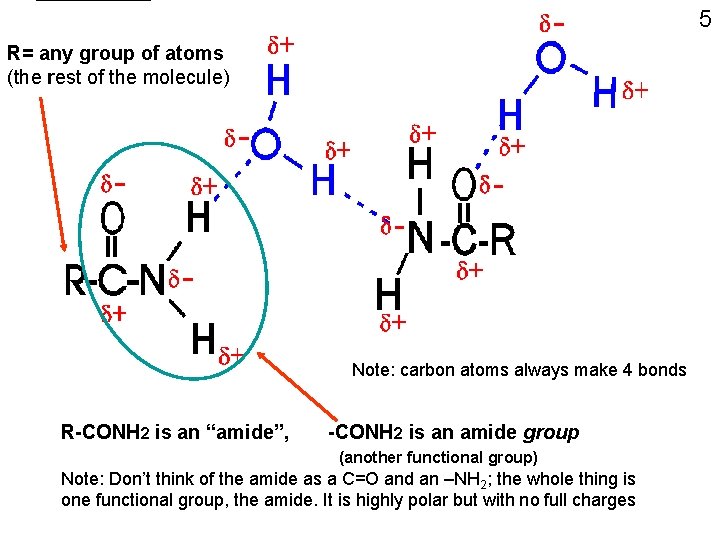
5 R= any group of atoms (the rest of the molecule) Note: carbon atoms always make 4 bonds R-CONH 2 is an “amide”, -CONH 2 is an amide group (another functional group) Note: Don’t think of the amide as a C=O and an –NH 2; the whole thing is one functional group, the amide. It is highly polar but with no full charges

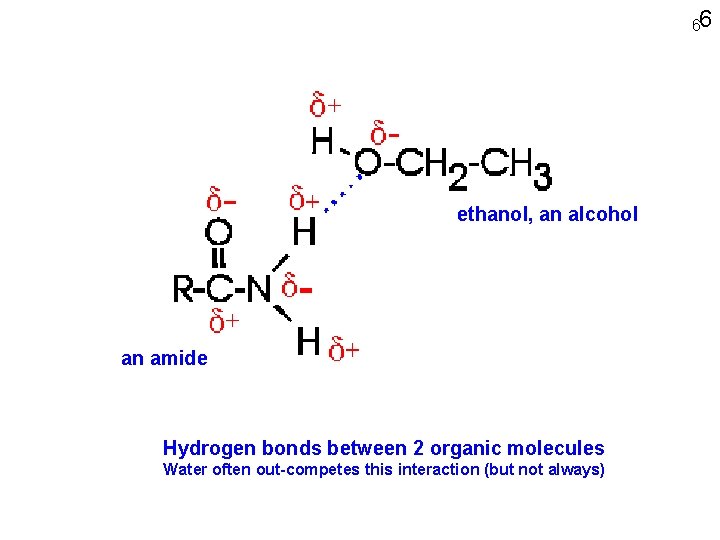
66 ethanol, an alcohol an amide Hydrogen bonds between 2 organic molecules Water often out-competes this interaction (but not always)

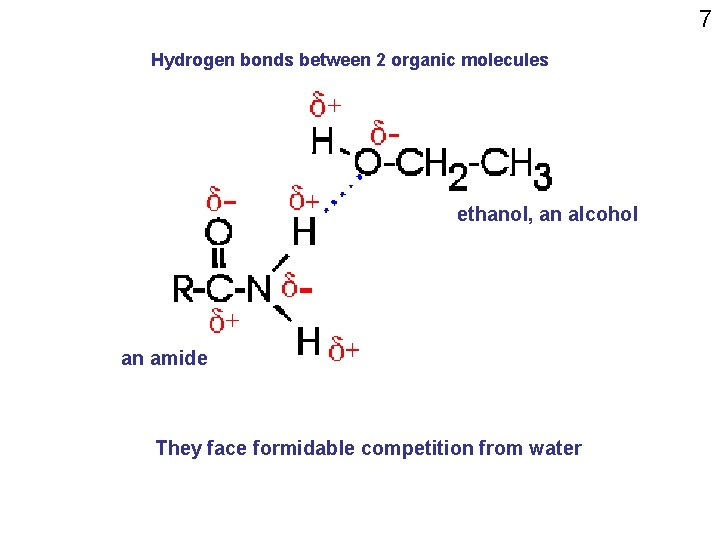
7 Hydrogen bonds between 2 organic molecules ethanol, an alcohol an amide They face formidable competition from water

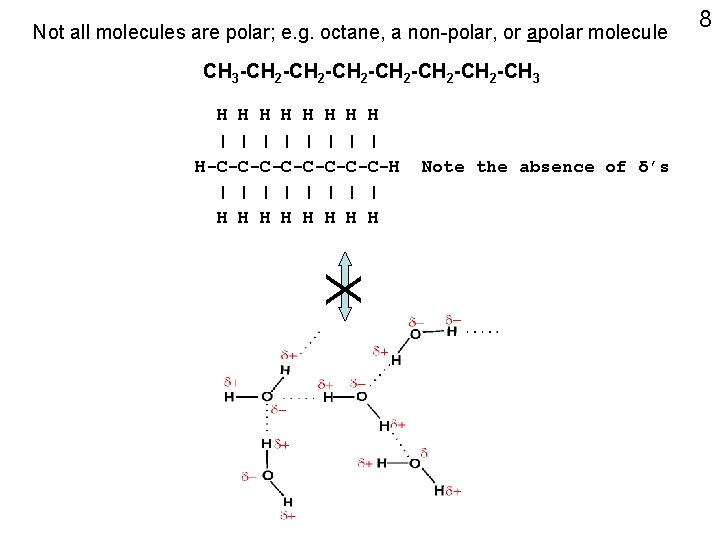
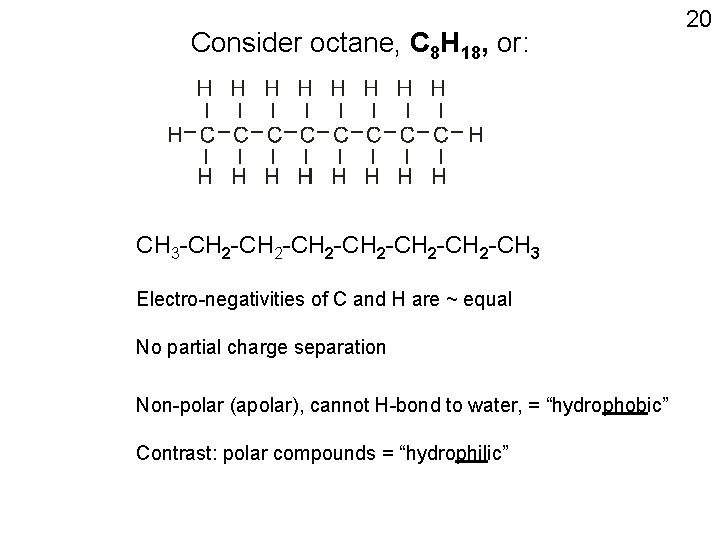
Not all molecules are polar; e. g. octane, a non-polar, or apolar molecule CH 3 -CH 2 -CH 2 -CH 3 H H H H | | | | H-C-C-C-C-H | | | | H H H H Note the absence of δ’s 8 X

