The Water Molecule Atoms the smallest units which






- Slides: 33

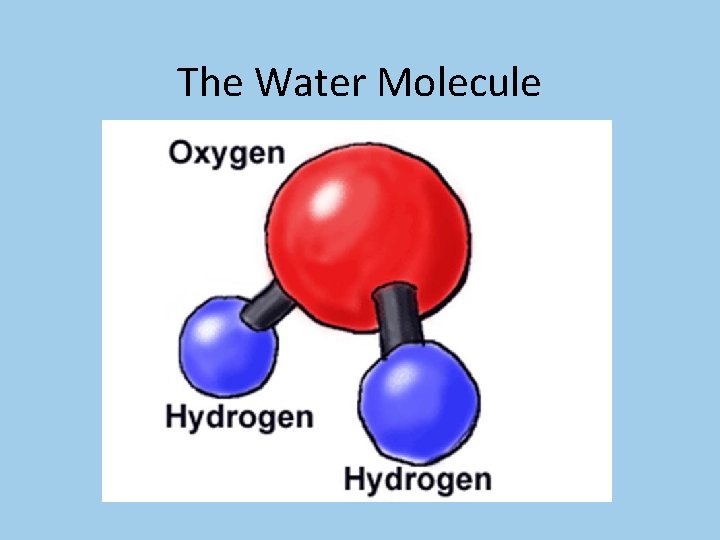
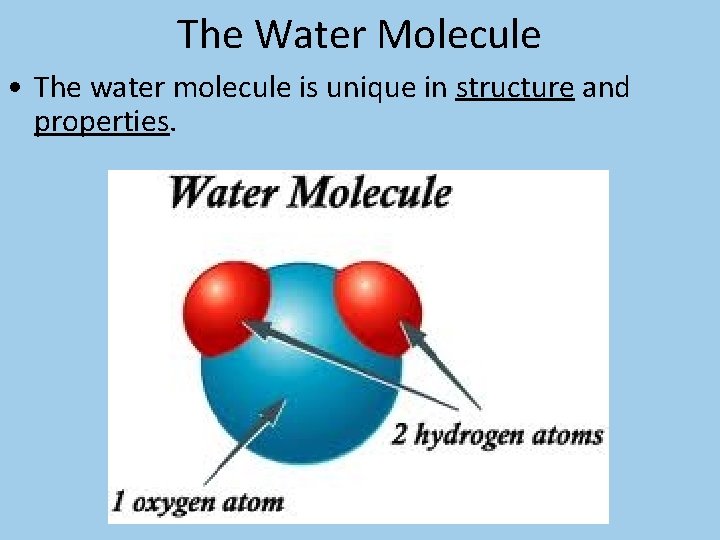
The Water Molecule