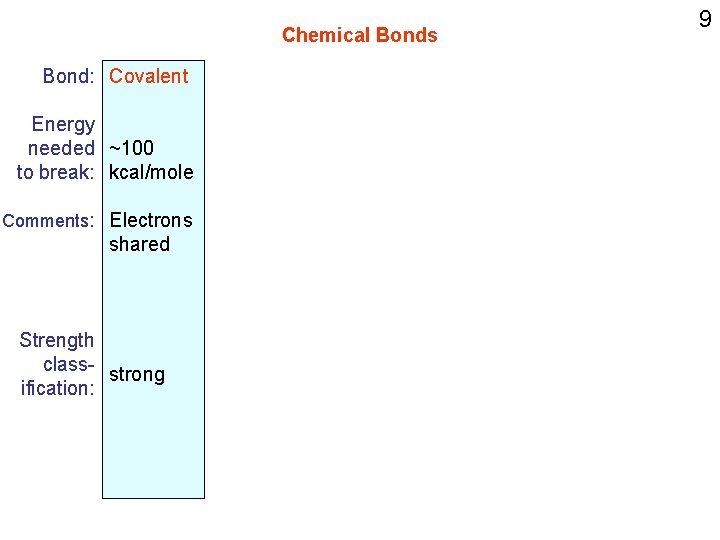
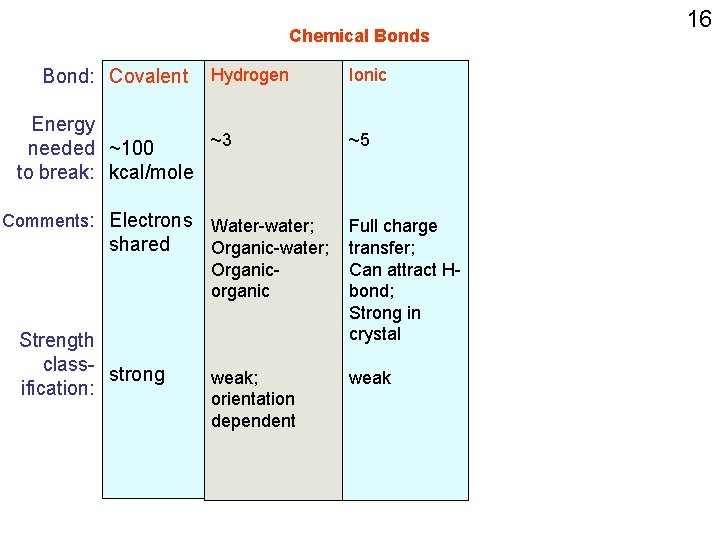
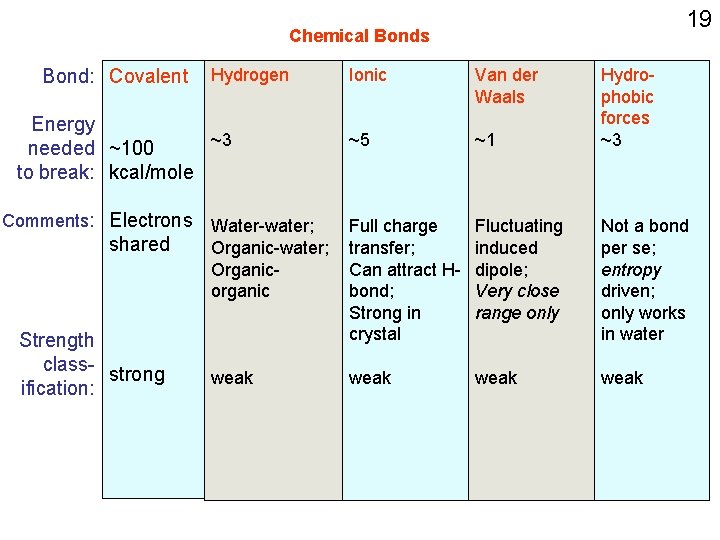
Chemical Bonds Bond: Covalent Energy needed ~100 to break: kcal/mole Comments: Electrons shared Strength class- strong ification: 9

10 • 1 calorie = amount of energy needed to raise the temperature of 1 gram of water (1 cc or ml. of water) one degree C • 1 Calorie = dietary calorie = 1000 calories • 1 kilocalorie (kcal) = 1000 calories

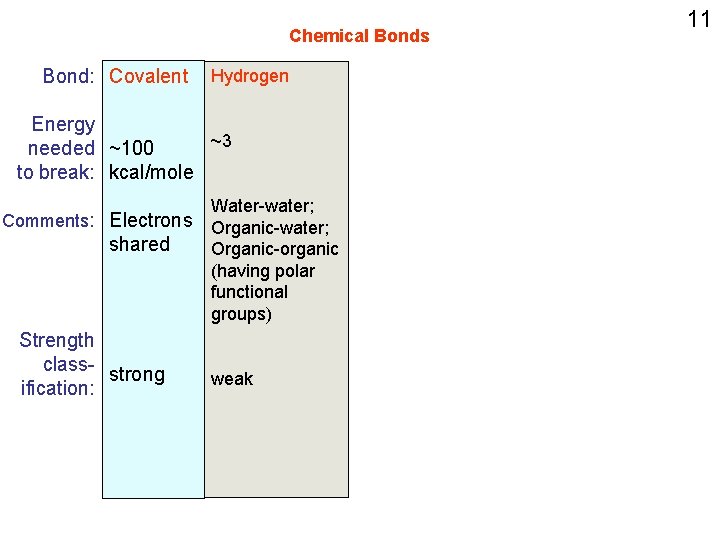
Chemical Bonds Bond: Covalent Hydrogen Energy ~3 needed ~100 to break: kcal/mole Water-water; Comments: Electrons Organic-water; shared Organic-organic (having polar functional groups) Strength class- strong ification: weak 11

Ionic bonds • Full loss or capture of an electron • Full charge separation • Full positive charge, or full negative charge (= charge of one electron) • E. g. Na. Cl = Na+: : : Cl- Strong bond between the ions in a crystal (e. g. , rock salt) • But: weak in aqueous solution • So the ionic bond of Na. Cl becomes weak in water • Is the bond between an Na+ ion and water ionic or an H-bond? Some characteristics of each: a “polar interaction” or an “ion-dipole interaction” 12