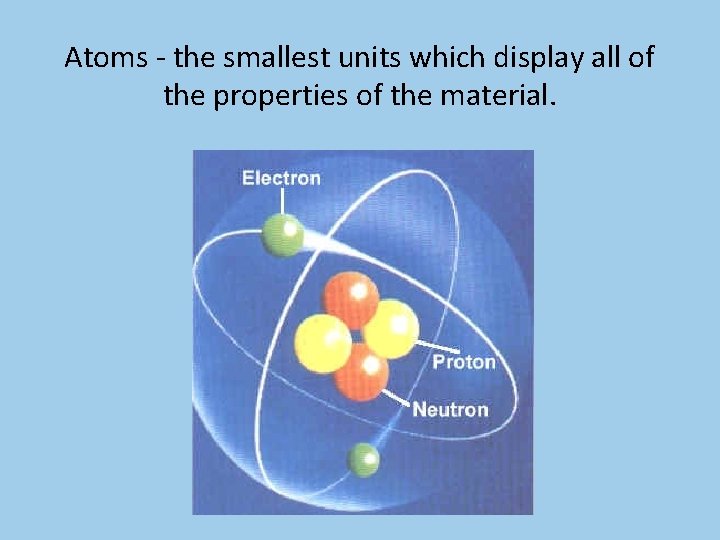
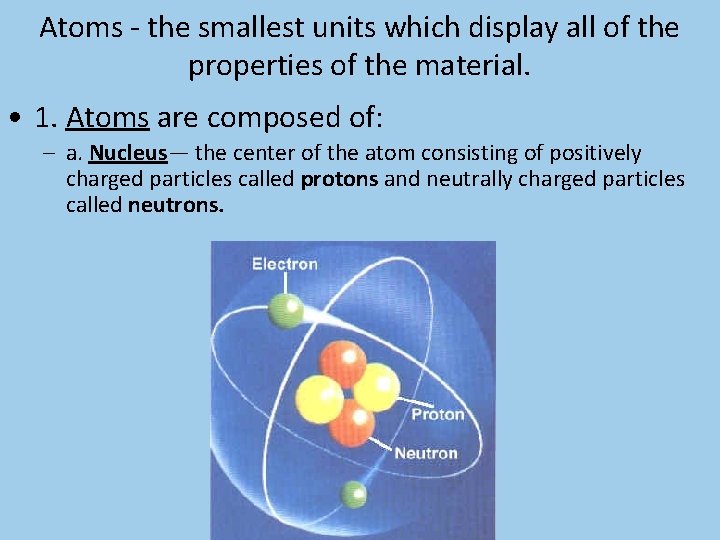
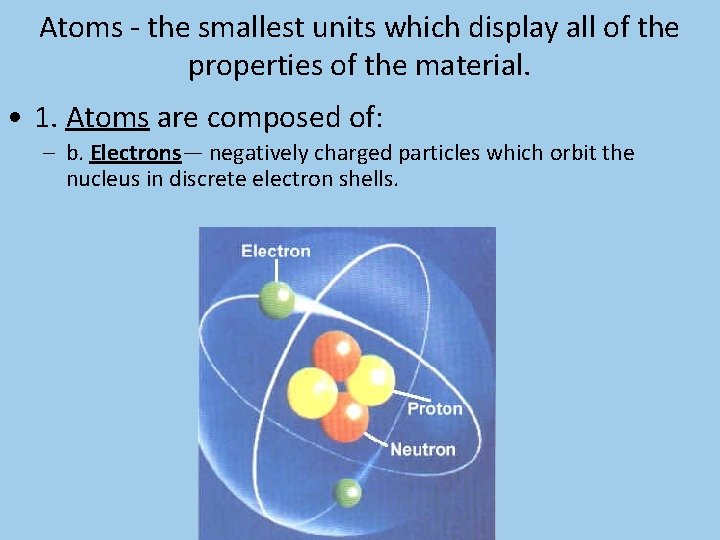
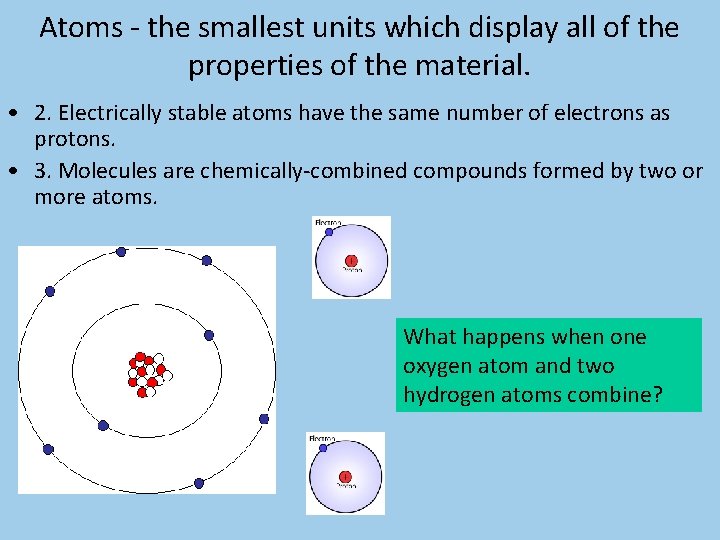
Atoms - the smallest units which display all of the properties of the material.

Atoms - the smallest units which display all of the properties of the material. • 1. Atoms are composed of: – a. Nucleus— the center of the atom consisting of positively charged particles called protons and neutrally charged particles called neutrons.

Atoms - the smallest units which display all of the properties of the material. • 1. Atoms are composed of: – b. Electrons— negatively charged particles which orbit the nucleus in discrete electron shells.

Atoms - the smallest units which display all of the properties of the material. • 2. Electrically stable atoms have the same number of electrons as protons. • 3. Molecules are chemically-combined compounds formed by two or more atoms. What happens when one oxygen atom and two hydrogen atoms combine?

Heat results from the vibrations of atoms (kinetic energy) and can be measured with a thermometer.

Heat results from the vibrations of atoms (kinetic energy) and can be measured with a thermometer. 1. In solids, the atoms or molecules vibrate weakly and are rigidly held in place. Draw this

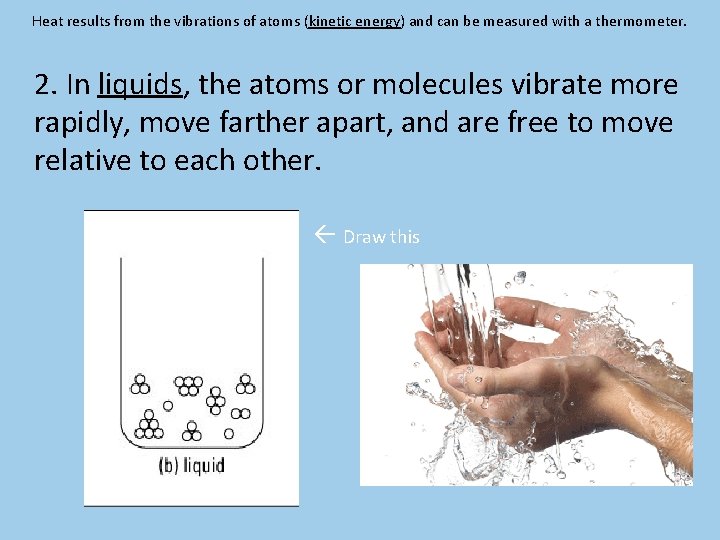
Heat results from the vibrations of atoms (kinetic energy) and can be measured with a thermometer. 2. In liquids, the atoms or molecules vibrate more rapidly, move farther apart, and are free to move relative to each other. Draw this