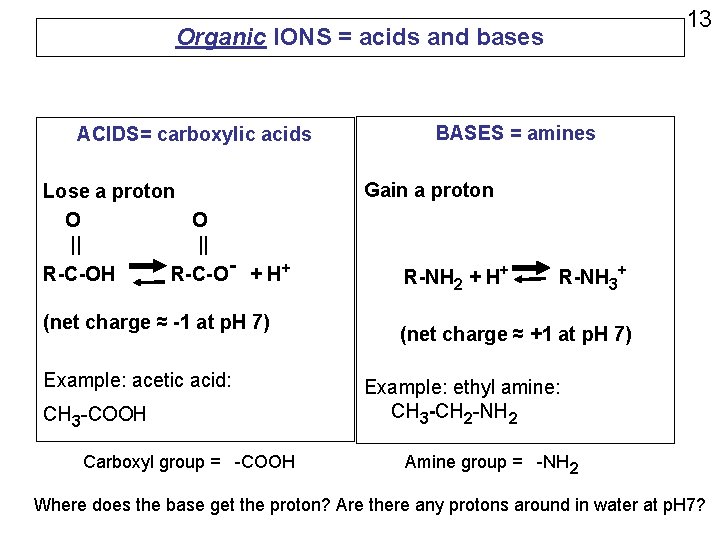
Organic IONS = acids and bases ACIDS= carboxylic acids Lose a proton O O || || R-C-OH R-C-O- + H+ (net charge ≈ -1 at p. H 7) Example: acetic acid: CH 3 -COOH Carboxyl group = -COOH 13 BASES = amines Gain a proton R-NH 2 + H+ R-NH 3+ (net charge ≈ +1 at p. H 7) Example: ethyl amine: CH 3 -CH 2 -NH 2 Amine group = -NH 2 Where does the base get the proton? Are there any protons around in water at p. H 7?

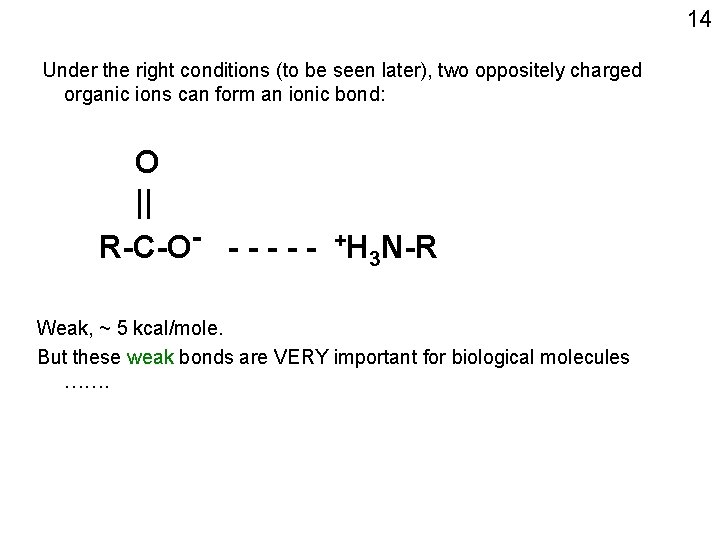
14 Under the right conditions (to be seen later), two oppositely charged organic ions can form an ionic bond: O || R-C-O- - - - +H 3 N-R Weak, ~ 5 kcal/mole. But these weak bonds are VERY important for biological molecules …….

15 15 The chemical structures of the functional groups used in this course must be memorized. See the Functional Groups handout. This is one of very few memorizations required. O || -C -- OH “carboxyl” Me You

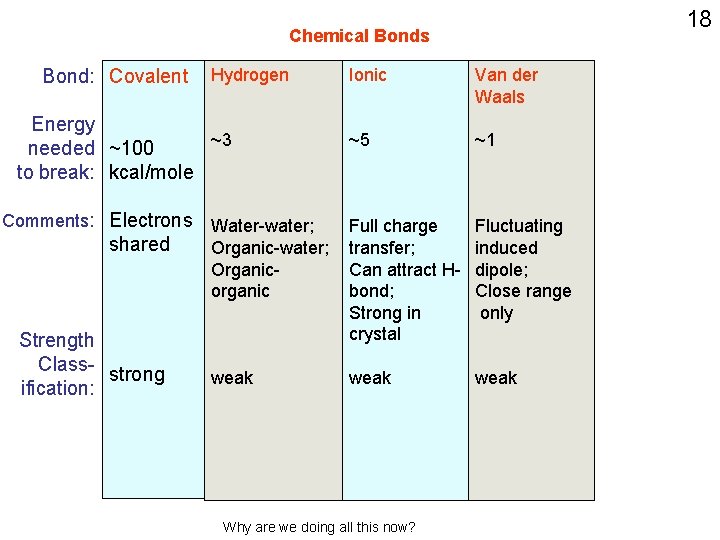
Chemical Bonds Bond: Covalent Hydrogen Energy ~3 needed ~100 to break: kcal/mole Comments: Electrons Water-water; shared Organic-water; Organicorganic Strength class- strong ification: weak; orientation dependent Ionic ~5 Full charge transfer; Can attract Hbond; Strong in crystal weak 16

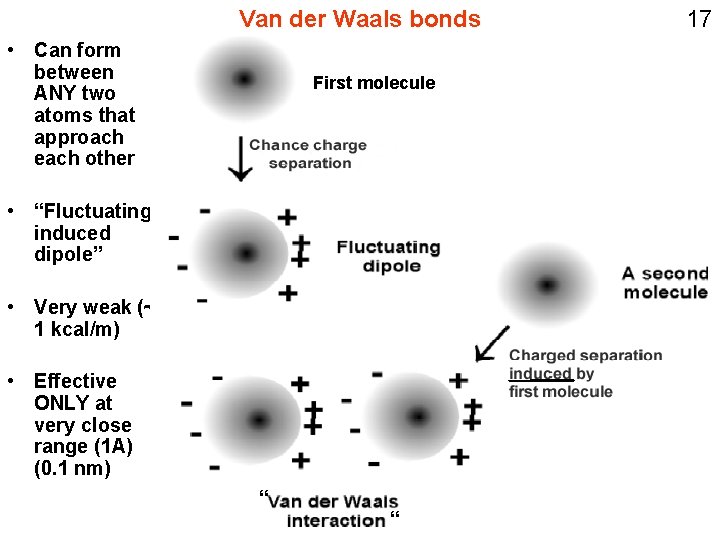
Van der Waals bonds • Can form between ANY two atoms that approach each other First molecule • “Fluctuating induced dipole” • Very weak (~ 1 kcal/m) • Effective ONLY at very close range (1 A) (0. 1 nm) “ “ 17

18 Chemical Bonds Bond: Covalent Hydrogen Energy ~3 needed ~100 to break: kcal/mole Ionic Van der Waals ~5 ~1 Organicorganic Full charge transfer; Can attract Hbond; Strong in crystal Fluctuating induced dipole; Close range only weak Comments: Electrons Water-water; shared Organic-water; Strength Class- strong ification: Why are we doing all this now?

19 Chemical Bonds Bond: Covalent Hydrogen Energy ~3 needed ~100 to break: kcal/mole Ionic Van der Waals ~5 ~1 Organicorganic Full charge transfer; Can attract Hbond; Strong in crystal Fluctuating induced dipole; Very close range only Not a bond per se; entropy driven; only works in water weak Comments: Electrons Water-water; shared Organic-water; Strength class- strong ification: Hydrophobic forces ~3

Consider octane, C 8 H 18, or: CH 3 -CH 2 -CH 2 -CH 3 Electro-negativities of C and H are ~ equal No partial charge separation Non-polar (apolar), cannot H-bond to water, = “hydrophobic” Contrast: polar compounds = “hydrophilic” 20

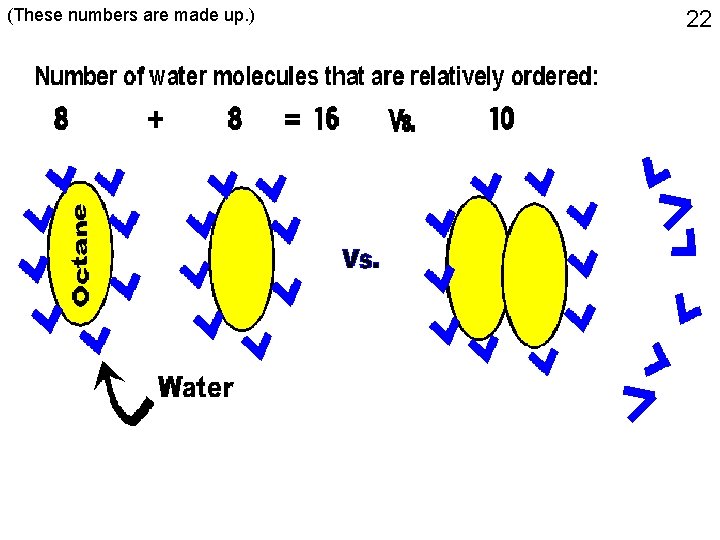
21 Octane in water (These numbers are made up. )

(These numbers are made up. ) 22

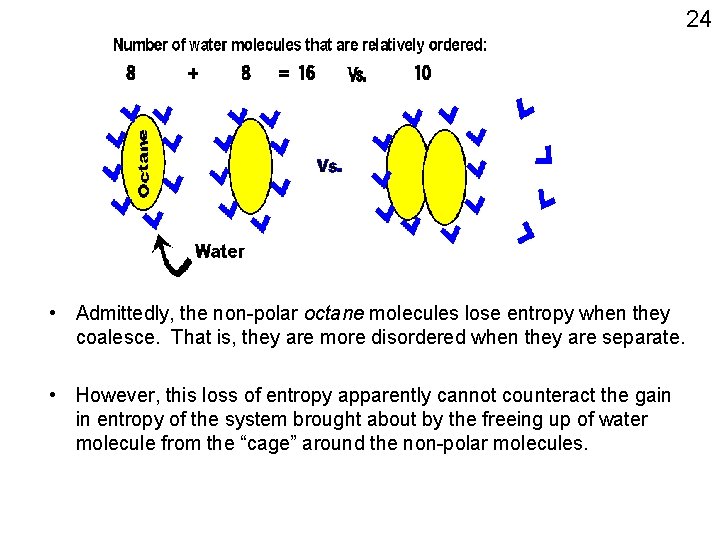
23 • ENTROPY: related to the number of different states possible • The water molecules around the non-polar molecule have a LOWER entropy (less choices, more ordered). • Systems tend to change to maximize entropy (no. ofdifferent states possible to occupy). • Aggregation of the non-polar molecules with each other minimizes the number of lower entropy water molecules that are on their surface, thus maximizing the entropy of the system

24 • Admittedly, the non-polar octane molecules lose entropy when they coalesce. That is, they are more disordered when they are separate. • However, this loss of entropy apparently cannot counteract the gain in entropy of the system brought about by the freeing up of water molecule from the “cage” around the non-polar molecules.

Hydrophobic “bonds” (forces) • Affects NON-polar molecules that find themselves in an aqueous environment (i. e. , must be in water) • They cannot H-bond with water molecules • The water molecules around the non-polar molecule are not able to constantly switch partners for H-bonding • The water molecules around the non-polar molecule are in a MORE ordered state. • Hydrophobic “forces”, not really “bonds” per se 25

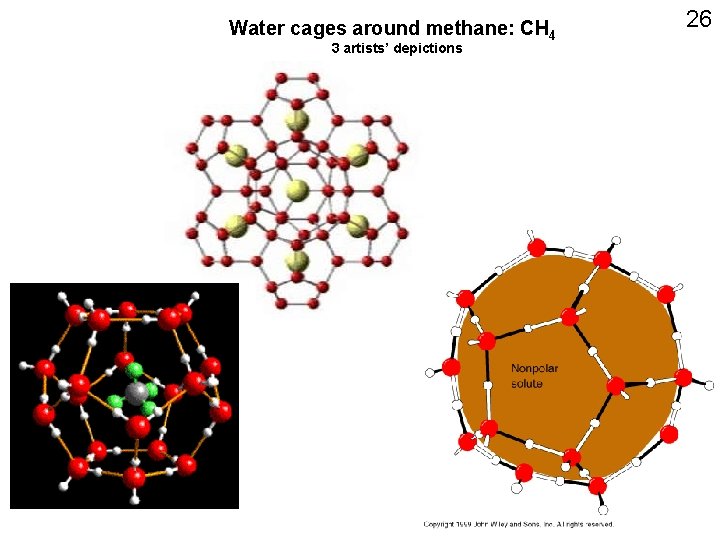
Water cages around methane: CH 4 3 artists’ depictions 26