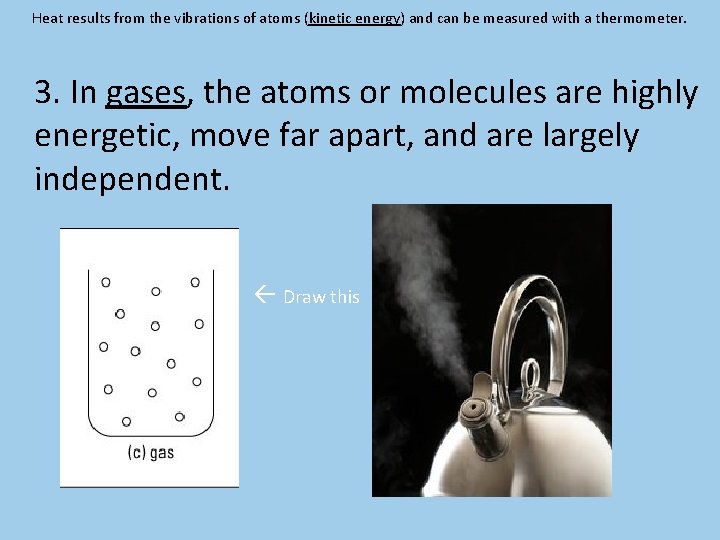
Heat results from the vibrations of atoms (kinetic energy) and can be measured with a thermometer. 3. In gases, the atoms or molecules are highly energetic, move far apart, and are largely independent. Draw this

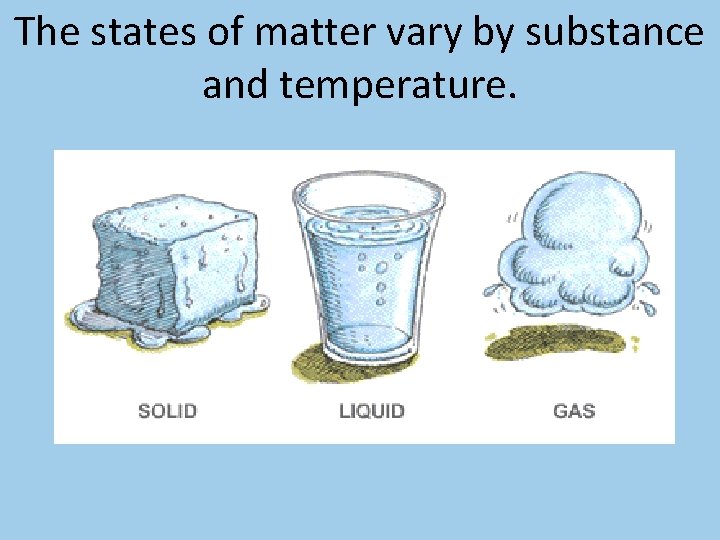
The states of matter vary by substance and temperature.

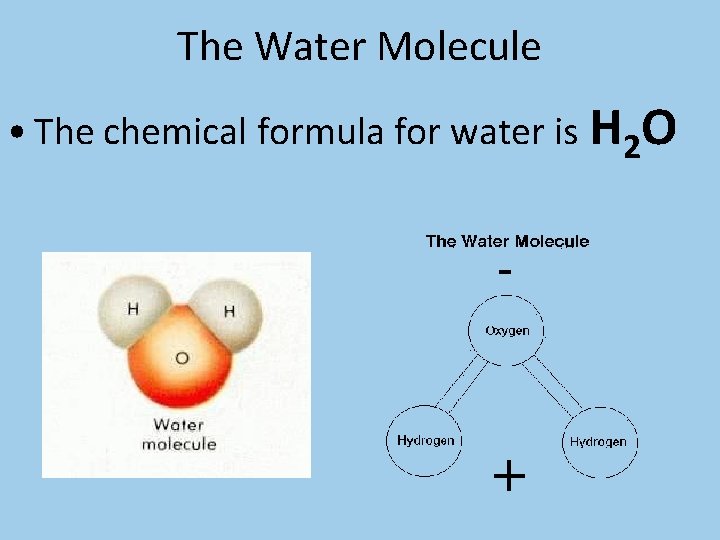
The Water Molecule • The chemical formula for water is H 2 O

Heat and States of Water • 1. Melting is the transition from solid to liquid; freezing is the reverse.