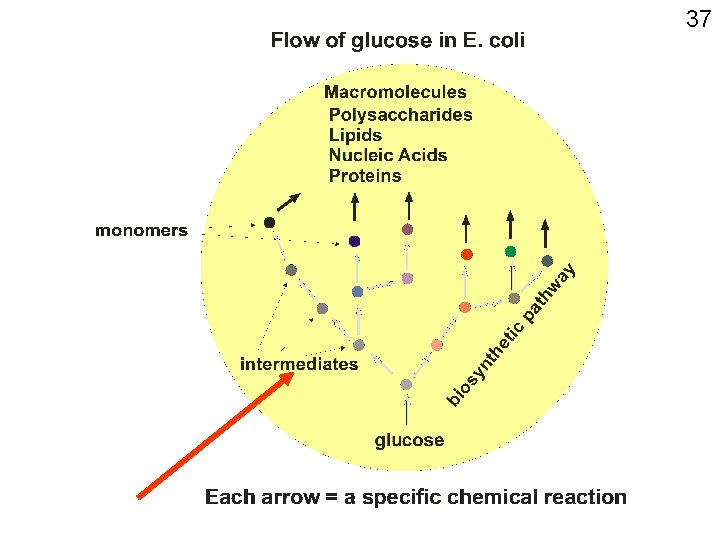
End of bonds, and water, our molecule #1 Now on to the next 4999 types of molecules found in an E. coli cell: First let’s categorize: Small vs. large molecules LARGE • >= ~5000 daltons SMALL • <= ~500 daltons (~ 50 atoms) • Called macromolecules • Called small molecules • Examples: proteins, polysaccharides, DNA • Size differences are rough, there are gray areas • Examples: water, ethanol, glucose, acetamide, methane, octane 27

28 Propylene CH 3 -CH=CH 2 Polypropylene, a polymer, a large molecule

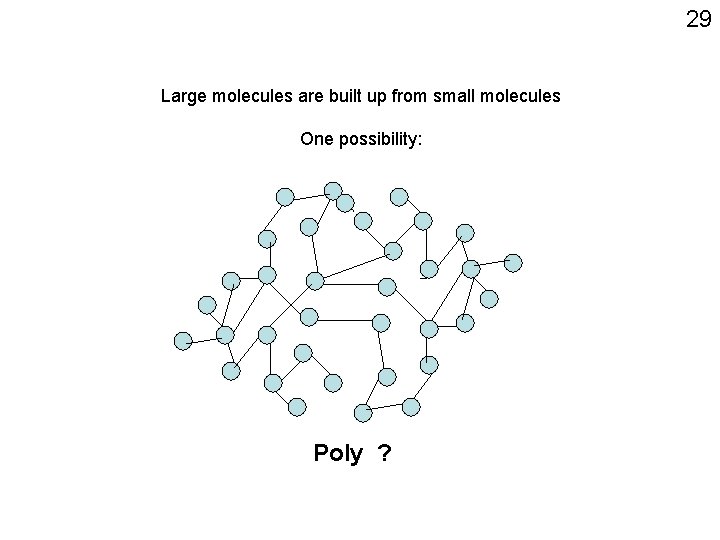
29 Large molecules are built up from small molecules One possibility: Poly ?

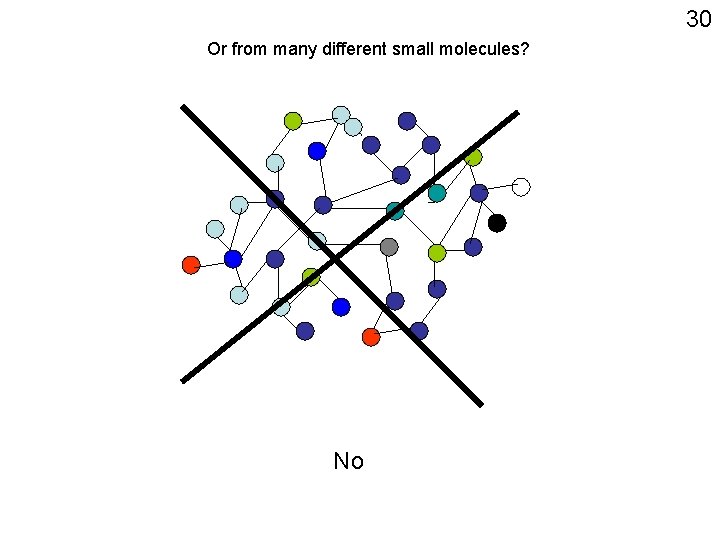
30 Or from many different small molecules? No

31 A great simplification: Large molecules are linear polymers of small molecules. O-O-O-O-………

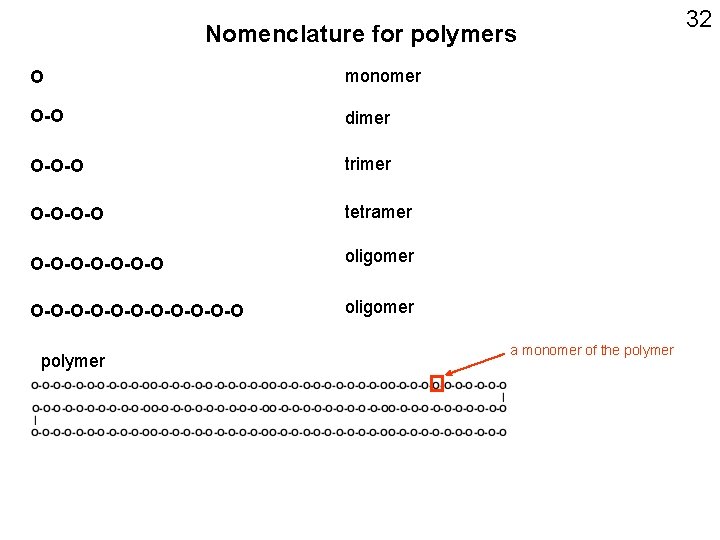
Nomenclature for polymers O monomer O-O dimer O-O-O trimer O-O-O-O tetramer O-O-O-O oligomer O-O-O-O-O-O oligomer polymer a monomer of the polymer 32

The large molecules, or macromolecules, of all cells can be grouped into 4 categories: • polysaccharides, • lipids, • nucleic acids, and • proteins. • Many important small molecules are the monomers of these polymers. • Only about 50 of these monomers, a small number to learn about. • About another dozen important small molecules are not monomers of polymers. Mostly vitamins. 33

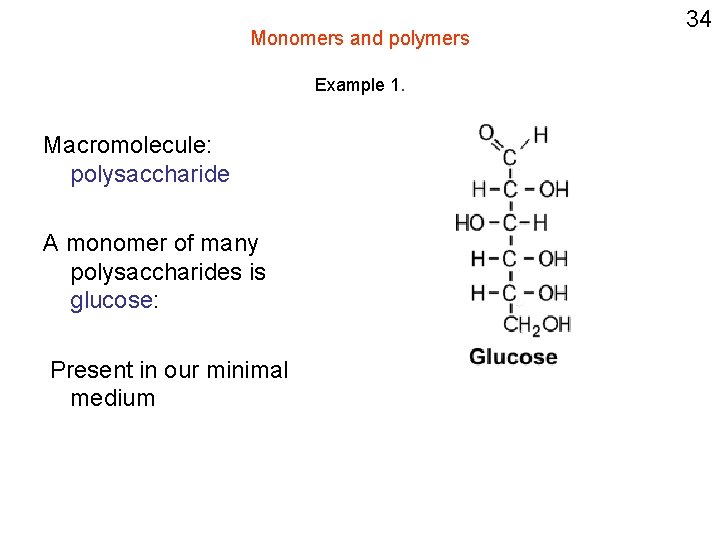
Monomers and polymers Example 1. Macromolecule: polysaccharide A monomer of many polysaccharides is glucose: Present in our minimal medium . 34