Heat and States of Water • 2. Evaporation (vaporization) is the transition from liquid to gas; condensation is the reverse.

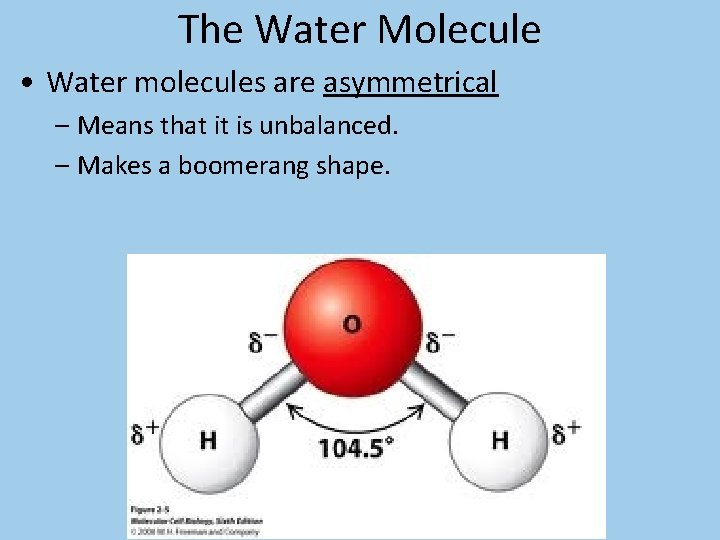
The Water Molecule • Water molecules are asymmetrical – Means that it is unbalanced. – Makes a boomerang shape.

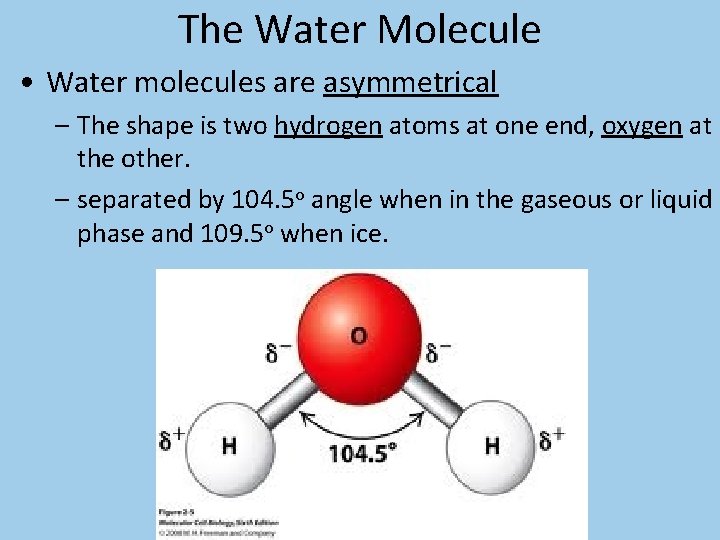
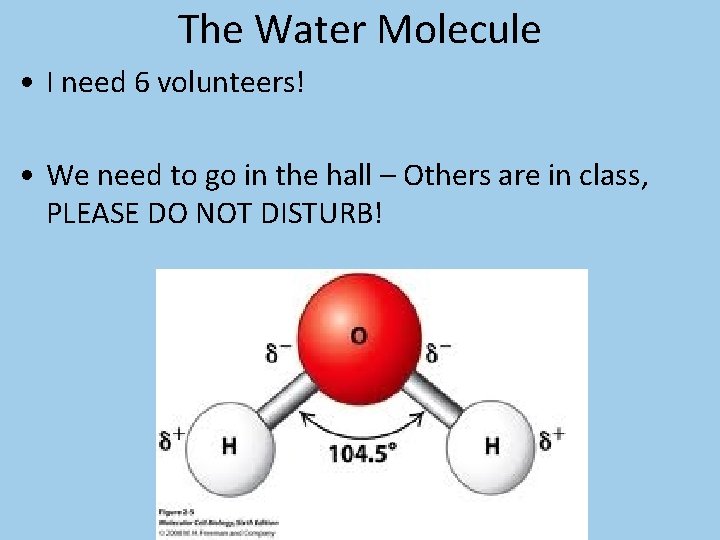
The Water Molecule • Water molecules are asymmetrical – The shape is two hydrogen atoms at one end, oxygen at the other. – separated by 104. 5 o angle when in the gaseous or liquid phase and 109. 5 o when ice.

Atom worksheet Using your notes, complete the atom worksheet. You will have a quiz next class on your notes so far!

The Water Molecule • The water molecule is unique in structure and properties.