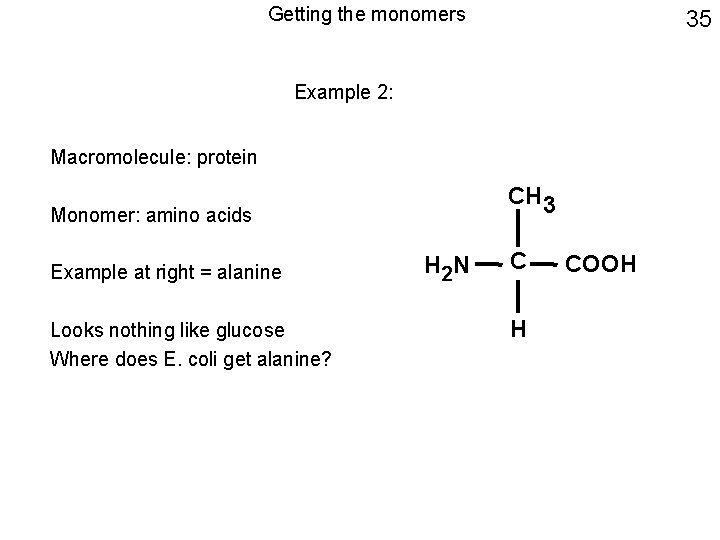
Getting the monomers 35 Example 2: Macromolecule: protein CH 3 Monomer: amino acids Example at right = alanine Looks nothing like glucose Where does E. coli get alanine? H 2 N C H COOH

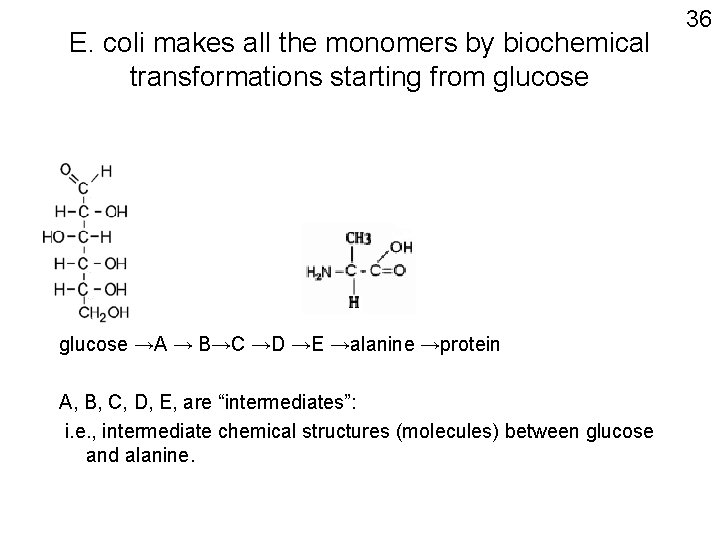
E. coli makes all the monomers by biochemical transformations starting from glucose →A → B→C →D →E →alanine →protein A, B, C, D, E, are “intermediates”: i. e. , intermediate chemical structures (molecules) between glucose and alanine. 36

37

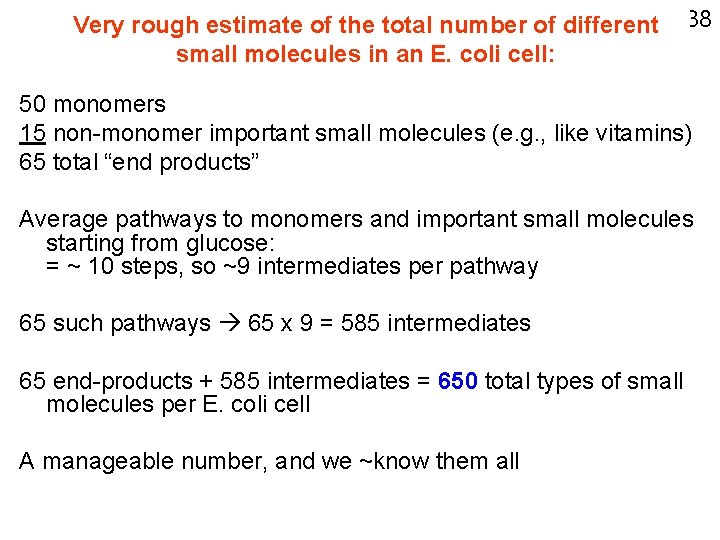
Very rough estimate of the total number of different 38 small molecules in an E. coli cell: 50 monomers 15 non-monomer important small molecules (e. g. , like vitamins) 65 total “end products” Average pathways to monomers and important small molecules starting from glucose: = ~ 10 steps, so ~9 intermediates per pathway 65 such pathways 65 x 9 = 585 intermediates 65 end-products + 585 intermediates = 650 total types of small molecules per E. coli cell A manageable number, and we ~know them all

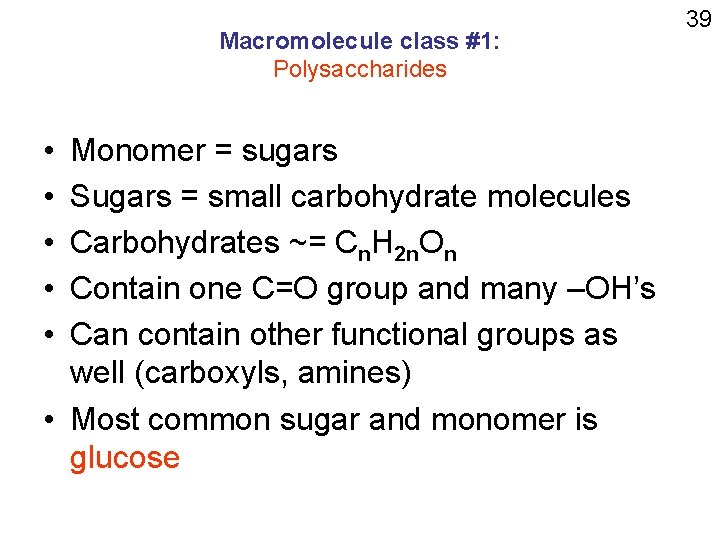
Macromolecule class #1: Polysaccharides • • • Monomer = sugars Sugars = small carbohydrate molecules Carbohydrates ~= Cn. H 2 n. On Contain one C=O group and many –OH’s Can contain other functional groups as well (carboxyls, amines) • Most common sugar and monomer is glucose 39