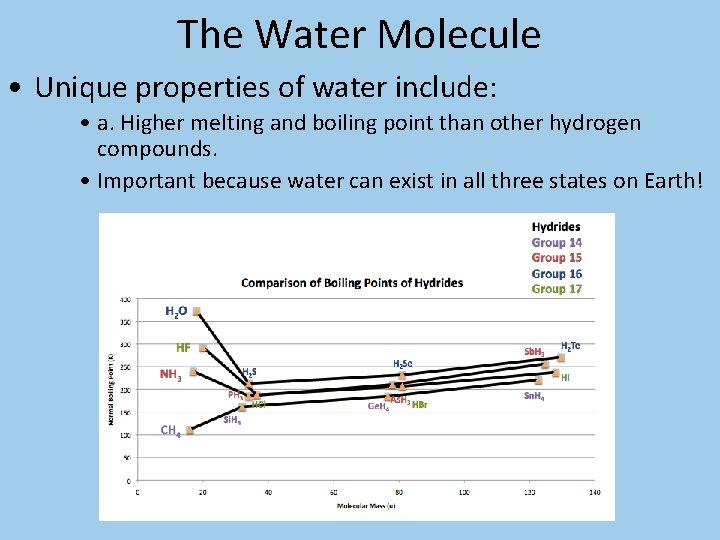
The Water Molecule • Unique properties of water include: • a. Higher melting and boiling point than other hydrogen compounds. • Important because water can exist in all three states on Earth!

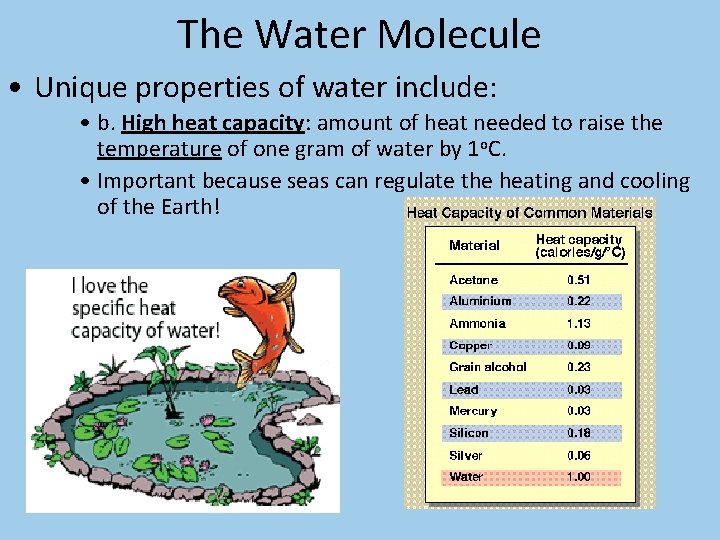
The Water Molecule • Unique properties of water include: • b. High heat capacity: amount of heat needed to raise the temperature of one gram of water by 1 o. C. • Important because seas can regulate the heating and cooling of the Earth!

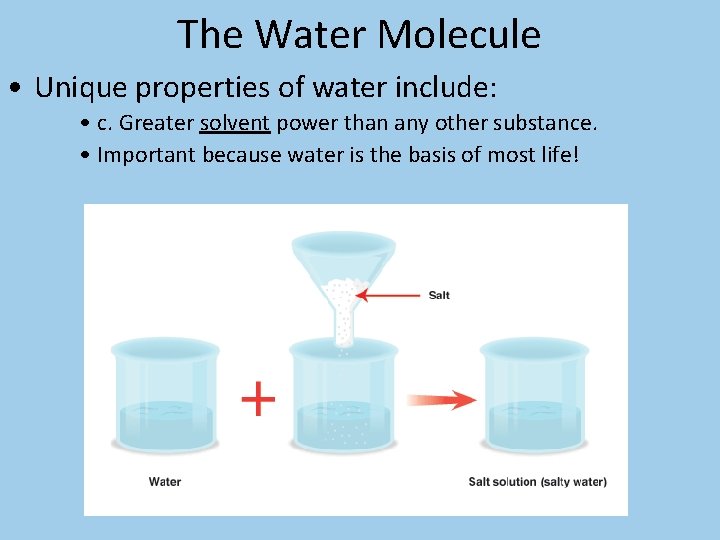
The Water Molecule • Unique properties of water include: • c. Greater solvent power than any other substance. • Important because water is the basis of most life!