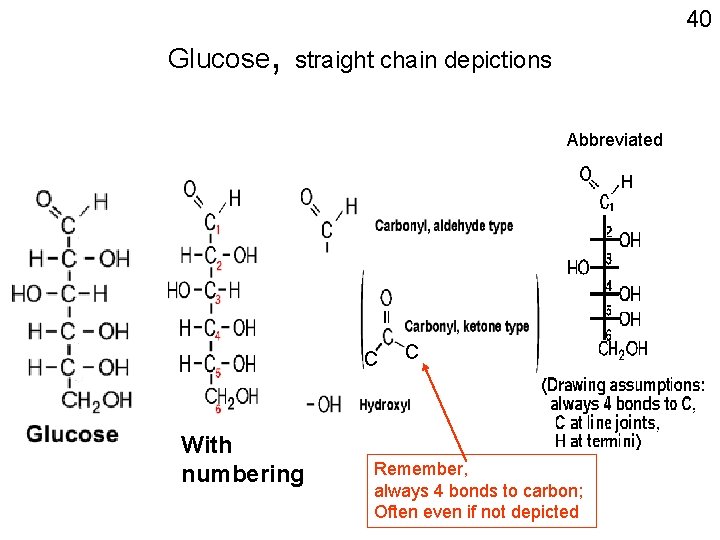
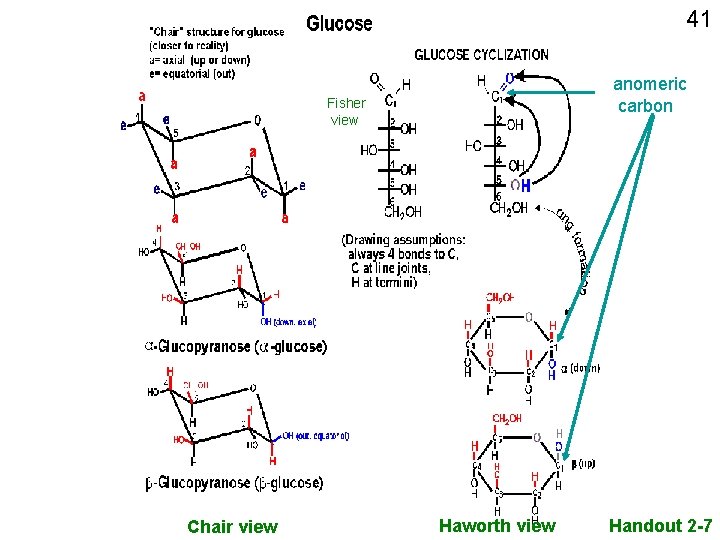
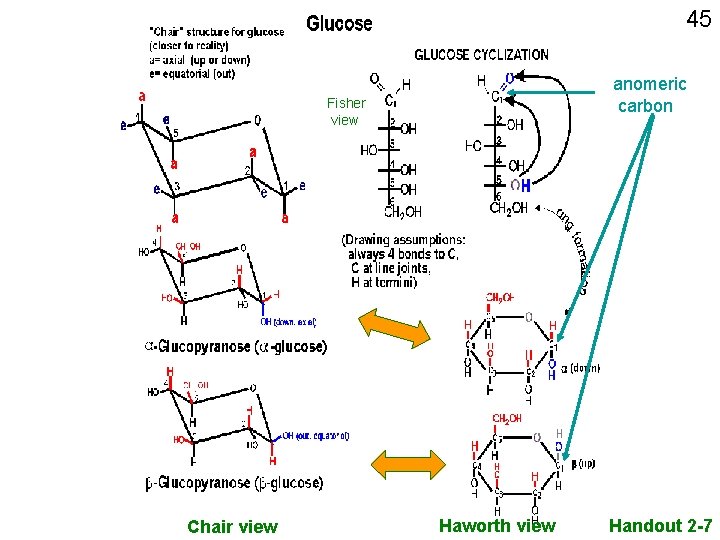
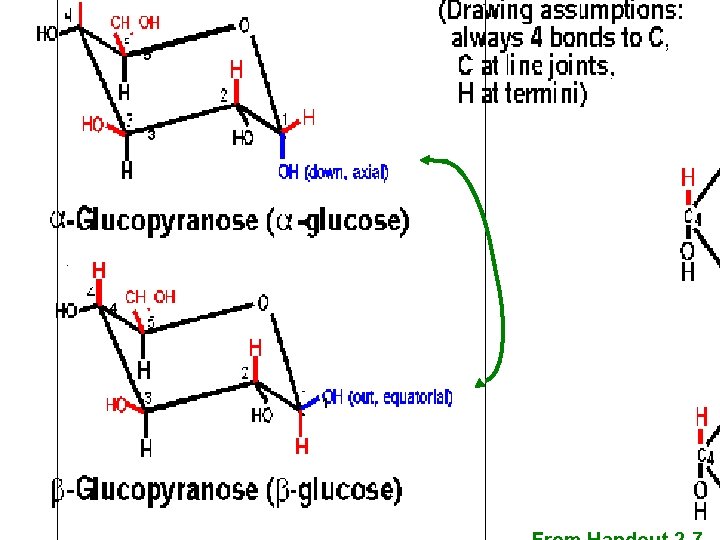
40 Glucose, straight chain depictions Abbreviated C With numbering C Remember, always 4 bonds to carbon; Often even if not depicted

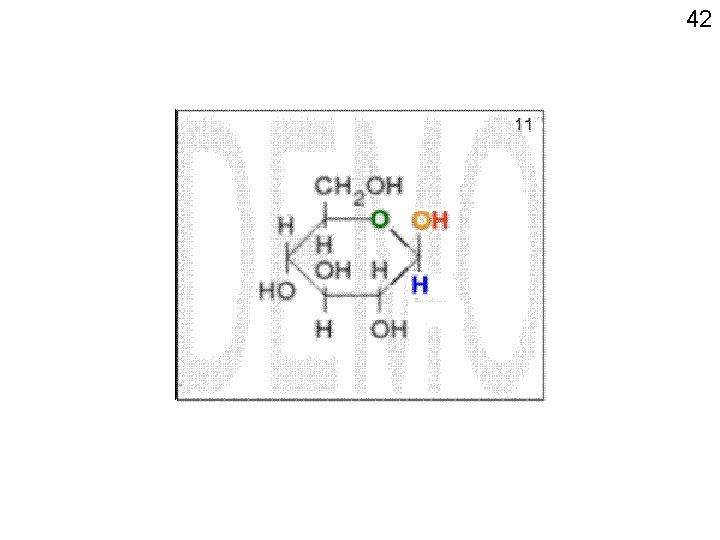
41 anomeric carbon Fisher view Chair view Haworth view Handout 2 -7