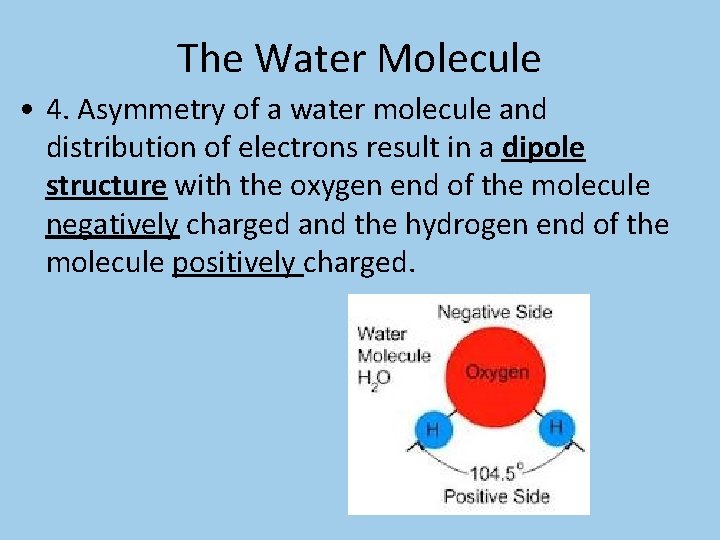
The Water Molecule • 4. Asymmetry of a water molecule and distribution of electrons result in a dipole structure with the oxygen end of the molecule negatively charged and the hydrogen end of the molecule positively charged.

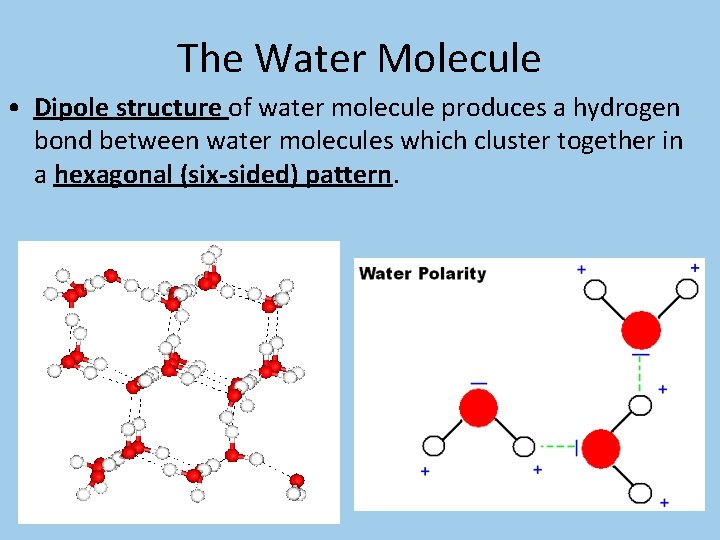
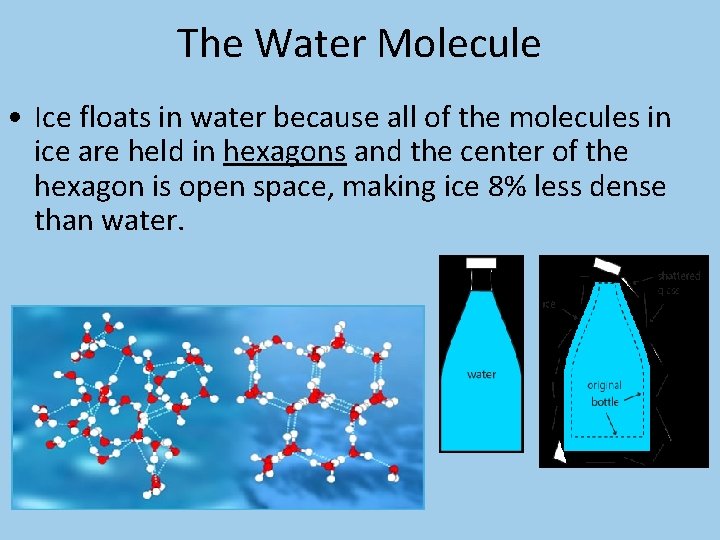
The Water Molecule • Dipole structure of water molecule produces a hydrogen bond between water molecules which cluster together in a hexagonal (six-sided) pattern.

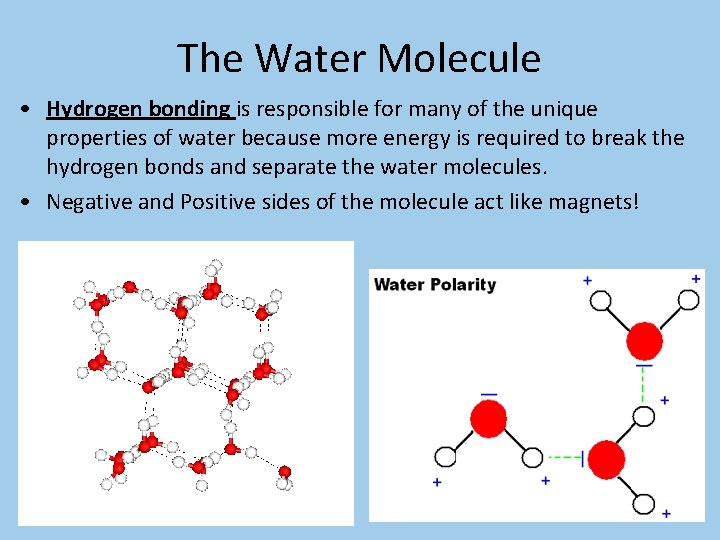
The Water Molecule • Hydrogen bonding is responsible for many of the unique properties of water because more energy is required to break the hydrogen bonds and separate the water molecules. • Negative and Positive sides of the molecule act like magnets!