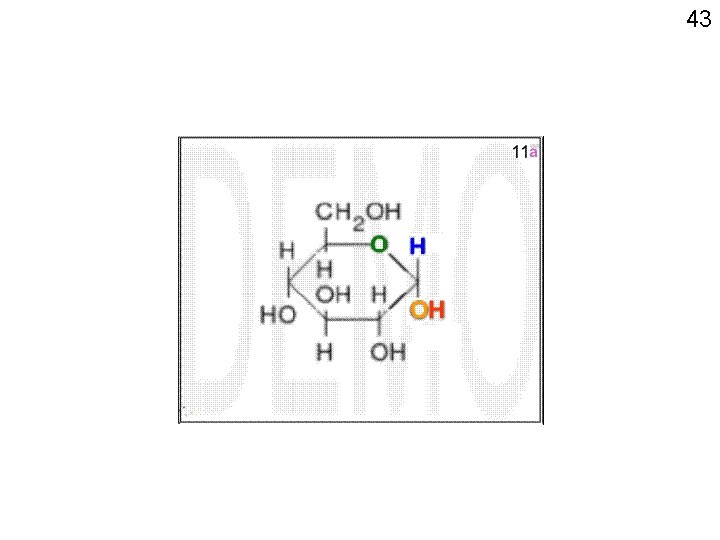
42 11 10 7 6 5 89 3 1 24

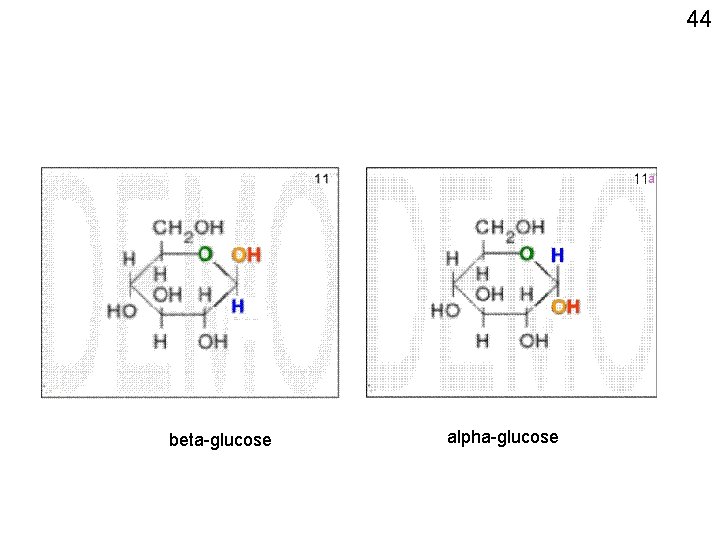
43 7 6 5 89 3 1 24

44 beta-glucose alpha-glucose

45 anomeric carbon Fisher view Chair view Haworth view Handout 2 -7

46 5 3 4 1

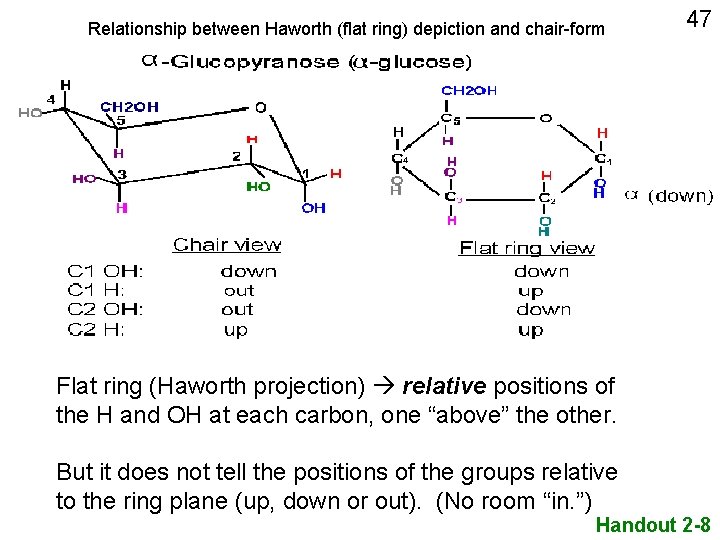
Relationship between Haworth (flat ring) depiction and chair-form 47 Flat ring (Haworth projection) relative positions of the H and OH at each carbon, one “above” the other. But it does not tell the positions of the groups relative to the ring plane (up, down or out). (No room “in. ”) Handout 2 -8

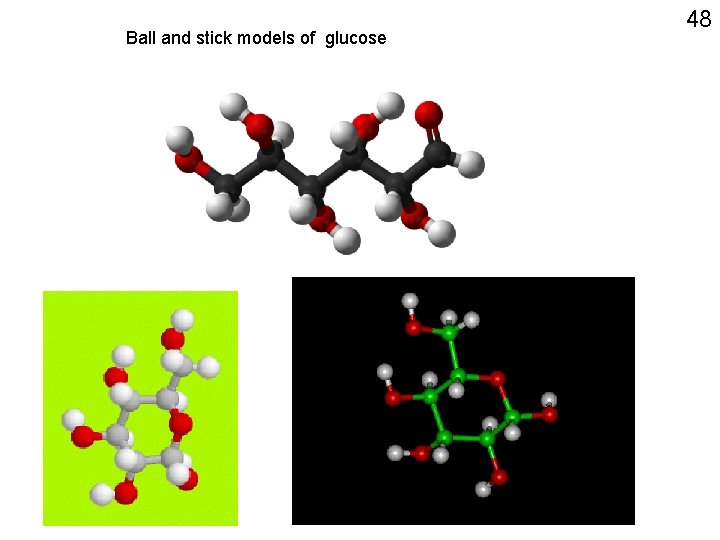
Ball and stick models of glucose 48

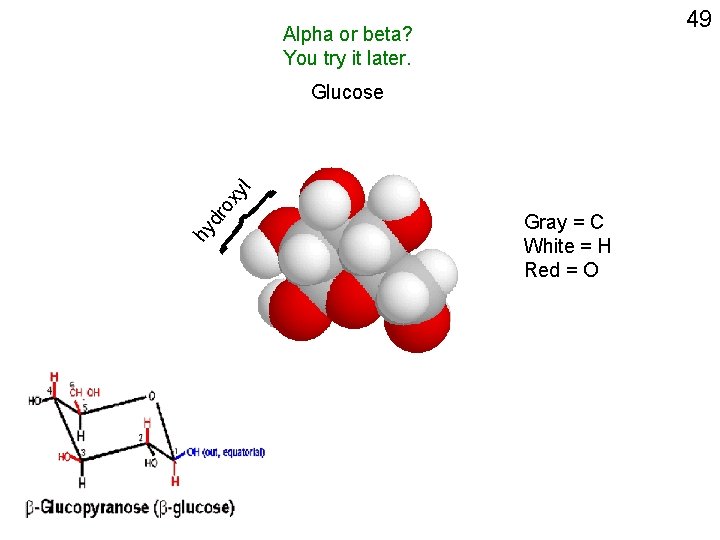
49 Alpha or beta? You try it later. hy dr o xy l Glucose Gray = C White = H Red = O

50