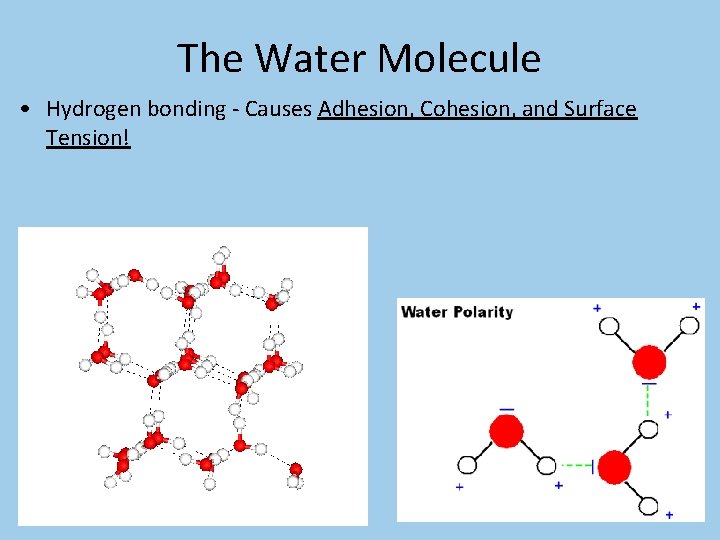
The Water Molecule • Hydrogen bonding - Causes Adhesion, Cohesion, and Surface Tension!

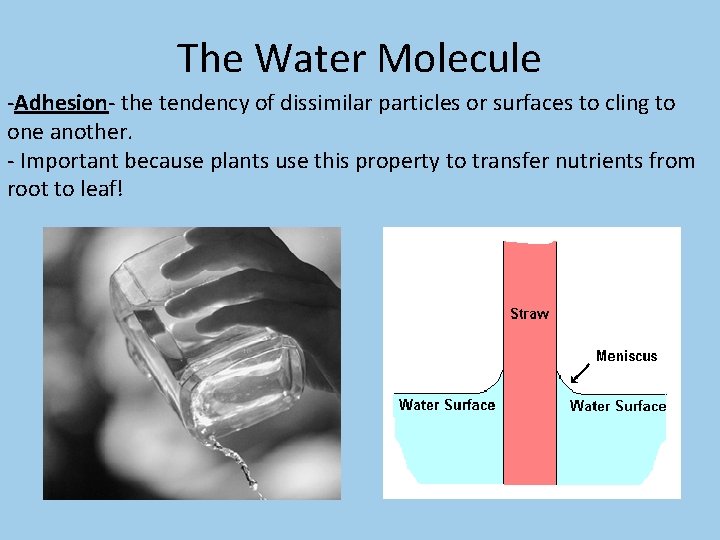
The Water Molecule -Adhesion- the tendency of dissimilar particles or surfaces to cling to one another. - Important because plants use this property to transfer nutrients from root to leaf!

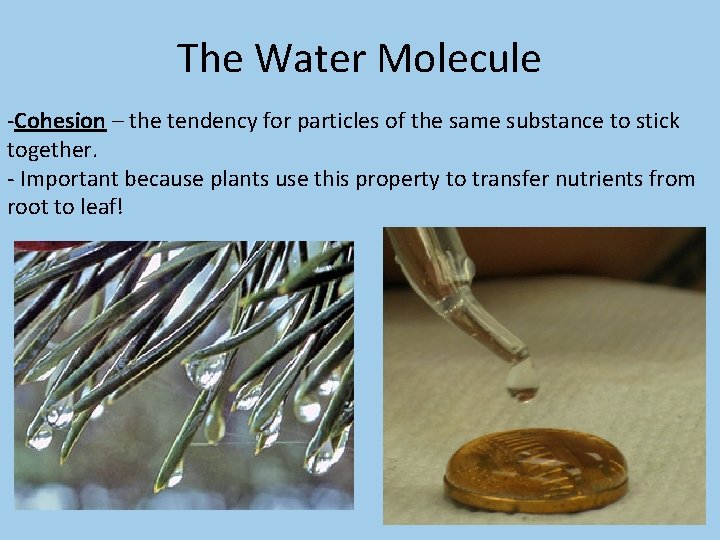
The Water Molecule -Cohesion – the tendency for particles of the same substance to stick together. - Important because plants use this property to transfer nutrients from root to leaf!

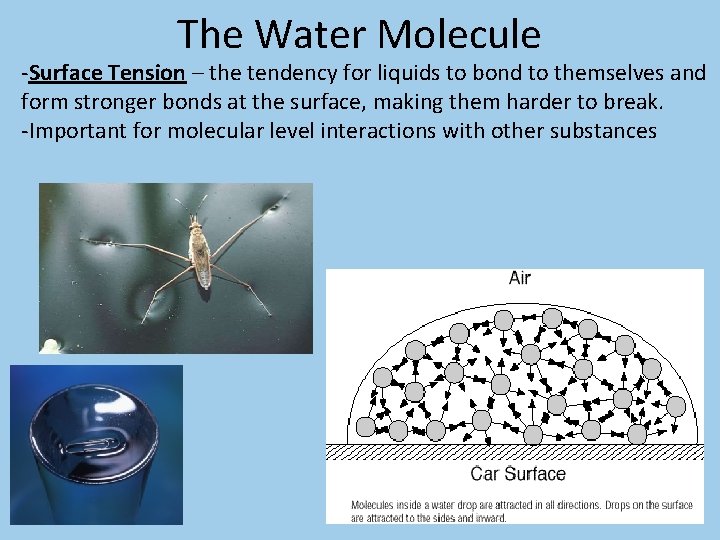
The Water Molecule -Surface Tension – the tendency for liquids to bond to themselves and form stronger bonds at the surface, making them harder to break. -Important for molecular level interactions with other substances

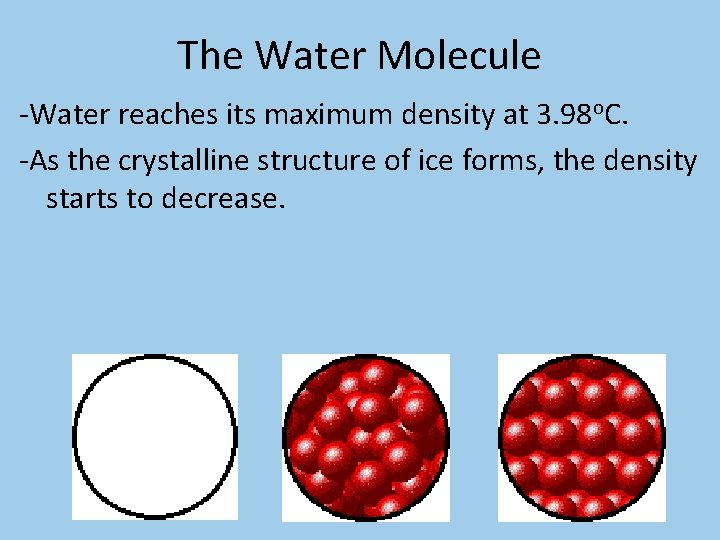
The Water Molecule • Ice floats in water because all of the molecules in ice are held in hexagons and the center of the hexagon is open space, making ice 8% less dense than water.

The Water Molecule -Water reaches its maximum density at 3. 98 o. C. -As the crystalline structure of ice forms, the density starts to decrease.

The Water Molecule -Unique property of water - Ice floats in water. . . but most solids will sink in their own liquids! -Important because if ice sank in the sea, it might never melt! Paraffin Water

The Water Molecule • I need 6 volunteers! • We need to go in the hall – Others are in class, PLEASE DO NOT DISTURB!

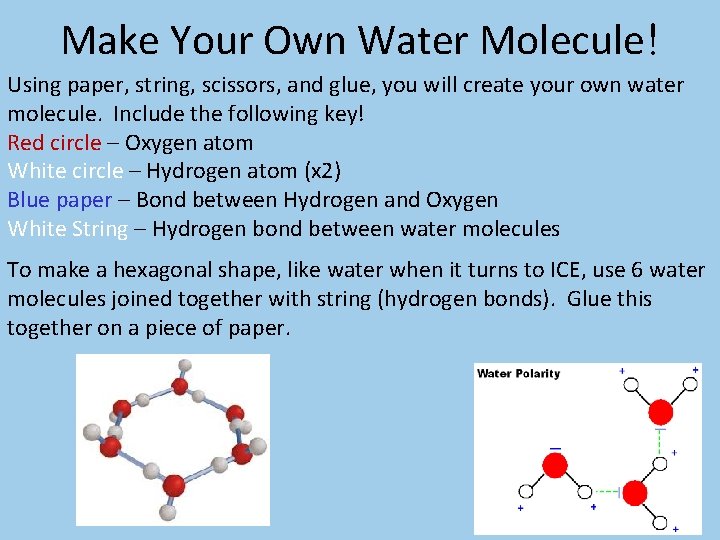
Make Your Own Water Molecule! Using paper, string, scissors, and glue, you will create your own water molecule. Include the following key! Red circle – Oxygen atom White circle – Hydrogen atom (x 2) Blue paper – Bond between Hydrogen and Oxygen White String – Hydrogen bond between water molecules To make a hexagonal shape, like water when it turns to ICE, use 6 water molecules joined together with string (hydrogen bonds). Glue this together on a piece of paper.

Water is Special! A Very Serious Video About Water A Very Silly Video About Water